Chemistry IGCSE - Electrochemistry
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
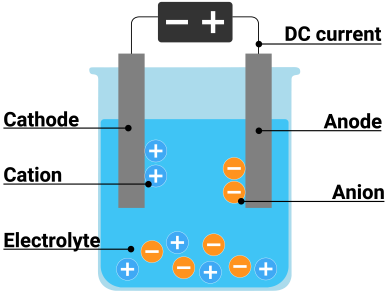
Electrolysis
decomposition of an ionic compound, when molten or in aqueous solution, by the passage of an electric current
Mnemonic for electrodes

Electrolyte
the molten or aqueous substance that undergoes electrolysis
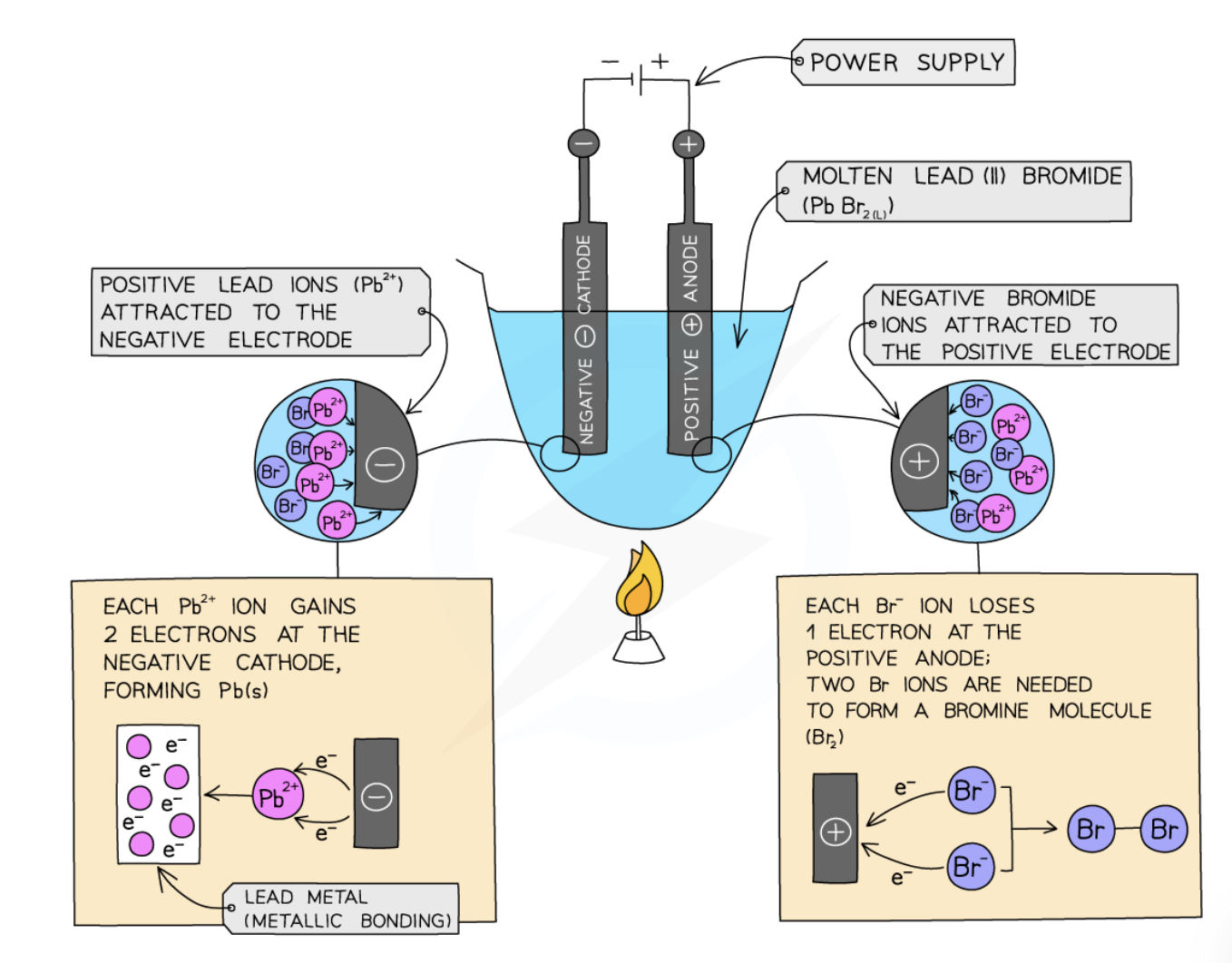
Electrolysis of molten lead(II) bromide (PbBr₂)
Cathode (–): Pb²⁺ + 2 e⁻ → Pb(l)
Lead metal (solid) collects at the cathode
Anode (+): 2 Br⁻ → Br₂(g) + 2 e⁻
Reddish brown bromine gas
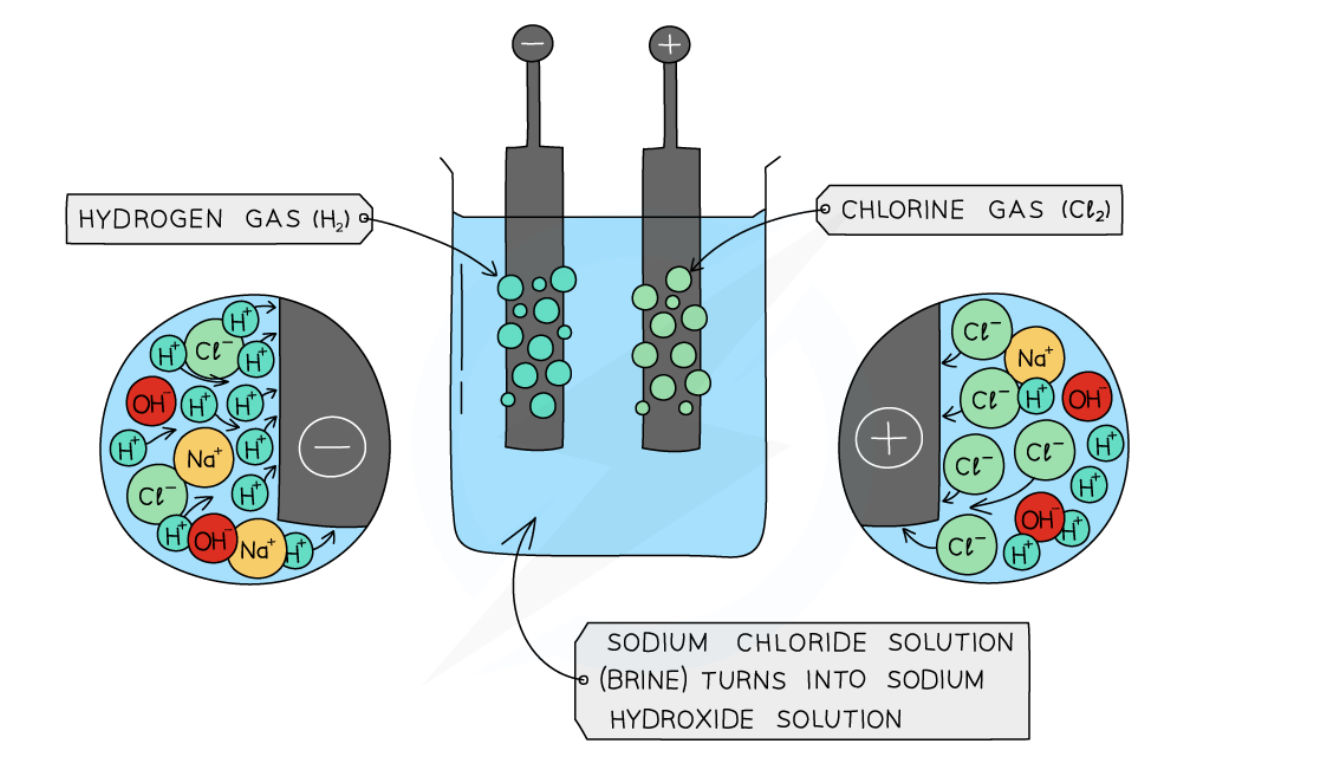
Electrolysis of concentrated aqueous sodium chloride (NaCl)
Cathode (–): 2 H₂O + 2 e⁻ → H₂(g) + 2 OH⁻
Hydrogen bubbles at cathode
Anode (+): 2 Cl⁻ → Cl₂(g) + 2 e⁻
Green - yellow chlorine gas bubbles at anode
Electrolysis of dilute sulfuric acid (H₂SO₄)
Cathode (–): 2 H₂O + 2 e⁻ → H₂(g) + 2 OH⁻
Bubbles of hydrogen gas at cathode
Anode (+): 2 H₂O → O₂(g) + 4 H⁺ + 4 e⁻
Oxygen bubbles at anode
Describe transfer of charge during electrolysis: the movement of electrons in the external circuit
