orgo hydrogenation mechanisms
1/19
Earn XP
Description and Tags
suicidal.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
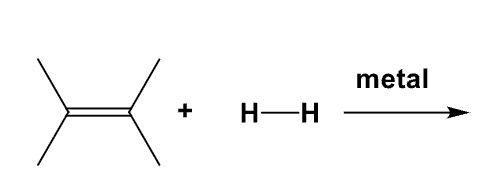
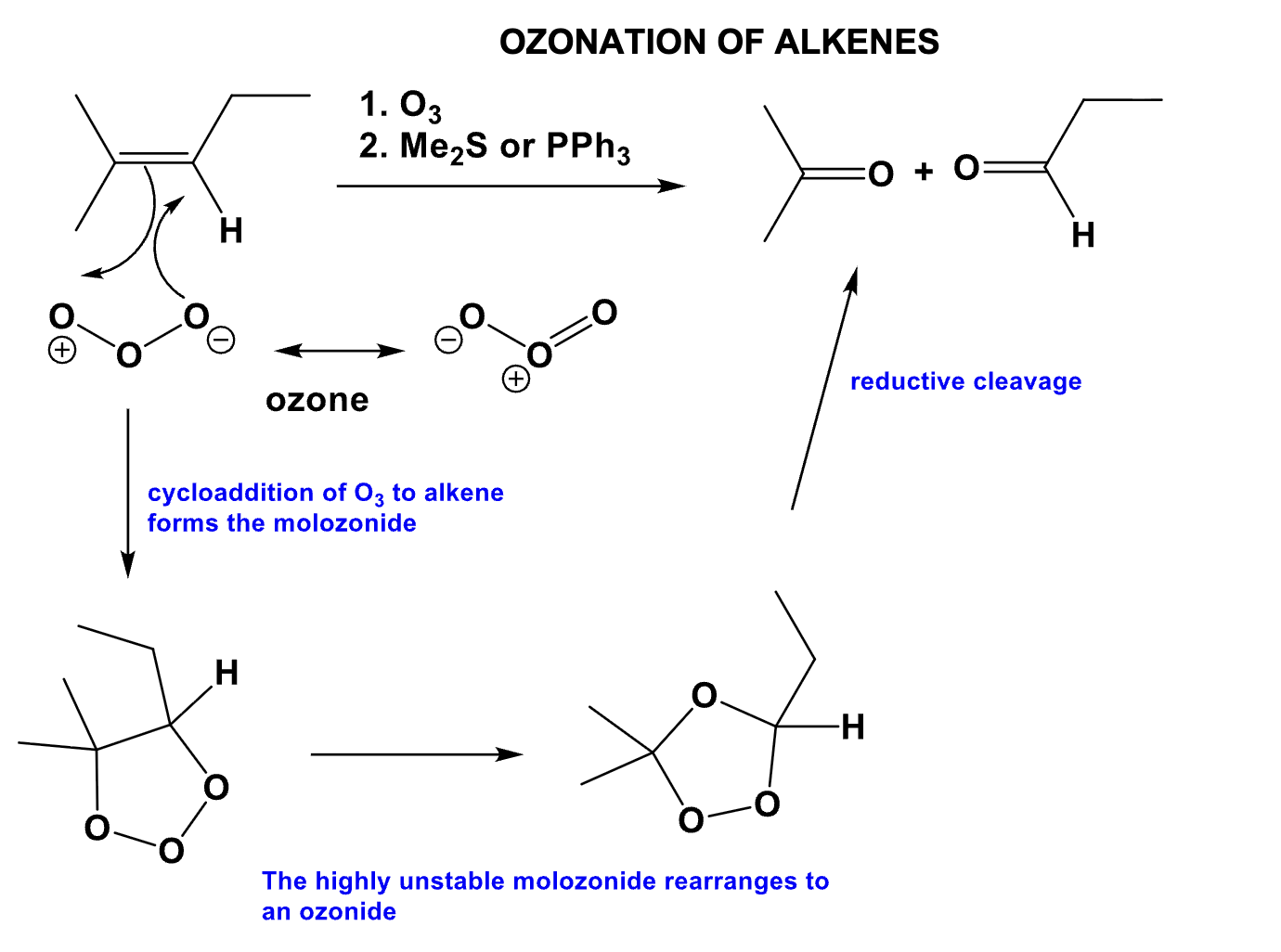
What is the product of this mechanism?
All catalytic mechanisms can be drawn as a cycle.
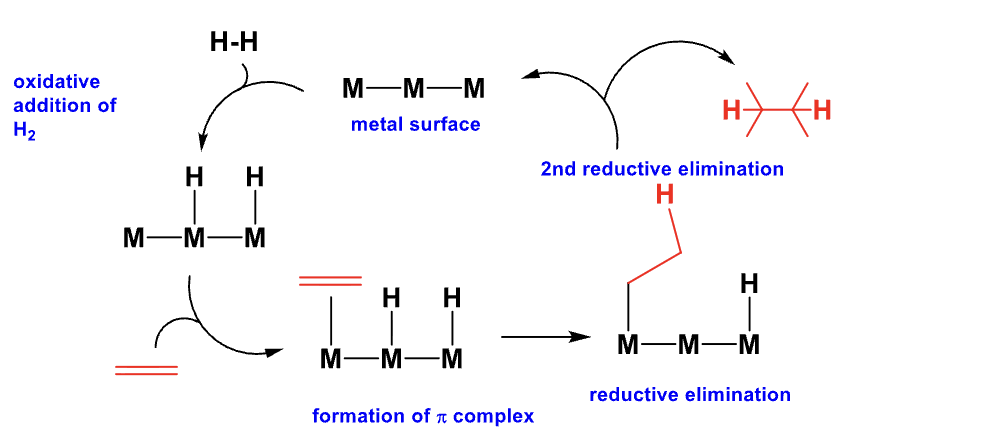
What is the product of this mechanism?
This is called SYN Addition of Hydrogen.

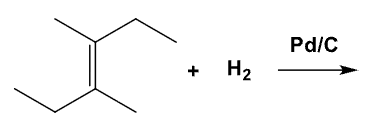
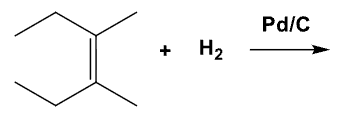
What is the product of this mechanism?
