crystal synthesis
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
90 Terms
what is the shake and bake/ ceramic method?
what does it make?
reagents ground up and heated until they react
whole range of materials including metal oxides, sulphites, nitrides, aluminosilicates etc
how do ions move in shake and bake method?
what does this mean about temperature and speed
reaction ions migrate between and through solid starting materials
slow processes requiring lots of heat
what does high temperature methods give for ceramic method?
what does lower temperature give?
higher = thermodynamic product
lower = kinetic product - lower Ea = faster reaction
why is the ceramic method intrinsically slow?
reagents are not mixed at the atomic level
reaction occurs by solid state diffusion between particles of reactant materials

diagram of reaction under ceramic method
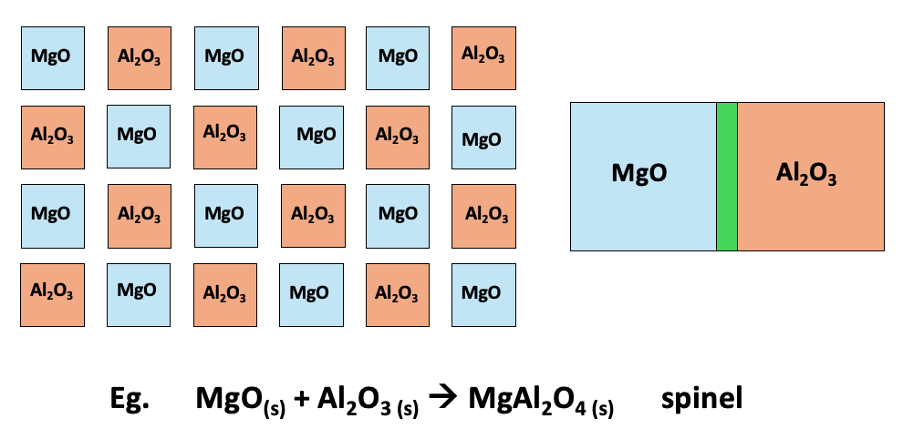
why are reactants ground up in ceramic method?
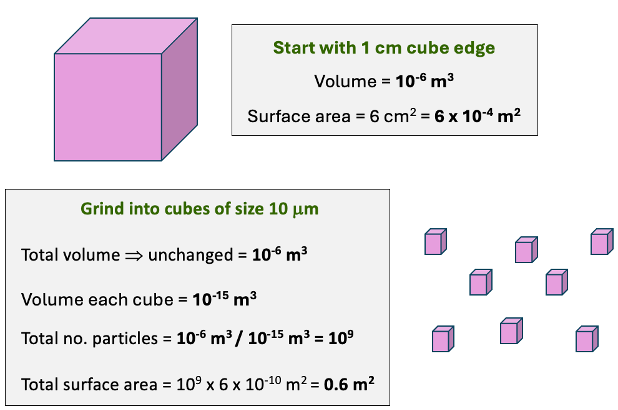
increases surface area
show volume and surface area for 1cm cube vs 10μm
what are the 2 steps in the formation of crystals?
nucleation and growth
what is flow charge of nucleation and growth showing ions to crystal
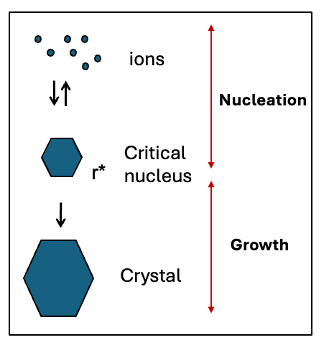
what is nucleation?
when do nuclei propagate?
formation of stable nucleus of product (which can grow)
nuclei only propagate when they reach critical size (critical nucleus). smaller particles are unstable and redissolve
what can make nucleation occur quicker?
if nuclei can form on another favourable surface (heterogenous) - e.g. if structure of precursor resembles the product
e.g. MgO(s) + Al2O3 (s) → MgAl2O4 (s) spinel
how does growth from MgO support nucleation? what type of growth is it?
both MgO and MgAl2O4 are based on CCP oxide ion arrays
conversion occurs by maintaining same oxide packing
this is topotaxial growth
what is topotaxy?
when a solid state reaction occurs with 3D orientational and structural resemblance between reagent and product crystals
e.g. MgO(s) + Al2O3 (s) → MgAl2O4 (s) spinel
how does growth from Al2O3 affect nucleation? what type of growth is it?
