CH167
1/197
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
198 Terms
epoxide functional group

hemiacetal functional group
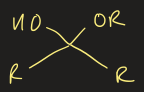
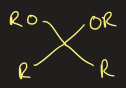
acetal functional group
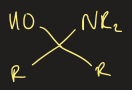
hemiaminal
imine functional group
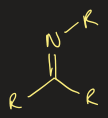
nitro group

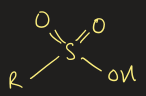
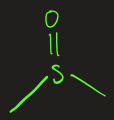
sulfonic acid
acetone

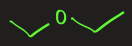
diethyl ether
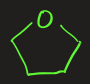
tetrahydrofuran (THF)
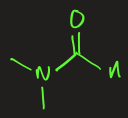
N,N-dimethylformamide (DMF)
dimethylsulfoxide (DMSO)
structural isomer definition
identical formulae but different connectivity of the atoms
double bond equivalents definition
the degree of unsaturation in a molecule based on its molecular formula
equation to work out double bond equivalents
DBE = C + N/2 - H/2 - X/2 + 1
X = any halogen
conformational isomers definition
structures that can be converted by rotation around a single sigma bond
what conformational isomer of ethane is most stable and why
staggered conformation is more stable sue to electrons repelling
what is a mesomeric effect
used to describe electrons withdrawing/donation properties of substituents based on relevant resonance structures
what is negative mesomeric effect and draw example
when the substituent is an electron-withdrawing group
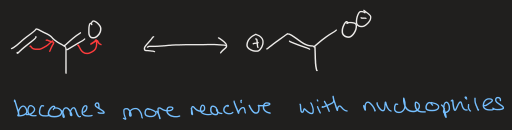
what is positive mesomeric effect and draw example
when the substituent is an electron donating group
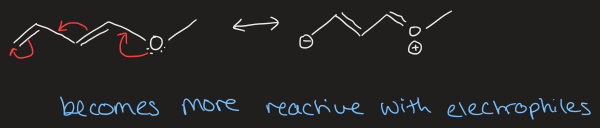
what is the inductive effect
shifting of electrons in a sigma bond due to difference in electronegativity of nearby atoms, resulting in bond polarisation
what is a polar molecule
a molecule that has an overall molecular dipole as a result of polarised bonds within their structure