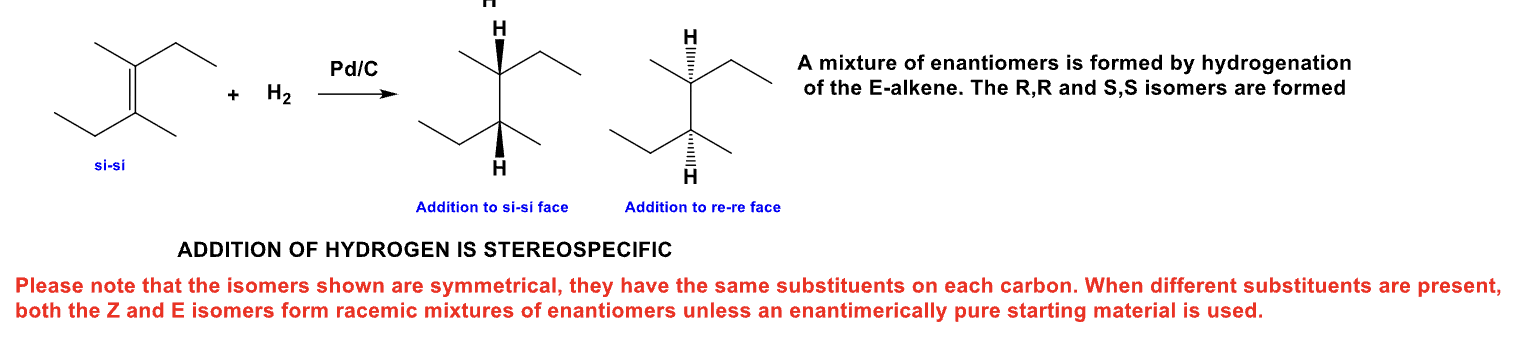
Explain the following:
- Name and what happens in the mechanism
- Where the halogen ends up at
- The rate rule and which halogens is better than others
The stereochemistry
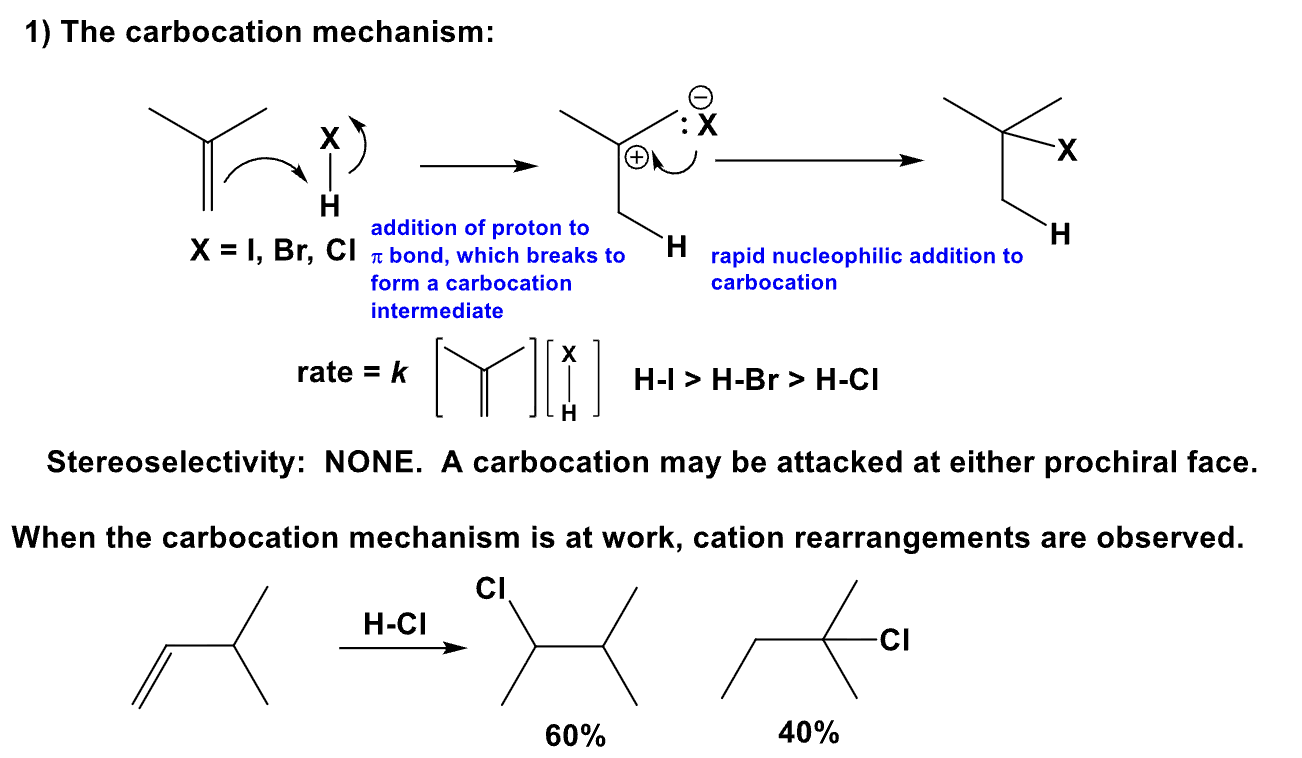
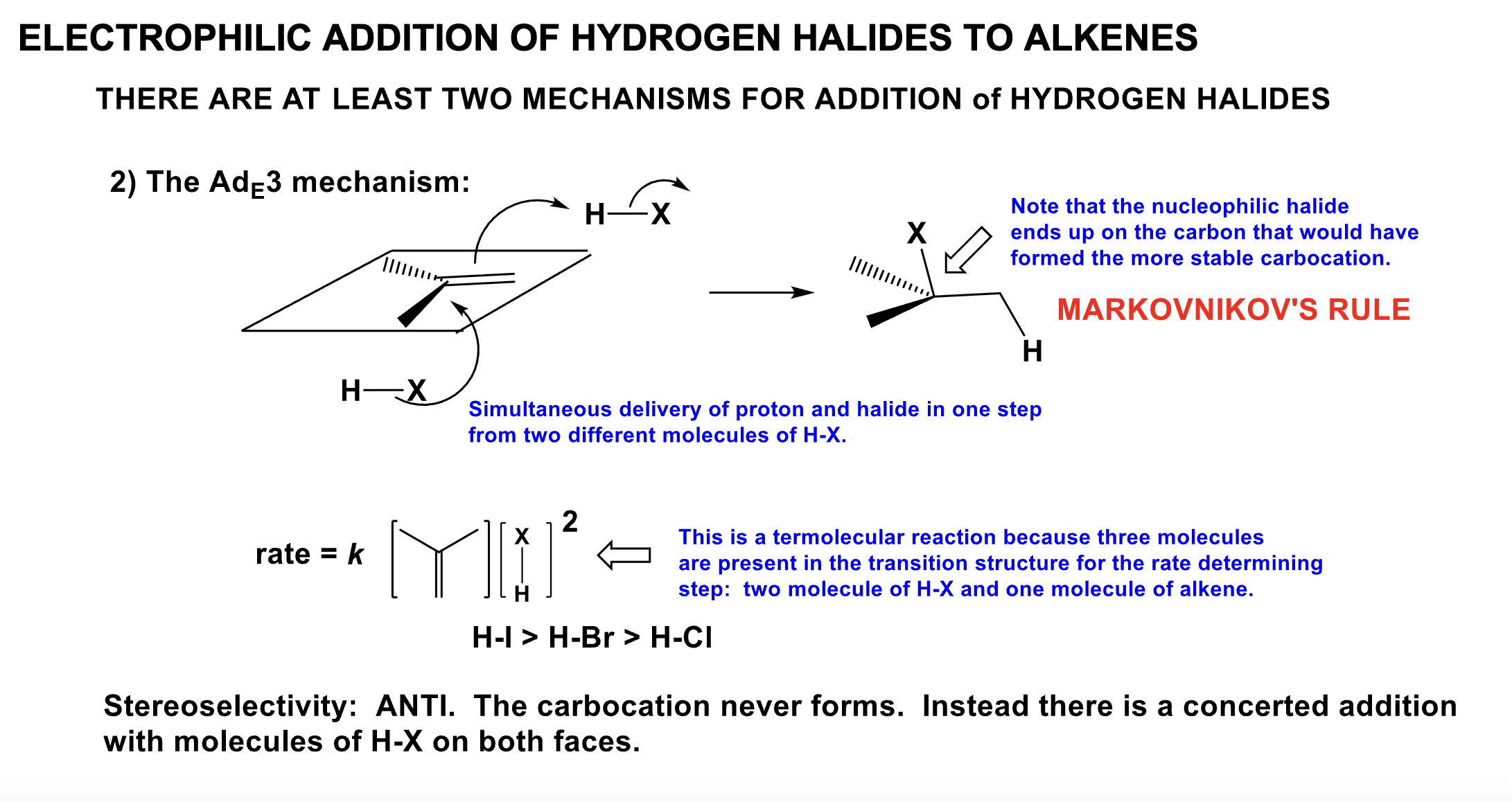
Explain the AdE3 Mechanism
- What is Markovnikov’s Rule?