Al2O3 structure is based on HCP oxide ions
nucleation of CCP MgAl2O4 on an HCP surface does not involve direct continuation of HCP lattice - however epitaxial growth occurs
what is epitaxy?
when crystal grows on crystalline substrate, with common in plane crystallographic orientation
there is a 2d correspondence
what is growth?
speed? distance?
after stable nucleus formed, ions diffuse into it from precursors so it can grow
slow but accelerated by high temperatures
even if reagents are finely ground, particles are large on atomic scale = ions diffuse long distances
what happens as grain of structure grows? (distance and speed)
distance the ions need to diffuse (path length) increases and reaction slows down
what is the advantage of the ceramic method?
easy: powder of starting material, combined in crucible
what are the disadvantages of the ceramic method?6
high temperatures needed (500-2000C)
may give incomplete reaction
compound may decompose at high T
little change of kinetic control as using high T (=thermodynamic product)
hard to control microstructure (crystal and grain size)
extremely pure starting materials - has to go completion = hard to purify
why are high temperatures required for ceramic method?
significant amount of energy to overcome lattice energy so cation or anion can diffuse into different site
what are the 4 ways of overcoming diffusion barrier/ intimately mixing reactants?
use reactants with small particle sizes
molecular precursor that has required elements in correct ratio
crystallise from gels (sol gel)
precipitate from solutions of metals (when no high T)
why are heated reactions carried out in sealed tubes? what are they made from?
starting materials/products can be sensitive to environments e.g. oxygen
sealed tubes made from fused silica, alumina or platinum
how are temperatures of 1300 reached?
electric furnaces
how are temperatures of 2300 reached?
electrical resistance heating in metal container - tantalum
passing electric current through sealed tantalum tube
how are temperatures of 4300 reached?
CO2 lasers
special atmospheres
how can higher ox state be prevented
how can higher ox state be formed
how can lower ox state be formed
argon can be used to prevent oxidation to higher ox state
may use oxidising gas (e.g. oxygen) to form high oxidation state
reducing gas (e.g. hydrogen) used to form lower ox state
how does microwave heating raise temperature?
what temp can it heat to?
raises temp of whole volume simultaneously - faster reaction
heated to over 1000C
do reactions need to be in solution for microwave heating?
what solids absorb microwaves well
carried out dry or in solution
V2O5, MnO2, Fe3O4, Co2O3, NiO, CuO, ZnO, WO3 and PbO2
what solids do not absorb microwaves?
CaO, Al2O3, TiO2, Fe2O3, La2O3, SnO and Pb3O4
what are the 2 ways a reactant can absorb microwave radiation?
conduction heating
dielectric heating
what is conduction heating?
electrons in lattice (high mobility) can move under influence of microwave field
resistance to movement causes energy to be transferred to surroundings as heat
what is dielectric heating?
material contains molecules with dipole - microwave field aligns them
oscillating field, the dipoles oscillate in sympathy (unable to follow rapid reversals in field)
power is dissipated in material which transfers heat energy to lattice
what is combustion heating?
heat generated by reaction is used to produce high temperatures
what are chimie-douche methods (soft chem)?
low temp methods of making solid materials
initial low temp, followed by firing at high temperatures
what are the advantages of chimie-douche methods?
products often higher purity
more uniform in texture and chemical homogeneity
what are the disadvantages of chimie-douche methods?
reagents are expensive
reaction conditions specific to given reaction and must be optimised
what are sol gel reactions? what are they an example of?
chimie-douche method
sol = colloidal suspension of particles in a liquid
gel = semi rigid solid in which solvent is entrapped in network of colloidal or polymeric material
how is sol and gel formed?