what is the difference in range between mesomeric and inductive effect
mesomeric can be very long range but inductive effect is only significant over a short range
how do you stabilise carbocations
carbocations with adjacent pi-bond or atom with lone pari can be stabilised through resonance (mesomeric)
more hyperconjugation = more stable
what stabilises/destabilises carbocations vs carbanions
Carbocations | Carbanions |
Stabilised by delocalisation into adjacent pi-systems | Stabilised by delocalisation into adjacent pi-systems |
Stabilised by adjacent atoms bearing lone pairs | Destabilised by adjacent atoms bearing lone pairs |
Stabilised by inductively donating groups | Destabilised by inductively donating groups |
Destabilised by inductively withdrawing groups | Stabilised by inductively withdrawing groups |
Occupy a vacant p-orbital (high energy unfilled) | Can occupy a p-orbital (if in conjugation with pi-system). Otherwise, stabilised by increased 's' character in orbital |
what is pKa
a measure of the ability of an acidic compound to give up a proton
strong acid = low pKa
what does the difference in pKa tell you
the log of the equilibrium constant
factors that affect pKa
intrinsic stability of the conjugate base
strength of HA bond
how well cations and anions are solvated
how can you stabilise an anion
electronegativity of atom bearing negative charge
negative charge better stabilised on a more electronegative element
hybridisation of atom bearing the negative charge
Better stabilised in an orbital with more s character
size of atom bearing negative charge
better stabilised on larger atom
delocalisation of negative charge (mesomeric effect)
more delocalisation = better stabilised
inductively electron-withdrawing
inductively electron-withdrawing groups stabilise negative charge
what is pKaH
pKa of corresponding conjugate acid
lower pKaH = weaker base
factors affecting pKaH