electrons leave battery’s negative side → travel down the external wire → into the cathode (-) → out of the anode (+) → back to the battery’s positive side
Describe transfer of charge during electrolysis: the loss or gain of electrons at the electrodes
Cathode (-) ⟶ + ions from the molten or aqueous electrolyte gain electrons ⟶ reduction
Anode (+): - ions give up electrons ⟶ oxidation
Describe transfer of charge during electrolysis: the movement of ions in the electrolyte
cations (+) move to cathode (-) ⟶ anions (-) move to anode (+) through the electrolyte
What is produced using aqueous copper (II) sulfate (CuSO₄)
Cathode (-): Copper metal ( Cu²⁺ + 2 e⁻ → Cu(s) )
Reddish brown deposit of copper forms
Anode (+): Oxygen gas (4 OH⁻ → O₂(g) + 2 H₂O + 4 e⁻)
Bubbles of oxygen gas appear
What is produced using aqueous copper (II) sulfate (CuSO₄) with copper electrodes
Cathode (-): Copper metal (Cu²⁺ + 2 e⁻ → Cu(s))
Cathode stays shiny or gains a fresh copper coating
Anode (+): Copper metal is oxidized back to Cu²⁺ (Cu(s) → Cu²⁺ + 2 e⁻)
Copper anode slowly dissolves → mass decreases → solution stays blue.