- Define the rate and its sterochemistry
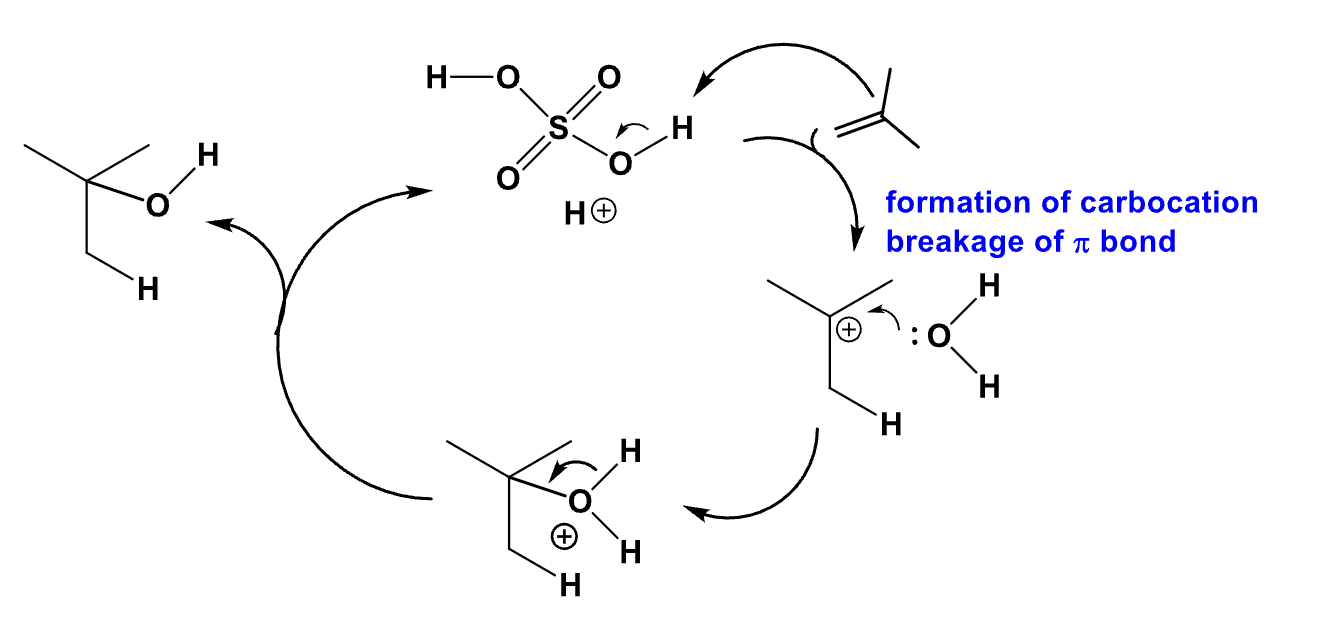
Draw the cycle of acid catalyzed hydration using the carbocation mechanism
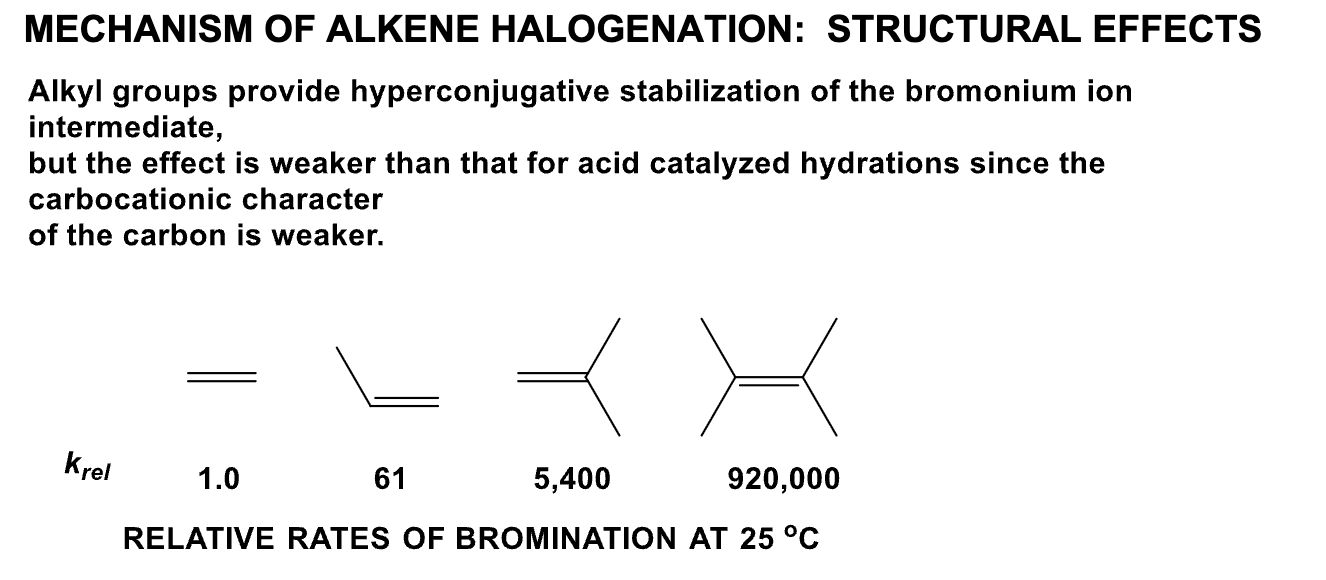
Since the carbocation is an intermediate, there will be a very strong dependence on electron density. The more electron rich an alkene is, the faster the reaction is.
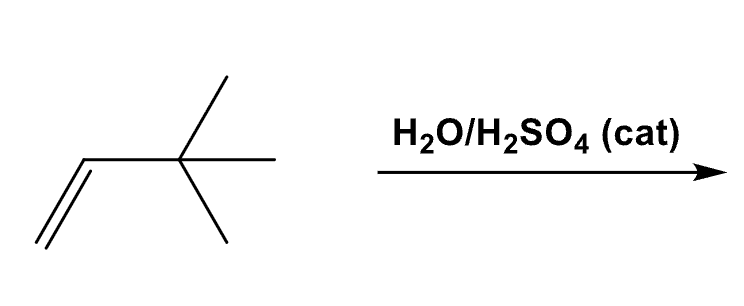
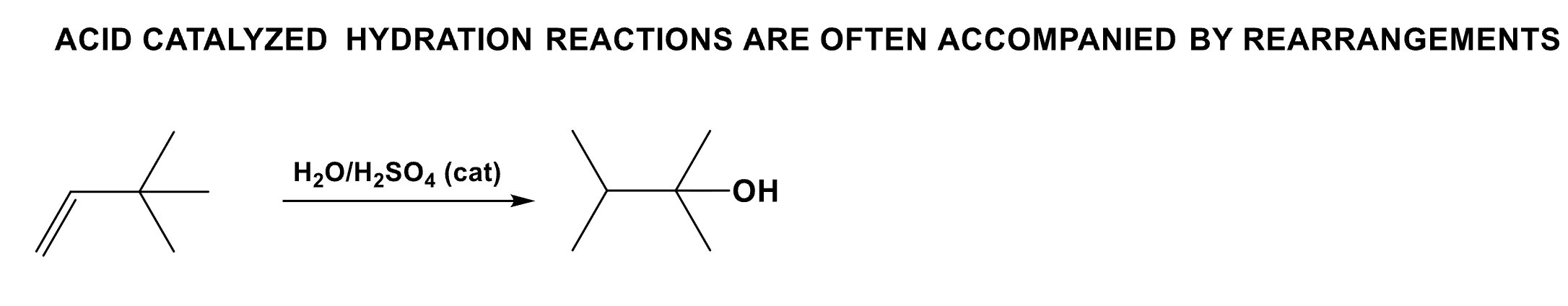
Draw the product
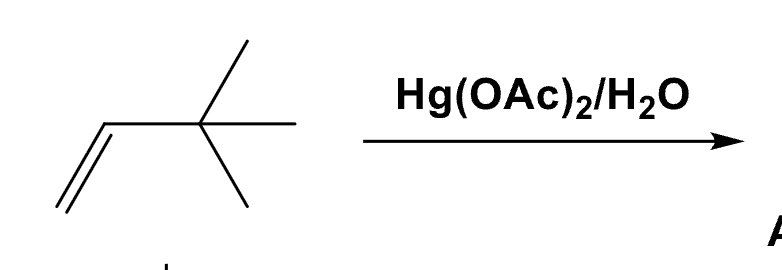
Draw the Oxymercuation-Demercuration of this alkene.