sol - formed by dispersion of insoluble particles in solvent or through reaction of precursor with solvent
gel - formed from sol by methods including concentration solution (eg eval), ageing or heating
how is aerogel and xerogel formed from sol-gel?
supercritically extracted - aerogel
evaporated to form xerogel
how is silica sol made? what is the precursor?
what are the 2 steps?
silica alkoxide (Si(OR)4) precursor
hydrolysis of alkoxide precursor to form Si(OH)4
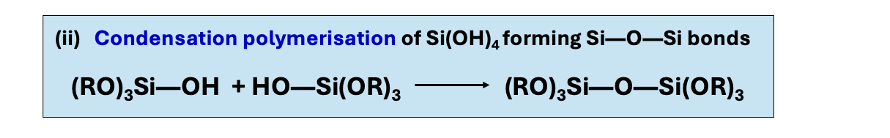
condensation polymerisation of Si(OH)4
hydrolysis of Si(OR)4 equation

condensation polymerisation of Si(OH)4 equation
what are the 5 steps of silica optical fibres from sol-gel
sol prep
gel formation
drying
purification and densification
fiber fabrication
how is gel formed? silica rxn
sol is cast into mould (often tube) and gelled
reducing pH = silica particles to aggregate/combine and form gel
what happens in drying? silica sol gel
wet gel is dried to remove liquid = giving porous silica gel
what is purification and densification? silica sol gel
porous gel heated to remove organic impurities and hydroxyl groups
sintered to create dense, transparent glass
what is fibre fabrication? silica sol gel to make optical fibres 2
preform creation : dense silica glass is used to create preform - larger thicker version of intended fibre
fibre drawing : preform heated and drawn into thin fibre with desired diameter and properties
what are the 4 benefits of sol gel of silica optical fibres?
compositional flexibility
purity - minimise impurities that can affect optical fibre performance
cost effectiveness
can create microstructures fibres - complex geometries with unique optical properties
how does the sol gel method give compositional flexibility?
allows dopants to be easily incorporated - controls optical properties of fibre
what are mesoporous solids?
how are they made?
pores of size 2nm-50nm
synthesised using templating methods
what is templating?
a scaffold is used to direct the formation of the solid = condense solid around it
template is removed to give product
why might you use an organic scaffold for templating?
what method can you use in conjunction with it?
can remove it at low temperatures
can use sol gel as low temp
what are thermotropic surfactants?
surfactants that change phase due to temperature
what are surfactant/solvent systems called?
what do surfactants do in solution?
lyotropic
surfactants self assemble to give different structures/phases
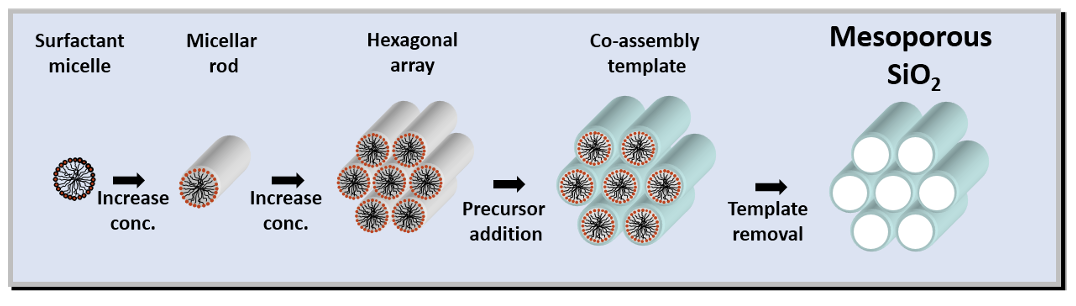
how to make mesoporous materials (e.g. silica) using surfactant
what does larger surfactant give
condense silica around surfactant
burn away surfactant to give mesoporous product
larger surfactant = larger pores
what is hydrothermal synthesis used for?
when you want to carry reactions out in solvents
at temperatures above their BP
what vessel would you use for hydrothermal synthesis? pressure? heating?
how is pressure controlled?
teflon lined cylinder or bomb - sealed/connected to pressure control
bomb is heated to 100-500C
p controlled either by degree of filling or externally
what is hydrothermal reactions but for organic solvents called
solvothermal reactions
what are zeolites?
what technique could you use to make them?
microporous aluminosilicate framework structures
use hydrothermal synthesis/sol gel reaction
what is the advantage of hydrothermal synthesis compared to other methods?
lower temp than ceramic / sol gel methods
what are the poles like in zeolites?
what are the applications?
well defined, interconnected
intersect at cavities / cages
used for many applications e.g. catalysis and molecular separation
what is the difference between zeolites and mesoporous substances?
zeolites are naturally occurring
what is the general formula for a zeolite? what is M for?
