A&P Chap 2- key elements, chemical bonds and reaction
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
12 and 99.9%
how many elements compose the human body
Major elements of the human body
Oxygen
Hydrogen
Carbon
Nitrogen
Calcium
Phosphorus
Lesser elements of the Human Body
Sulfer
Potassium
Sodium
Chlorine
Magnesium
Iron
Matter
anything that occupies space
Atom
smallest unit of an element
chemical symbol
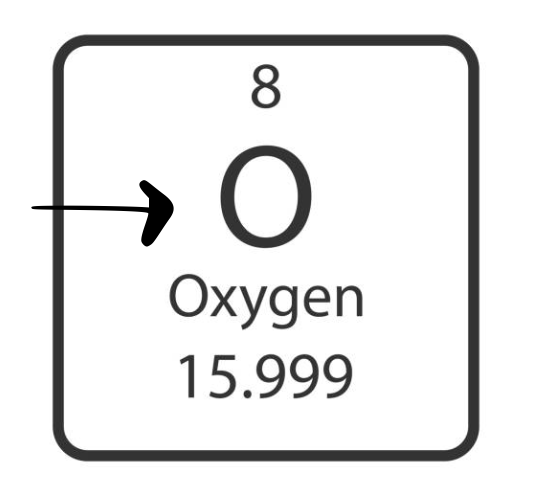
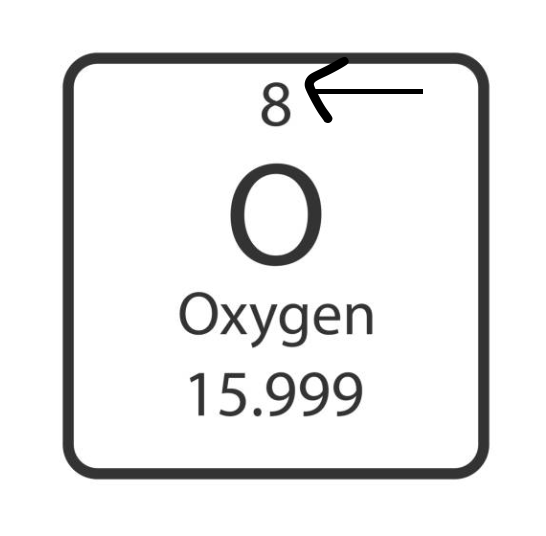
atomic number
number of protons
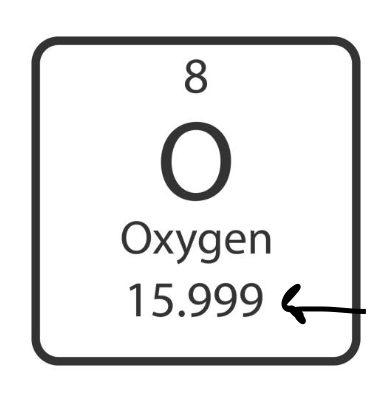
atomic mass
the sum of protons and neutrons
subatomic particles