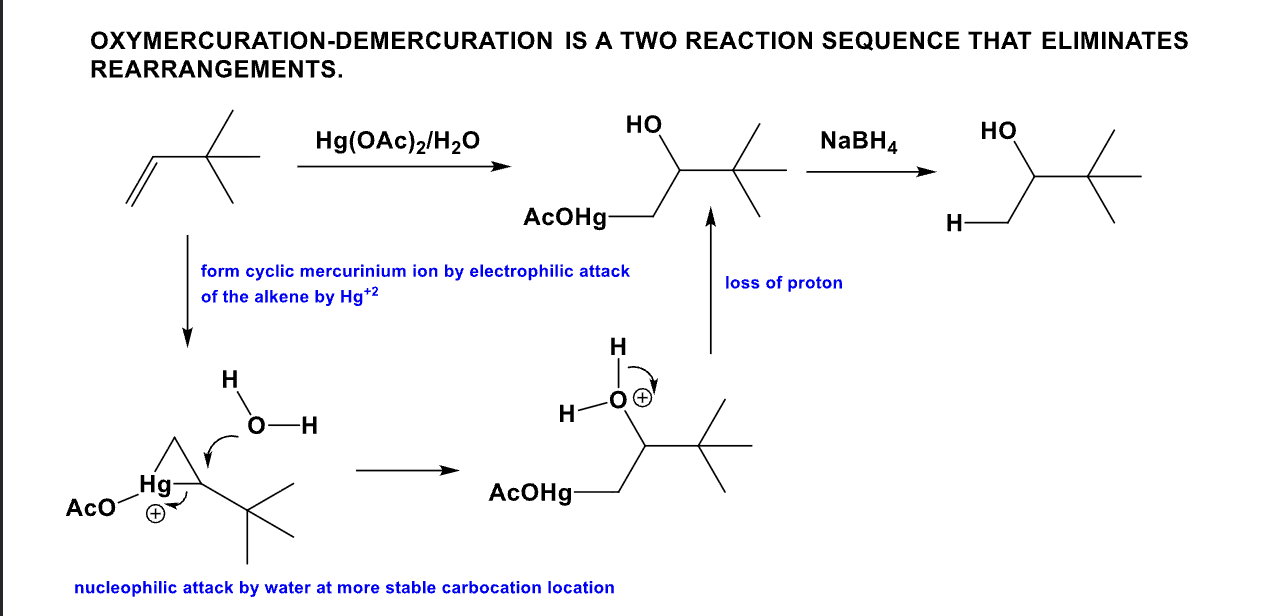
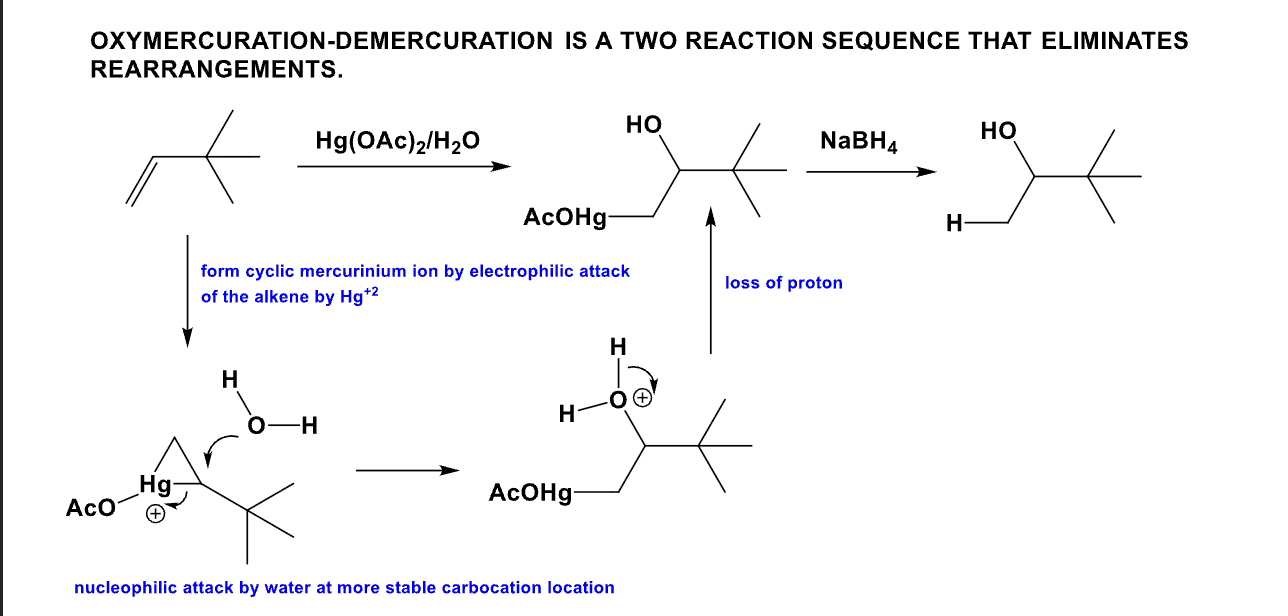
What is the product from this mechanism?
(Hint: Anti-Markovnikov rule)
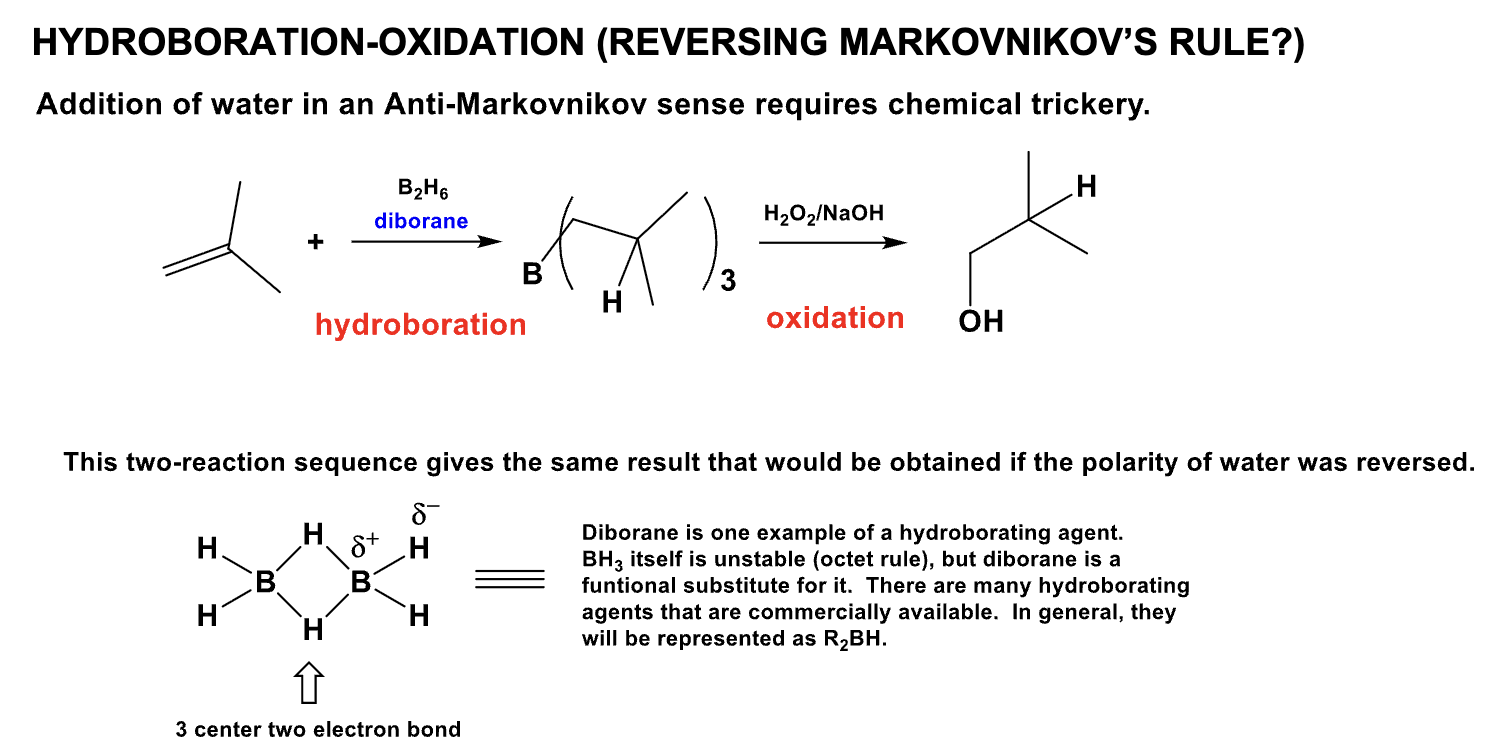
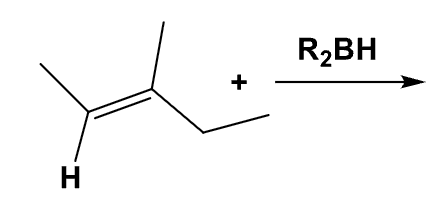
What is the product from this mechanism?
