3.2 First Law of Thermodynamics and Enthalpy
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
On what does the first law of thermodynamics applies on ?
applied mainly to gases enclosed in reservoirs that change from an initial state (i) to a final state (f)
Give the formula for the principle of work and energy which is applied at a microscopic scale :
ΔEk denotes the change in kinetic energy
ΔEp denotes the change in potential energy
ΔEth denotes the change in internal thermal energy between the molecules of the system

What is the formula for the change in internal energy ?
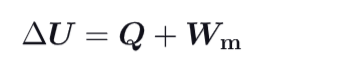
Is the change in internal energy impacted by the path between initial and final ?
No but the exchanged heat Q or the exchanged mechanical work Wm does.
What is the formula for the external work done on a gas ? Show demonstration
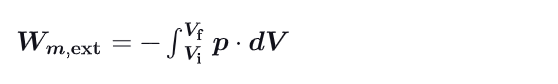
What is an expanding gas ?
Vf superior to Vi
area under the curve is positive (so the gas does positive work on the environment)
environment does negative work on the gas(external force is resisting the expansion)
What is a Compressed Gas ?
Vf inferior to Vi
area under the curve is negative
environment does positive work on the gas (environment adds energy to the gas)
What is an Isothermal Process ?
Process for which T is a constant, meaning that the gas follows the equation pV = constant (Boyle’s Law).
the curve follows a hyperbola in the p-V plane
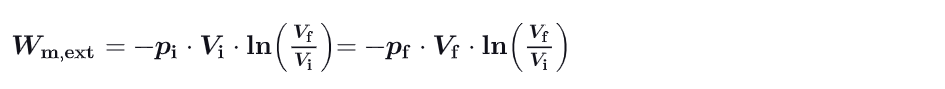
What is an isochoric process ?
The area under the pV curve is 0, no work is done.
What is an Isobaric Process ? Write the demonstration
p is a constant (the system expands or compresses at a constant pressure). The formula for the work done is then equal to -pΔV.
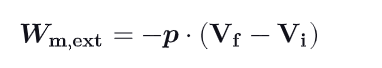
What is an adiabatic process ?
A process that does not impact the mechanical work. Zero heat is exchanged with the system.
the equation of the first law reduces to ΔU=Wm
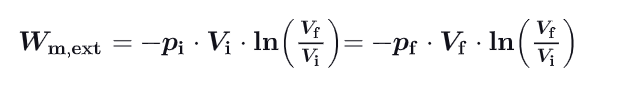
What is enthalpy (of a system) ?
expressed in J

What is enthalpy for an isobaric process ? Write the demonstration
dH = dQ