availability of lone pair
increasing s-character of lone pair = held tighter to nucleus so less available = weaker base
stability of cationic ammonium
more resonance forms = more stable = more basic
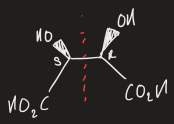
what is a chiral object
objects that cannot be superimposed onto their mirror image
what is an achiral object
object that can be superimposed onto their mirror image (contains an internal mirror plane)
what are enantiomers
non-superimposable mirror images, that rotate plane polarised light in equal and opposite directions
opposite configuration at all stereocentres
how to convert between enantiomers
invert all stereocentres
racemic mixture definition
an equimolar mixture of two enantiomers
how to assign absolute configuration
prioritise 4 substituents based on atomic number
arrange molecule such that lowest priority is pointing away
if the other substituents priorities are clockwise then it is R configuration and anticlockwise = S
how to interchange between R and S configuration
either:
draw mirror image
or
swap any two substituents
how to work out how many stereoisomers a molecule will have depending on number of stereogenic centres
number of stereoisomers = 2N
N = number of stereogenic centres
diastereoisomers definition
stereoisomers that are not enantiomers:
non-superimposable, non-mirror images
different chemical and physical properties
opposite configuration at some but not all stereocentres
how to convert between diastereoisomers
invert at least one, but not all stereo centres
meso compounds definition
a compound that contains two or more stereocentres but is superimposable on its mirror image
contain internal mirror plane
identical substituents on each stereo centre must be identical
achiral
conformation definition
any one of the infinite number of possible spatial arrangements of atoms in a molecule
how to convert between conformations