(Hint: Anti-Markovnikov rule)
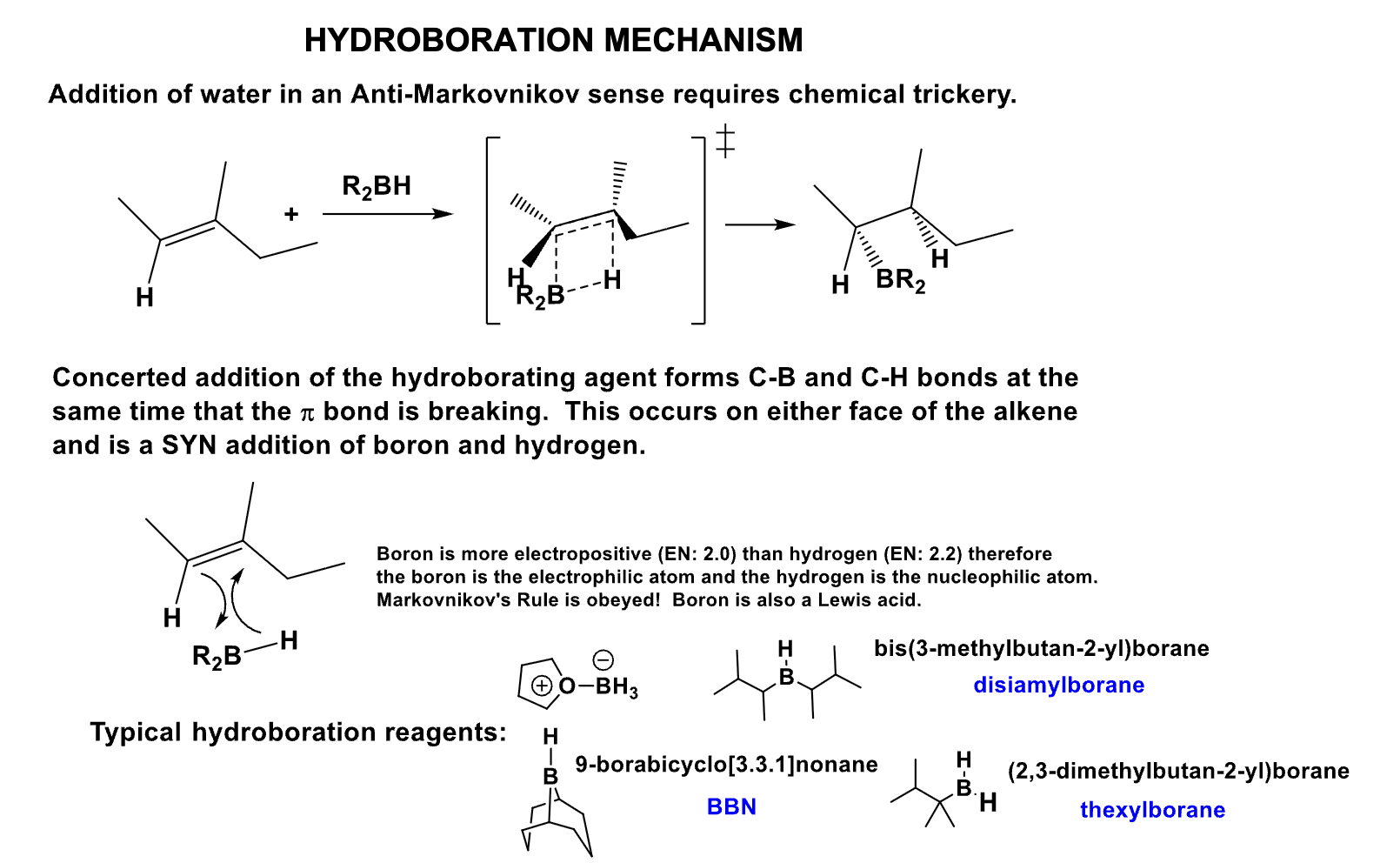
What is the mechanism of this alkylborane?
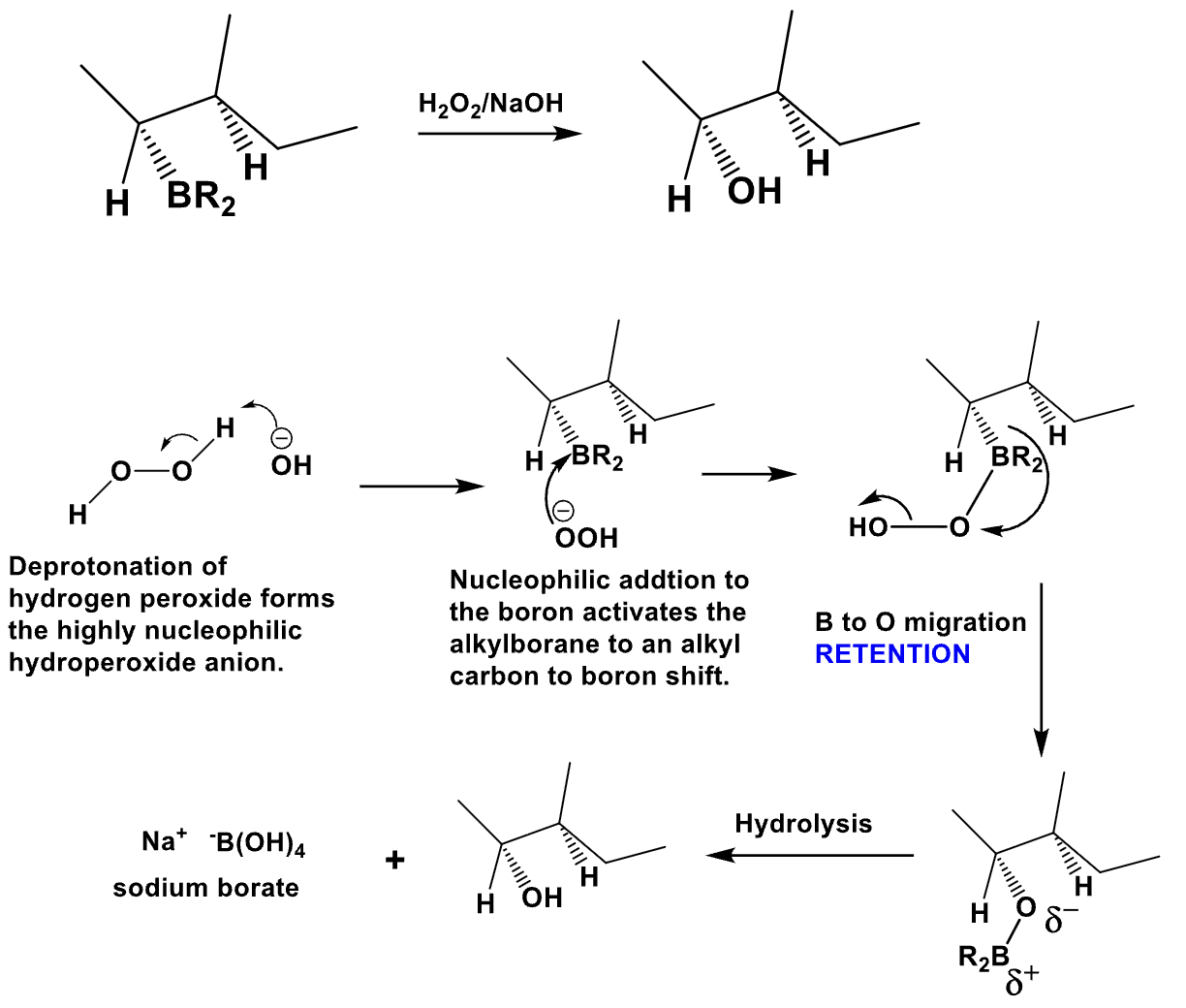
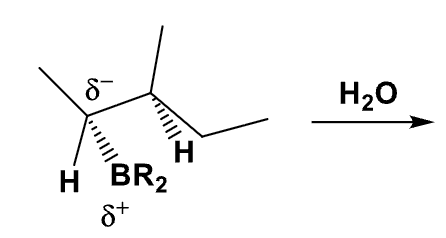
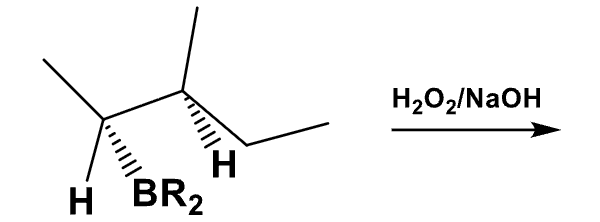
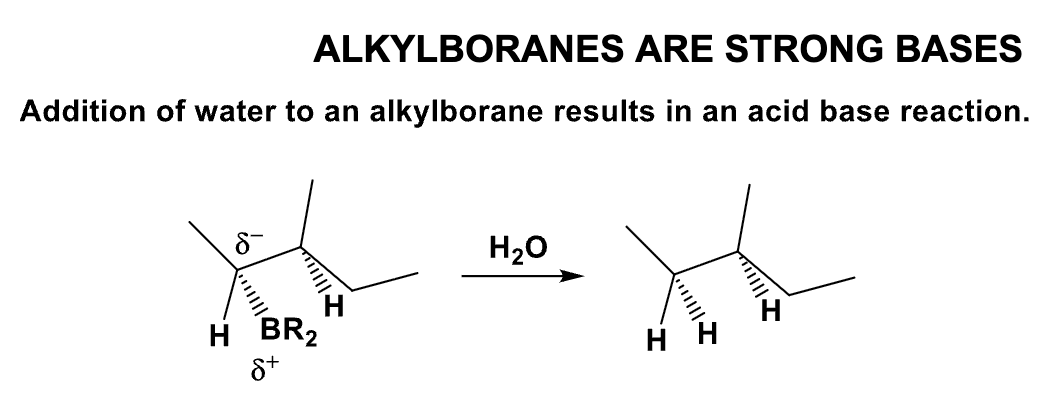
What is the product of this reaction? Explain what happens?