Protons
Neutrons
Electrons
Electrons
Number of Protons is equal to the number of
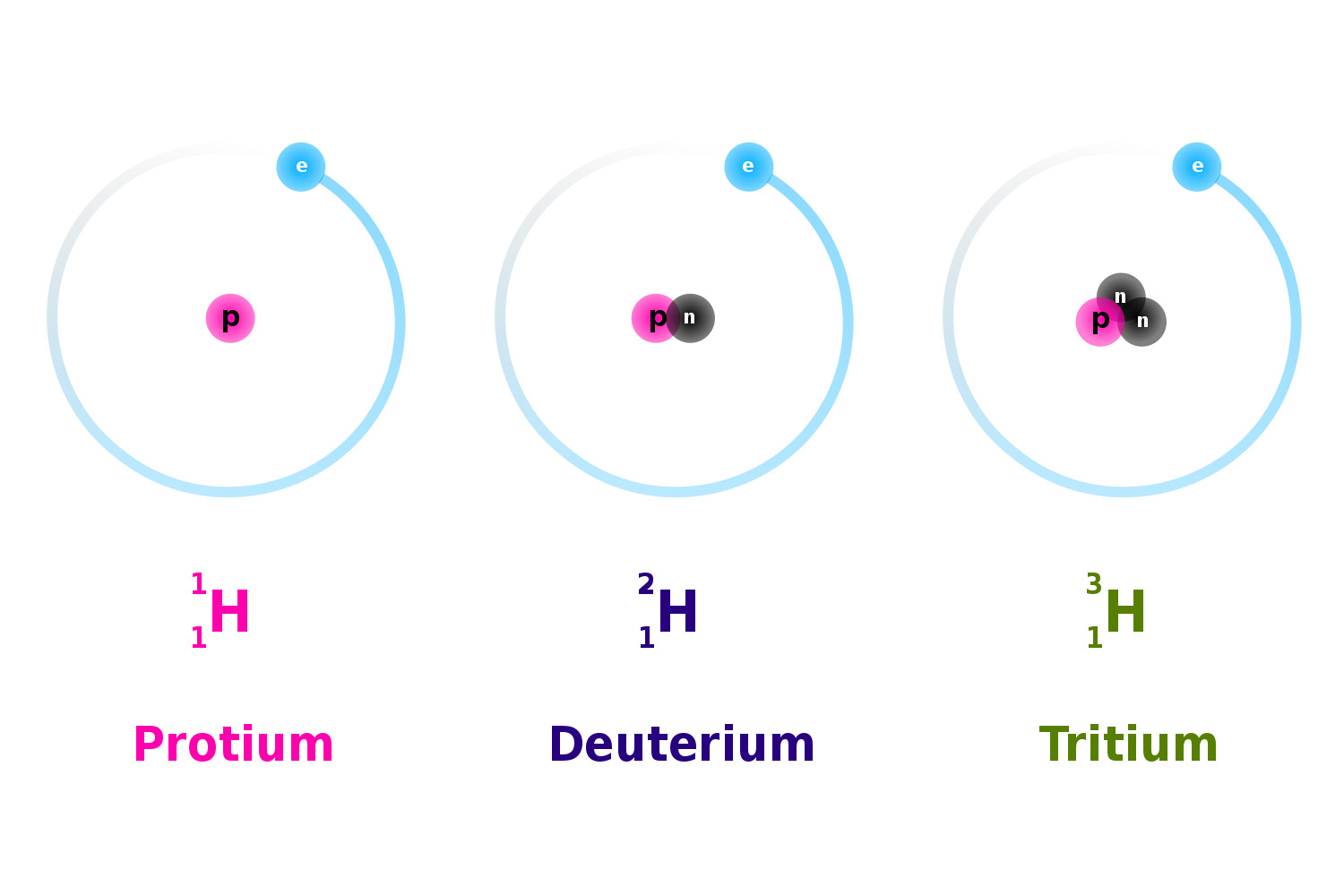
Isotope
-Atoms of the same element with the same number of protons but different number of Neutrons
Molecule
Substance formed by 2 or more atoms
bonded by covalent bonds ONLY
ex.) O₂ , H20
Compound
Substance formed by 2 or more elements
bonded by ionic or covalent bonds
ex.) NaCI, H20
Chemical bond
A force of attraction between 2 atoms
Atom combines with another to fill its valence shell
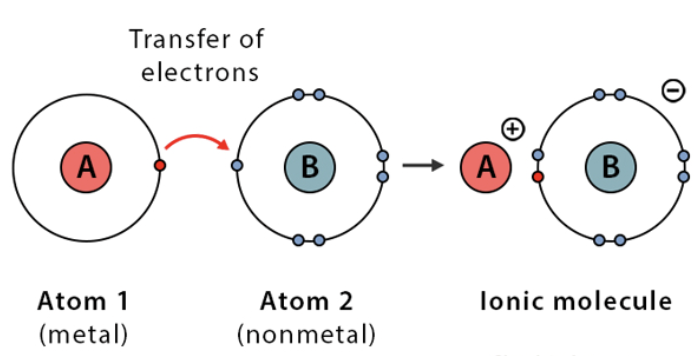
Ionic bonds
transfers one or more valence electrons to another
between oppositely charged ions