rotation about sigma bonds
configuration definition
spatial arrangement of atoms in a molecule
finite number of possible configurations
can only be converted by breaking bond s
what are achiral diastereoisomers
trans/cis alkenes are non-superimposable non-mirror images and are diastereoisomers even if they don’t contain stereocentres
what is a cis alkene
Z alkene
what defines whether geometric isomers can be cis or trans
restricted rotation
atoms bearing substituents have at least one substituent the same
what does the Hammond Postulate state
the transition state will be most similar in structure to the species it is closest to in energy
how to predict a reaction mechanism
electrons start somewhere electron-rich (nucleophile)
order of choice
negative charge
lone pair
multiple bond
weak single bond
electrons move somewhere electron-poor (electrophile)
order of choice
positively charges carbon or hydrogen
an atom directly attached to a positively charged heteroatom
partial positive charge on a carbon or hydrogen
electrons end up somewhere goof
either:
quenched by C+ and H+ by a new bond being formed to C or H
by breaking pi bond to a heteroatom, leave it with a single bond
by breaking sigma bond to a heteroatom, disconnecting it from the molecule
charges must be balanced
may have to reapply rules 1-3 again
nucleophile definition
a reagent that forms a bond to its reaction partner by donating both bonding electrons
electrophile definition
a reagent that forms a bond to its reaction partner by accepting both bonding electrons
bronsted base definition
a reagent that forms a bond to a proton by donating both bonding electrons
bronsted acid definition
a reagent that donates a proton to form a new bond
what is the priority of where electrons start
anion
lone pair
multiple bond
weak single bond
how to choose between electrons starting from reactive sites at the same level