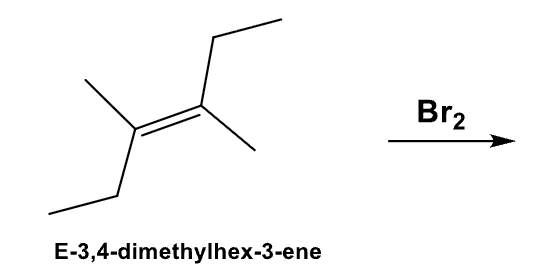
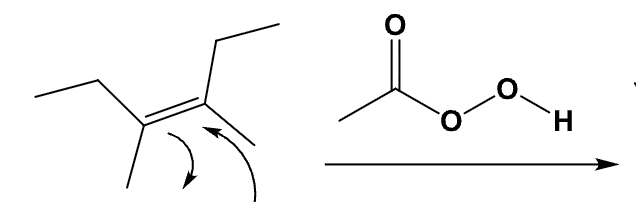
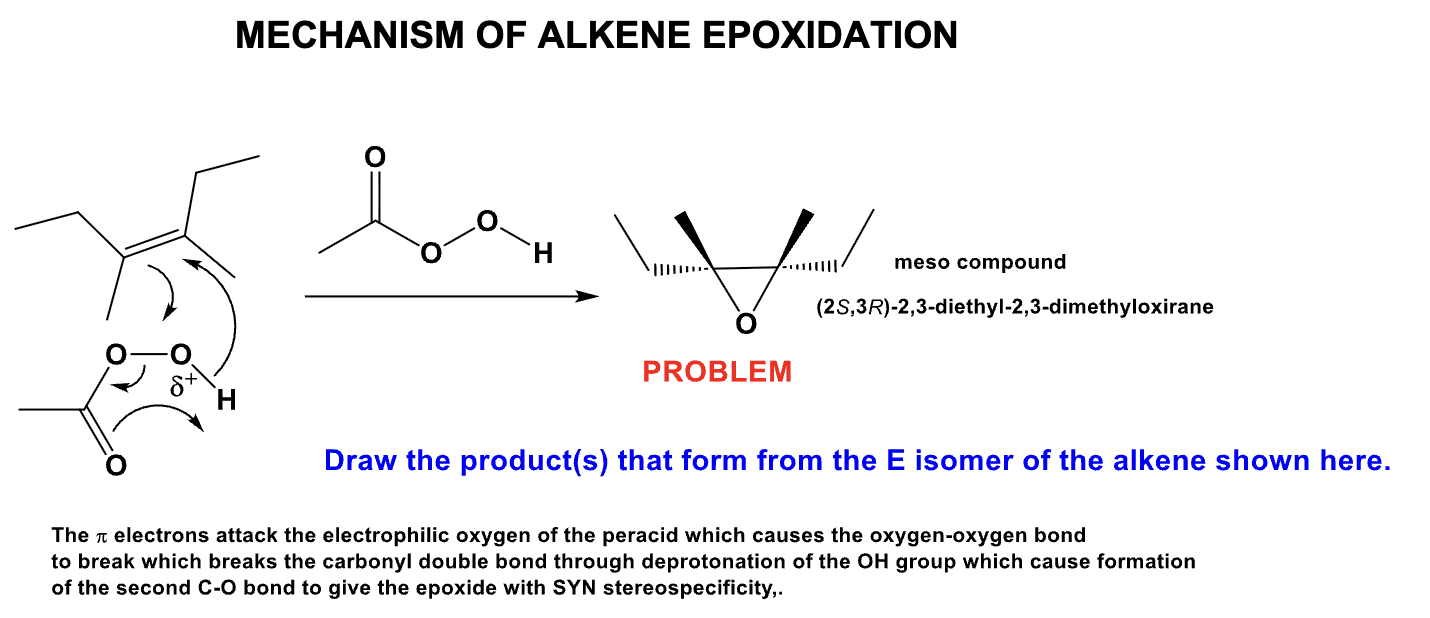
What is the product and define its stereospecificity?
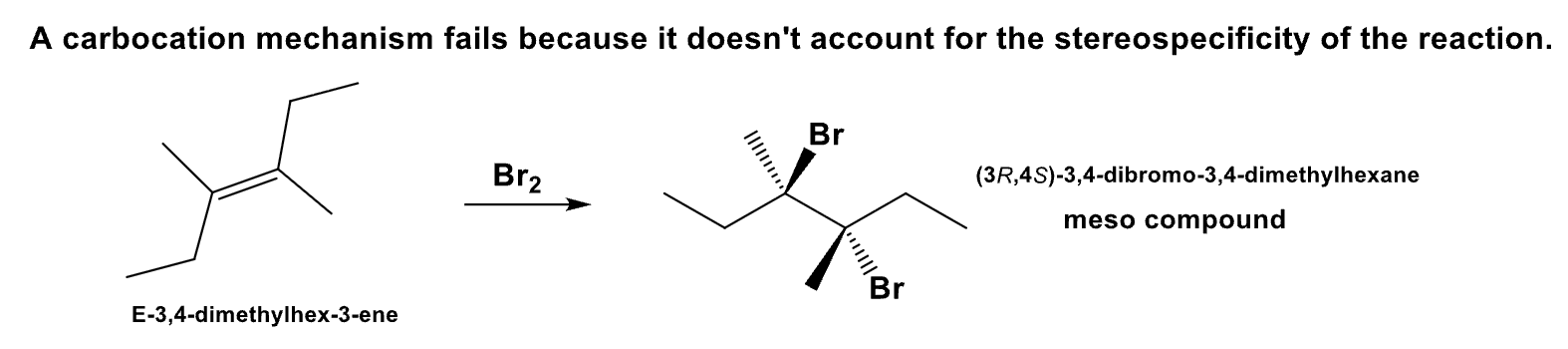
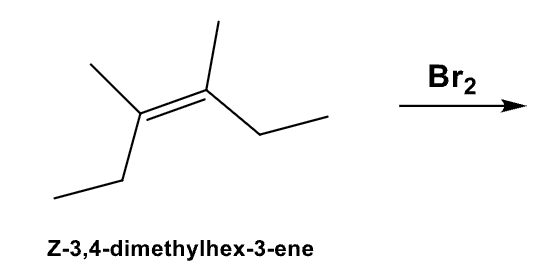
What is the product and define its stereospecificity?