Ionic Bond Process
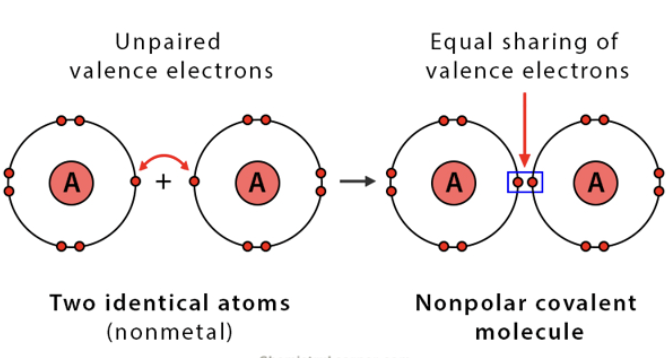
Non Polar covalent bond
two atoms share a pair of electrons equally
atoms with identical or very similar electro negativities
EX.) hydrogen (H2) Oxygen (O2)
hydrophobic
Covalent Bonds
atoms that form molecules by sharing electrons
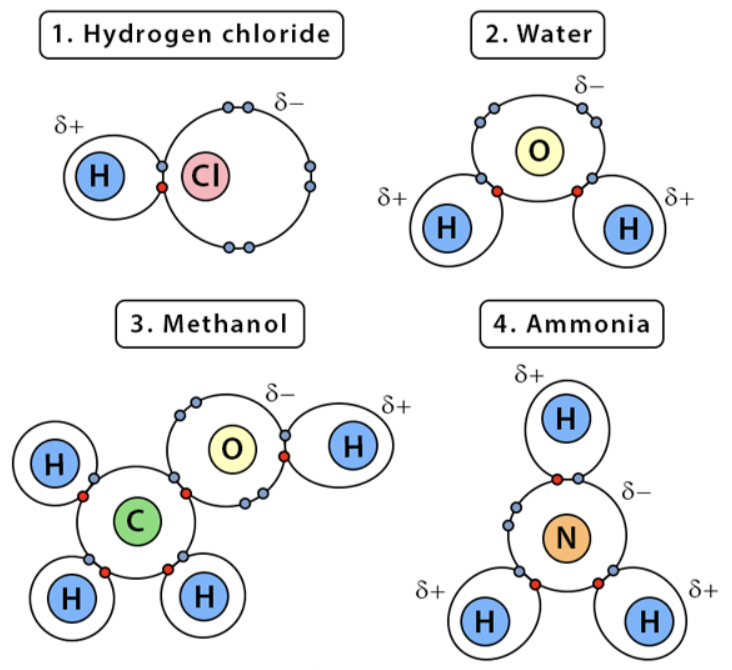
Polar Covalent Bond
electrons are shared unequally between two atoms
different electro negativities
Hydrophilic
ex.) water, hydrogen chloride
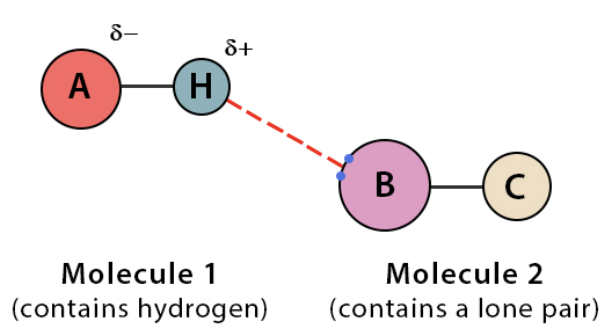
Hydrogen bonds
weak intermolecular attraction between a hydrogen atom
slightly positive and slightly negative