Mx/n[(AlO2)x(SiO2)y].mH2O
M present to neutralise negative charges on aluminosilicate framework
what does changing Si/Al ratio change in zeolites?
changes the cation content
what are zeolites with high Si content like?
hydrophobic
how are zeolites made?
why does gel form? why is it heated and in what?
reactive silica and alumina reagents under hydrothermal conditions - high pH
gel forms due to copolymerisation of silicate and aluminate ions
heating gel in autoclave generates zeolite (few days)
what is chemical vapour deposition? how are reactants and products delivered?
reactants are delivered in gas phase
product phase is deposited as thin film on substrate
what can chemical vapour deposition be used to make?
high purity thin films
what starting materials are used for chemical vapour deposition?
how are they transported? where do products form?
volatile - typically hydrides, halides and organometallic compounds - heated to produce vapours
mixed and transported to substrate using carrier gas
product deposits as thin film
what are the properties of diamond?5
hardness
chemical inertness
optical transparency
thermal conductivity at room temp
electrical insulation
what is a problem that arises when making diamond thin films using chemical vapour deposition?
how is this avoided?
graphite forms under ordinary conditions
in diamond chemical vapour deposition, need to deposit carbon while suppressing formation of graphitic sp2 bonds
establish high concentration of non diamond etchants e.g. atomic H
how are large single crystals of Si made?
what conditions? how is silicon prepared?
Czochralski process
normally performed in vacuum under inert atmosphere of Ar in inert chamber e.g. quartz
Si melted in crucible and then single crystal is position on surface of melt
crystal acts as seed which continues to grow, slowly withdrawn
how is large single Si crystal withdrawn?
pulling an ever lengthening crystal in same orientation as original seed
what are single crystals of potassium dihydrogen phosphate (KDP) used for?
how are they grown?
special optical properties - converting laser beams from IR to UV
grown from large 0.3m seeds. placed in tanks containing aq KDP. temp lowered to make supersaturated solution = driving force
what are the 3 problems stopping rapid crystal growth of KDP?
impeded transport of dissolved KDP from bulk of solution to crystal
insufficient supersaturation of solution
inaccurate measurement of supersaturation as can’t control it
what are the 3 solutions for KDP rapid crystal growth problems?
improved transport of dissolved KDP by putting seed in middle of slowly rotating turbine
improved model of crystal growth rates
turbine to give better mixing and suppress undesirable spontaneous nucleation
what are the two approaches for making nanomaterials?
bottom up
top down
what is bottom up nanomaterial synthesis?
when nano materials are made from smaller compounds
chemical synthesis or forming arrays of nanoparticles by self assembly
what is top down nanomaterial synthesis?
formed by the processing of larger (macroscopic) strucutres
e.g. etching through a mask or ball milling
is bottom up or top down more powerful?
bottom up is more powerful
-control material structure from atomic level
how is CdSe nanoparticles made?
how does nucleation occur?
hot injection methods
dissolved in tri-alkyl phosphine. solution injected into hot trioctyl phosphine oxide (TOPO). nucleation occurs in a burst
injection causes solution to cool. nuclei can grow but no knew can form.
in nanoparticle synthesis, what controls growth of nanocrystals after injection?