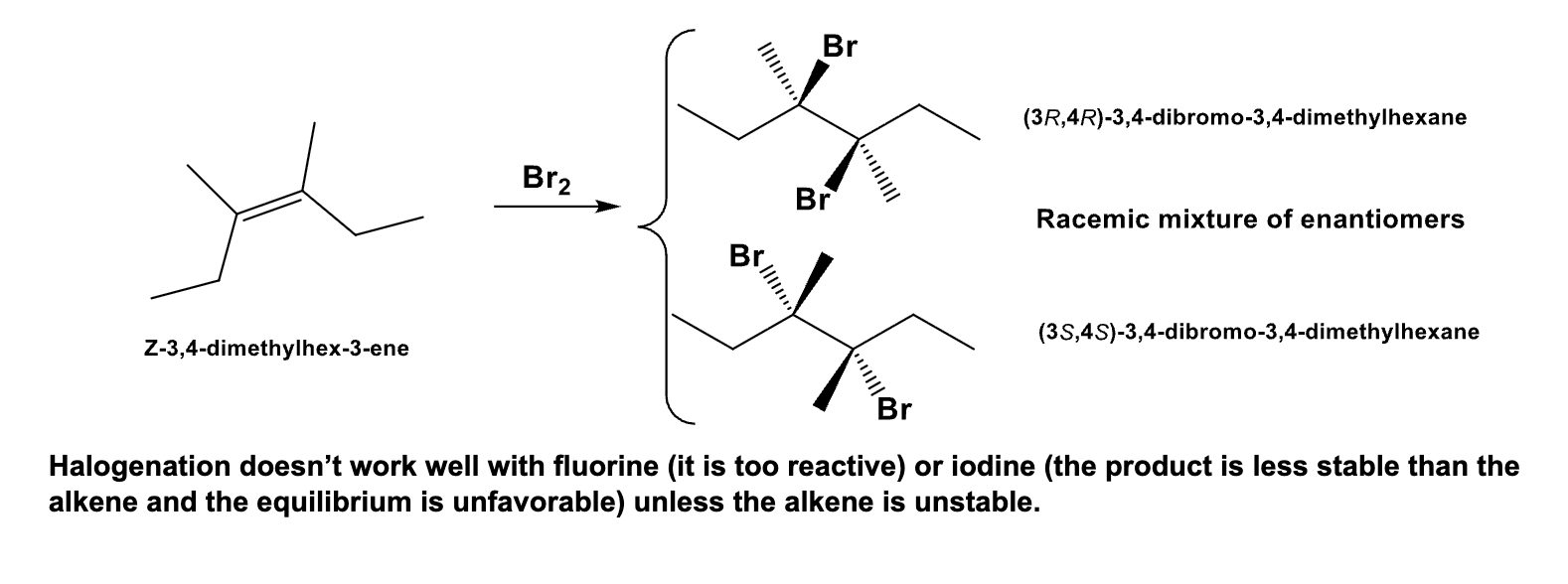
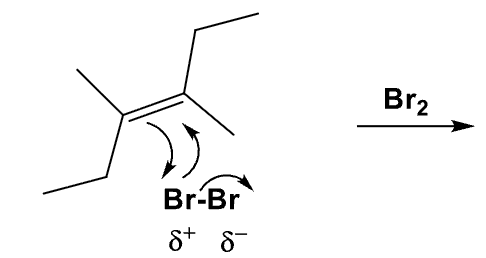
What is the product? Explain how you got ur answer
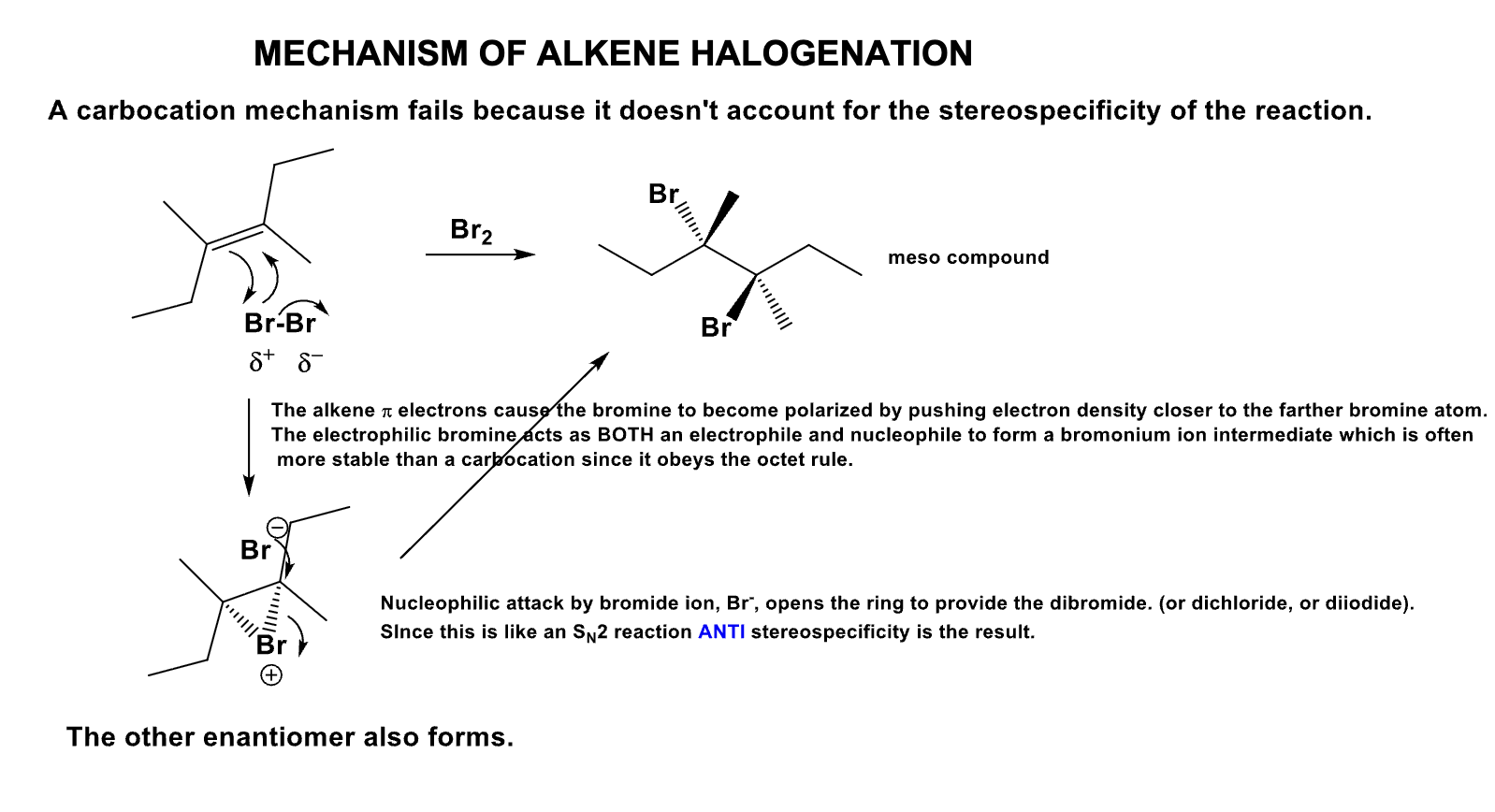
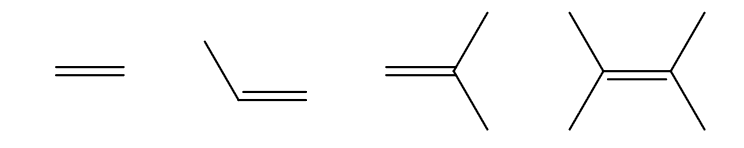
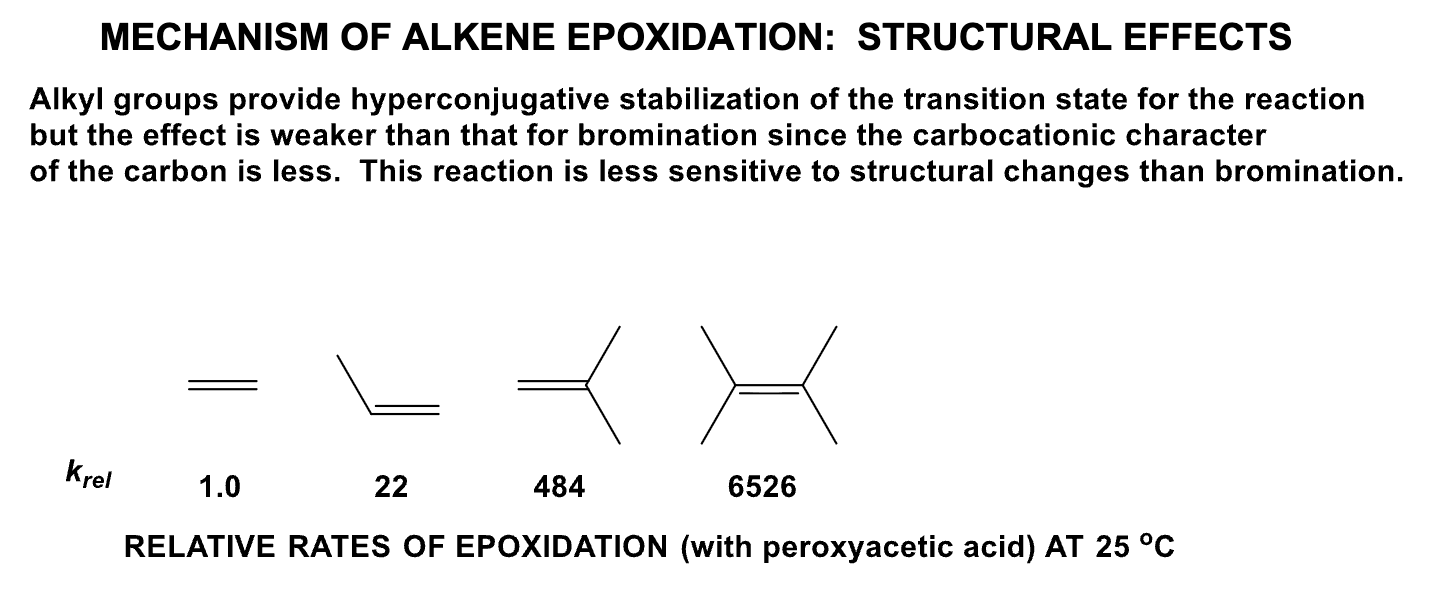
Which is the most reactive and which is the least reactive in terms of alkene halogenation? Why?
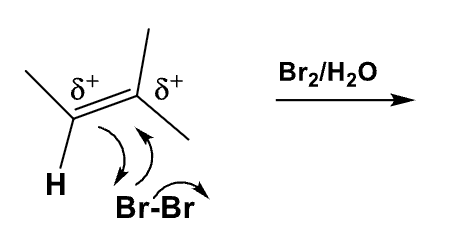
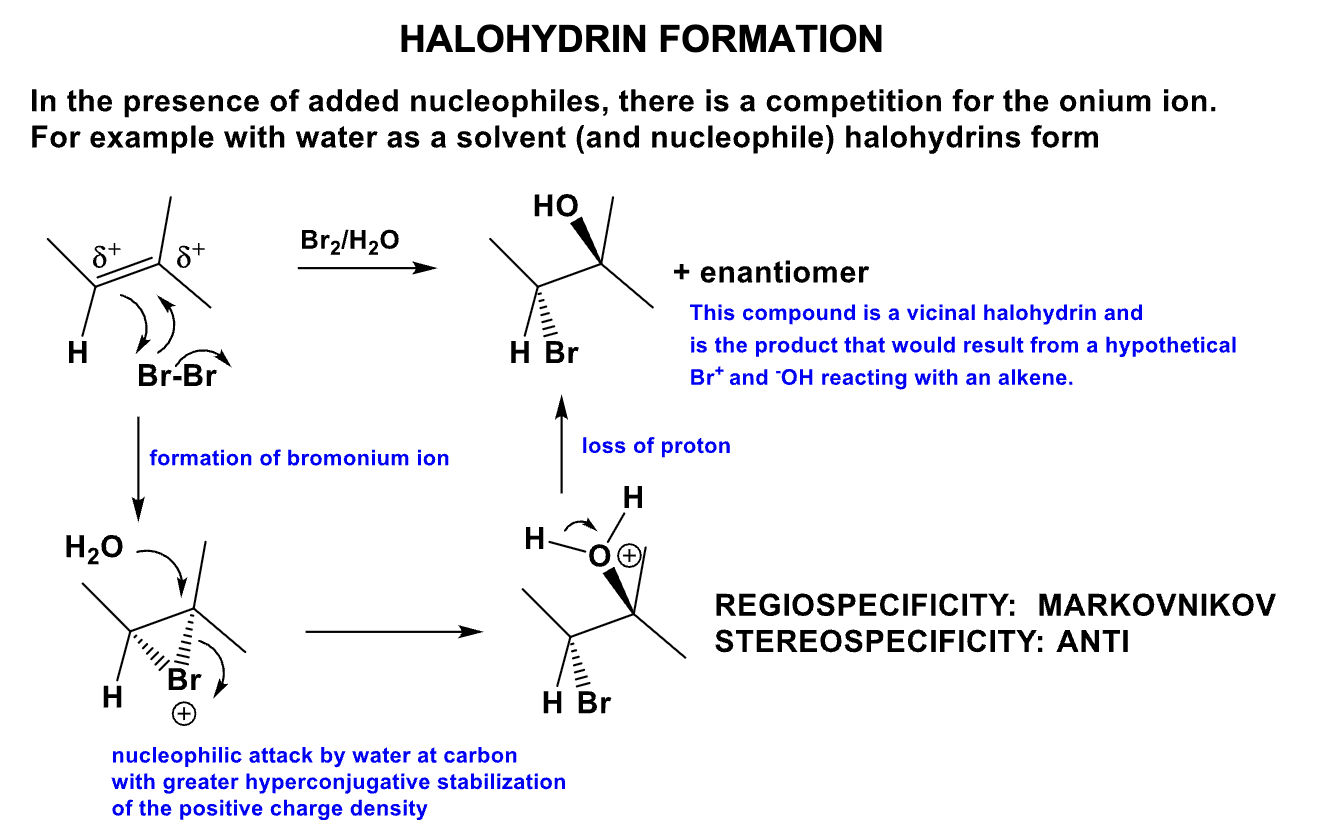
What is the formation of the product? What about the regiospecificity and the stereospecificity?
What kind of mechanism is this? What is the product? Explain whats happening
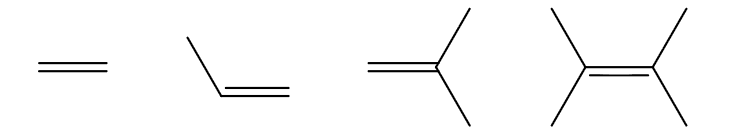
Which is the most reactive and which is the least reactive in terms of alkene halogenation? Why?
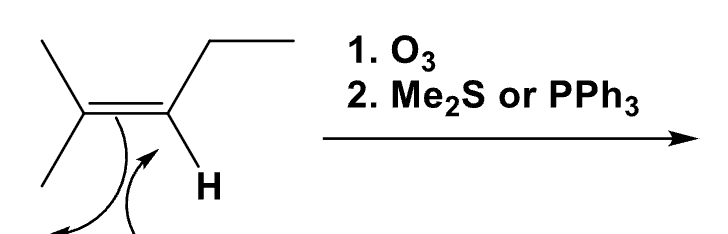
What mechanism is this? What is the product? Draw all steps