electronegativity
less electronegative = more reactive as they need to give away a pair of electrons
mesomeric effects (resonance)
resonance stabilises anion or lone pair = less nucleophilic
steric effects
less hindered = most nucleophilic
order of priority of where electrons move to
electron deficiency
more electron deficient = more electrophilic
steric effects
least sterically hindered = more favoured site of reaction
regioselectivity definition
the preference of chemical bonding or breaking in one direction over all other possible directions
what does an SN2 reaction mean
S = substitution
N = nucleophilic
2 = bimolecular rate determining step
what are the factors affecting SN2 reactions
reactivity of nucleophile
leaving group ability of X
substitution at the electrophilic carbon
nature of the solvent
what affects the reactivity of the nucleophile
less electronegative elements are more nucleophilic
steric hinderance reduces nucleophilicity
higher pKaH = better nucleophile
what factors affect leaving group ability
strength of C-X bond
stability of the leaving group anion
strong acids readily dissociate to form stabilised anions
how do you know anion stability
check pKa
lower pKa = more stable anion
what happens to rate of reaction as degree of substitution increases
more substitution = slower rate of reaction so tertiary substituted atoms are probably SN1 because its so slow
what type of solvent favours SN2
polar aprotic solvents (e.g. DMF and acetone)
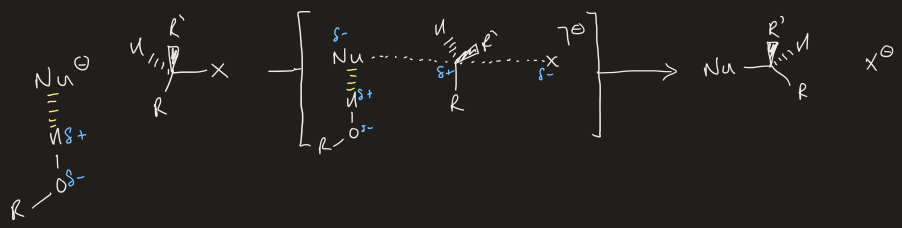
what is the Walden Inversion
when electrophile of SN2 reaction is chiral, reaction occurs with inversion of stereochemical configuration at C
draw a generic mechanism for SN1 reaction
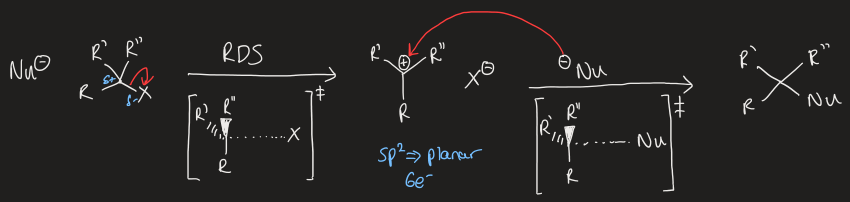
what factors affect SN1 reactions
leaving group ability of X
substitution at the electrophilic carbon
nature of the solvent
how does leaving group ability affect SN1 reactions