metals or hydrogen are formed at the cathode (-)
Non metals (OTHER THAN HYDROGEN) are formed at the anode (+)
Cathode (+) → reduction → (RED CAT)
Anode (-) → oxidation
Order of reactivity
If metal is more reactive than hydrogen (ONLY FOR AQUEOUS SOLUTION) → hydrogen gas discharge at the cathode (-)
In DILUTE solutions → oxygen produced at the anode (+)
Hydrogen - oxygen fuel cell
uses hydrogen + oxygen → produce electricity with water as the only chemical product
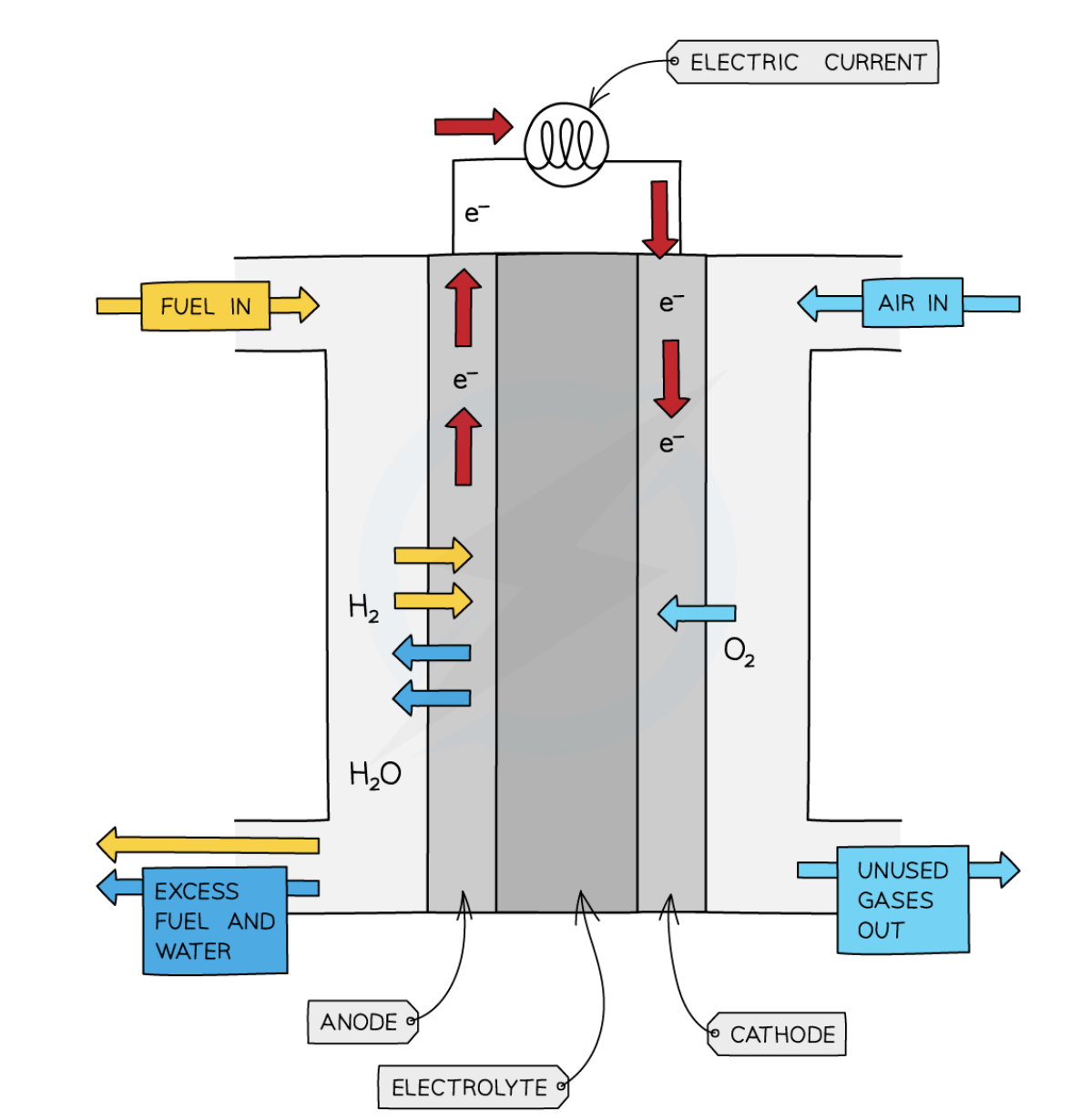
Pros + cons of a hydrogen - oxygen fuel cell
Pros | Cons |
|
|