surfactant molecules control growth
aggregation is suppressed by the presence of surfactants on the particle surface
growth of nanoparticles - concentration of reagents vs reaction time graph
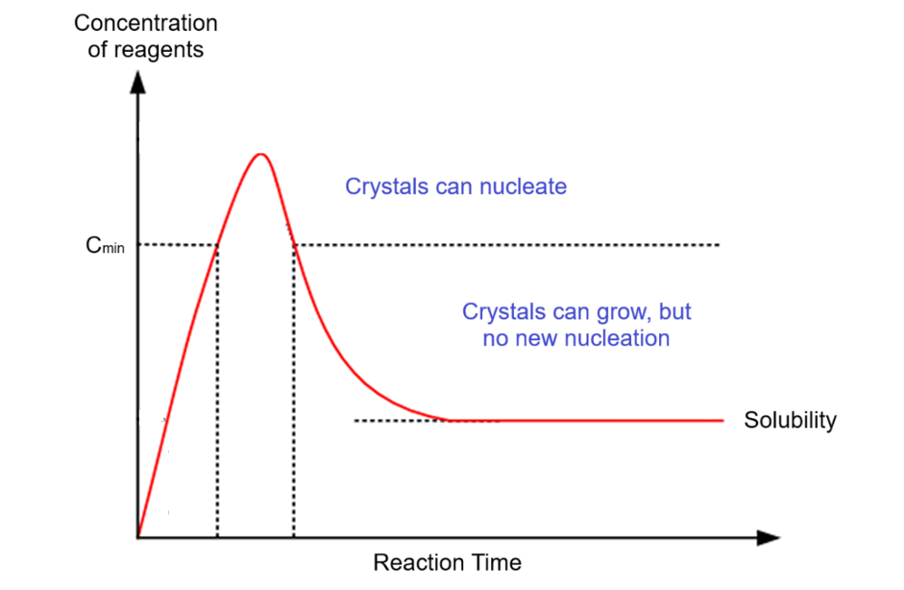
how does a burst of nucleation form a narrow size distribution?
all nuclei form at same time - reduces conc of reactants to a level where no new nuclei form
NCs continue to grow. since all formed at the same time and no new nuclei can form later, they all have same size
how can existing lattices be modified?
how is charge neutrality maintained? what must the reactant material be?
adding extra ions into vacant sites (intercalation)
removal of ions (de-intercalation)
electrons must be added or removed - reactant material must be a conductor to allow flow of ions or electrons
how is potassium graphite formed?
melting potassium over graphite power in inert atmosphere
intercalated ions in AxWO3
intercalated ions are mobile
how can LixWO3 be prepared 3
what is reducing agent
reacting WO3 with BuLi (Bu- red agent)
reacting WO3 with lithium vapour (Li0 red agent)
electrochemically reducing WO3 in presence of Li+
what is structure of WO3 and LixWO3
how does structure change as x increases?
edge centred cubic lattice
Li+ intercalates into centre of cubes
as x increases, turns into perovskite lattice
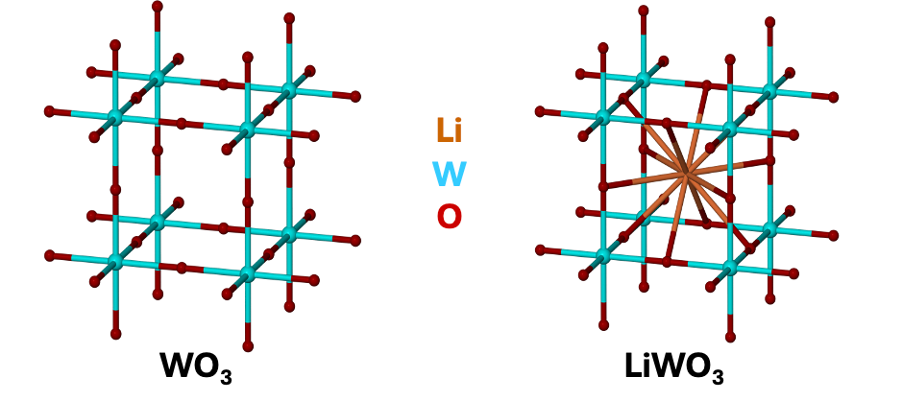
what is tungsten bronze made up of?
solid solution of perovskite LiWO3 in simple cubic WO3