rate of reaction increases if leaving group ability increases
how does increasing number of substituents change reaction rate of SN1 reactions and why
rate of reaction increases
substituents stabilise cationic intermediate through hyperconjugation
how does increasing mesomerically donating groups affect rate of reaction
increasing rate of reaction as charge is allowed to spread out
what type of solvent favour SN1 mechanism
polar protic solvents due to stabilisation of both ion pairs in intermediate
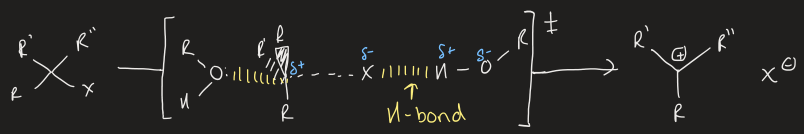
draw generic E2 mechanism
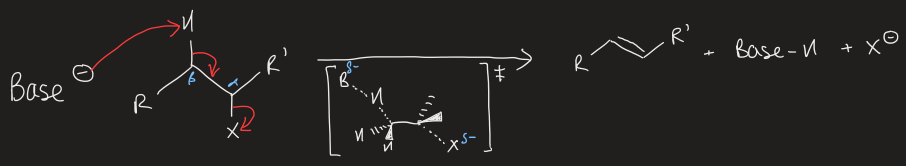
what two orbital interactions occur in E2 mechanism
2 electrons form base donate into σ*C-H breaking C-H bond
2 electrons form σC-H donate into empty σ*C-X and forming C=C bond
what favours E2 over SN2
more basic nucleophiles/bases
more sterically hindered nucleophiles/bases
higher reaction temperatures
what is the effect of temperature on elimination vs substitution reactions
in elimination, number of molecules increase so products are more disordered than reactants
elimination reactions involve positive change in entropy
become favourable at higher temperature
often performed above room temp
draw generic E1 mechanism
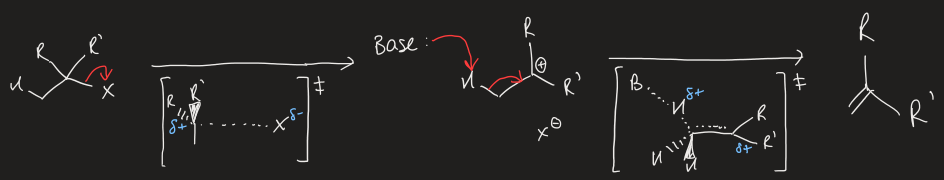
what is E1 favoured by over SN1
higher reaction temperatures
weak, non-nucleophilic bases
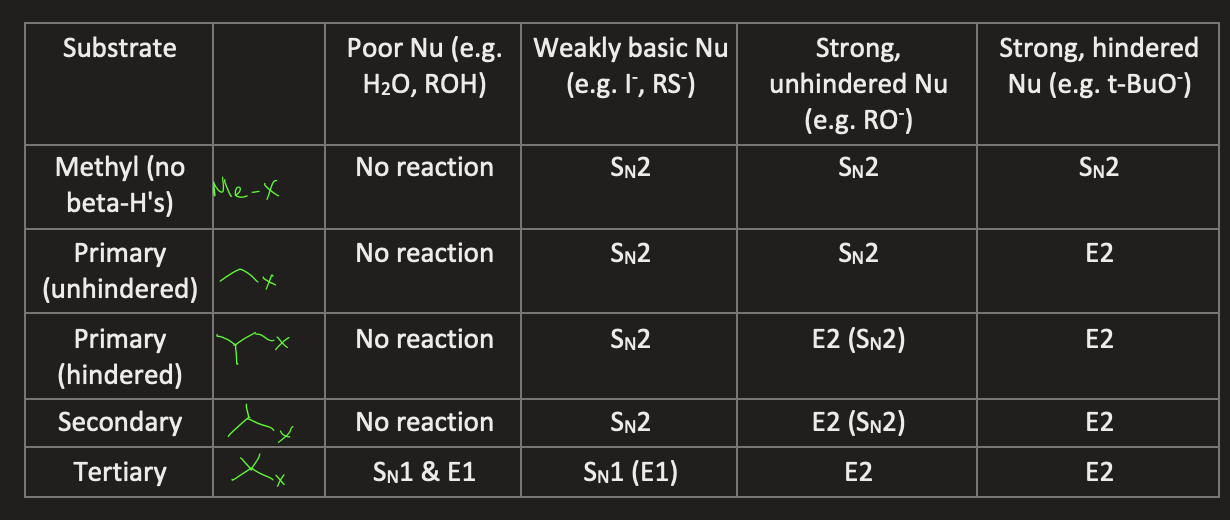
draw a table summary for substitution reactions vs elimination reactions for different substrates (methyl, unhindered primary, hindered primary, secondary and tertiary) and different nucleophiles (poor nu, weakly basic nu, strong unhindered nu, strong hindered nu)
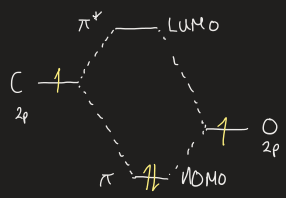
draw MO diagram for C=O
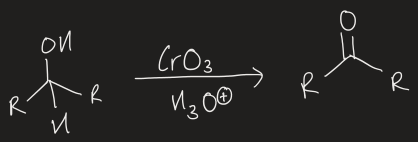
what reactants are needed to go from a 1o alcohol to a carboxylic acid
CrO3, H3O+ in acetone
how to go from a 1o alcohol down to an aldehyde, without going down to a carboxylic acid
use pyridinium chlorochromate (PCC) in anhydrous conditions
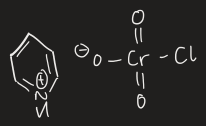
how to oxidise from 2o alcohol to ketone
use CrO3 and H3O+
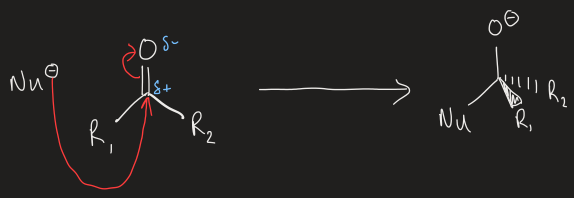
what is the general reaction mechanism for the addition of nucleophiles to aldehyde/ketone
what is the Burgi-Dunite angle
angle of attack
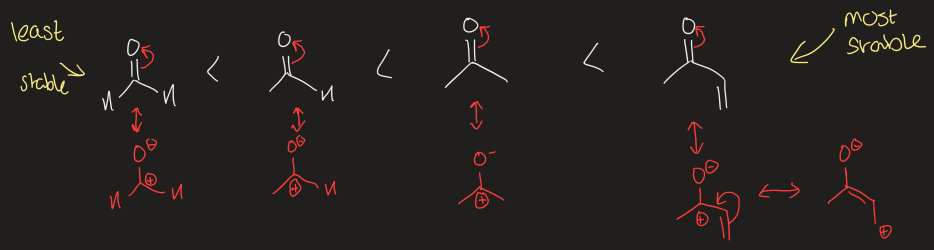
how does reactivity change for aldehydes and ketones
increased hyperconjugation/resonance = increased stability
inductive effect due to O being more electronegative than C
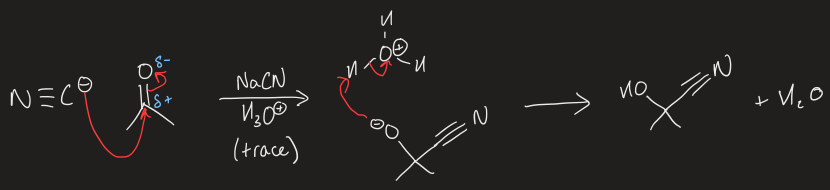
mechanism to form C-C bond on aldehyde/ketones using cyanide and acid
mechanism to remove cyanide using base to a ketone/aldehyde
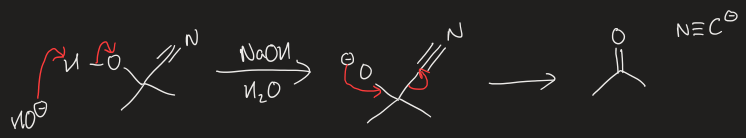
how do sterics affect K
planar molecules cause less crowding so larger K
larger substituents are felt more so lower K
mechanism for addition of organolithiums to aldehydes/ketones
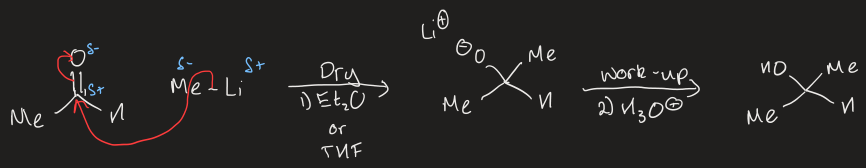
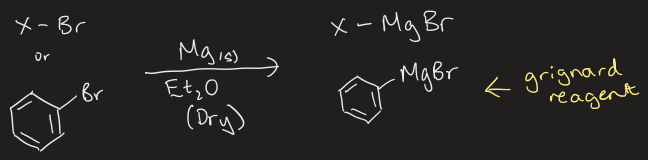
reaction to form Grignard reagent
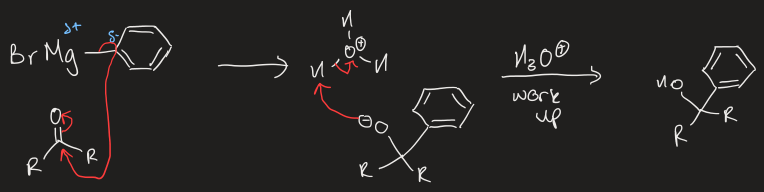
mechanism of Grignard reagent to aldehyde/ketones
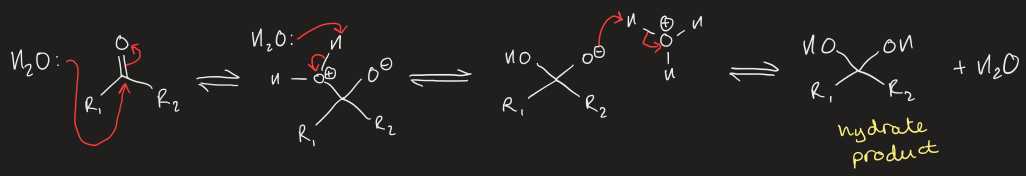
mechanism for nucleophilic addition of water to aldehyde/ketone (uncatalysed)
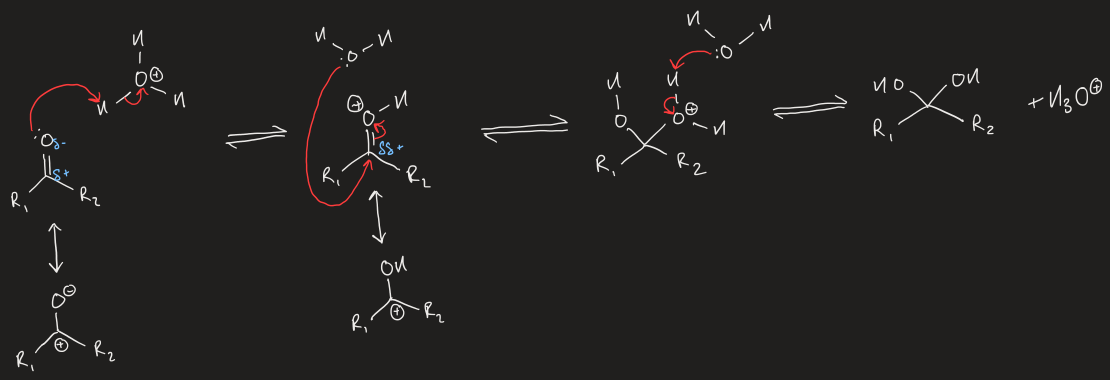
acid catalysis of protonation of carbonyl
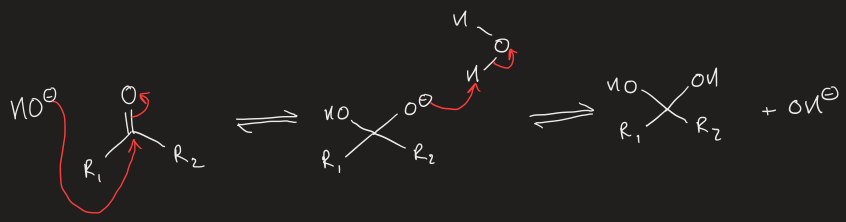
base catalysis of nucleophilic addition of aldehyde/ketone
mechanism for acid catalysed acetal/ketal formation from aldehyde/ketone
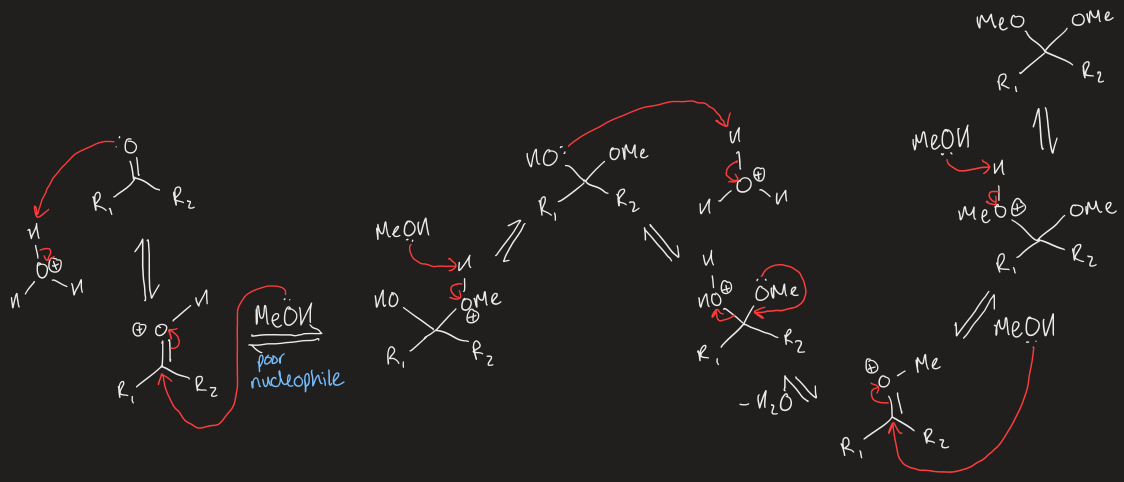
PADPEAD
what are acetals used for
protect C=O bond from nucleophilic attack
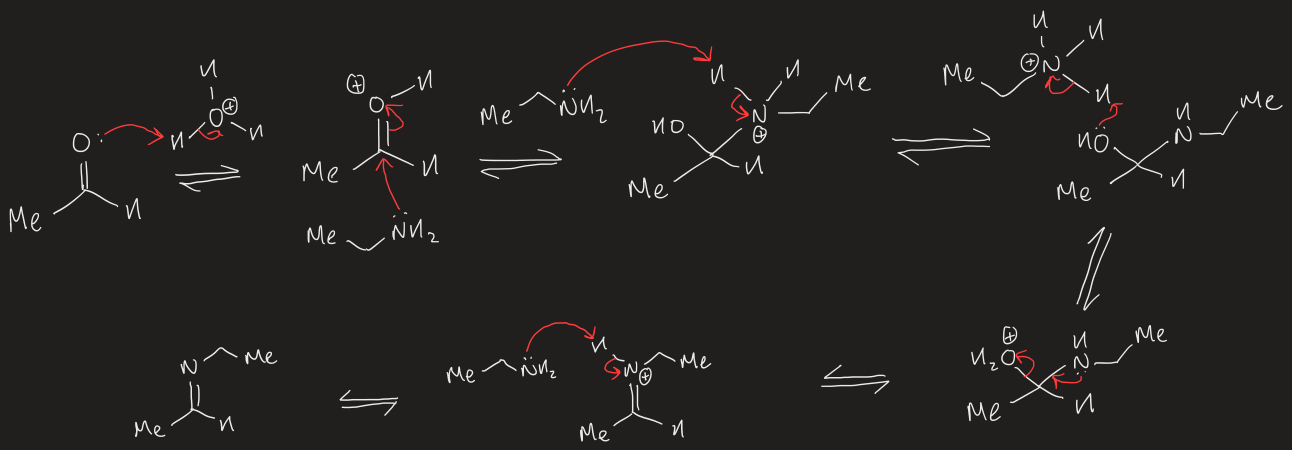
reaction to form aldimine

reaction to form ketimine
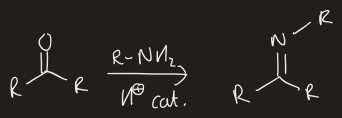
mechanism to form imines from aldehydes/ketones