Innate and Adaptive Immune System
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
54 Terms
First layer of innate immunity
Barrier
Prevents entry of pathogens, so immune system does not have to get into action
What are humoral effectors?
Any part of the innate immune system that is soluble, but not cellular
Adaptive innate immune cells
Name 3 things that make skin a good barrier
Thick + dead
Impermeable (due to keratin)
Releases antimicrobial secretions
Dry surface
What is the mucociliary elevator?
The portion of cilia along the epithelia of the lungs and bronchi that sweep mucus (and debris/pathogens within) up and out.
What stem cells do all immune cells come from?
Bone marrow stem cells —> multipotent progenitor cells
What are sentinel cells and 3 examples of them
They monitor tissues constantly for signs of infection or injury.
Once they detect danger signals (like microbes, damaged cells, or foreign substances), they activate and alert other immune cells.
Dendritic cells
Macrophages
Mast cells
What do dendritic cells do
Captures antigens and migrates to lymph nodes to activate T-cells
What do macrophages do
Engulfs pathogens and releases cytokines
What do mast cells do
Releases inflammatory molecules, such as histamine to increase blood flow, and vascular permeability
Why do immune cells release inflammatory cytokines and chemokines
to recruit and activate other immune cellsW
What is the most common granulocyte
Neutrophil
What pathogens do granulocytes defend against
Bacteria and fungi
Function of monocytes
They circulate in bloodstream, and when an infection is detected, it goes to that location and differentiates, depending on what is needed (macrophages or dendritic cells).
Function of natural killer cells
NK cells detect any cells that have abnormal surface markers, or have downregulated MHC I molecules. And destroy they cells by triggering apoptosis via release of cytotoxic granules
What is MHC I
A molecule on the surface of every body cell. Gets downregulated when infected/cancerous and acts as a marker for NK cells.
Inhibitory molecule that stops NK cells killing cells containing MHC I surface receptors.
What are neutrophils
Most common white blood cell (~60-70%)
They are non-dividing (don’t under mitosis) end stage cells (fully differentiated)
They eat and kill invading microbes using NETs. They follow chemotaxic gradients to reach infection sites.
Usually only in blood, found in tissue only when it is infected.
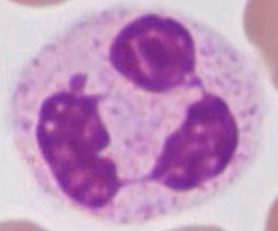
What are NETs?
Neutrophil extracellular traps - trap and ensnare bacteria using chromatin. Excess NETs can cause blood clotting and organ failure (e.g. in sepsis)
What are complement proteins
Proteins found in high concentration in plasma and tissue fluids, responsible for initiating an enzyme cascade
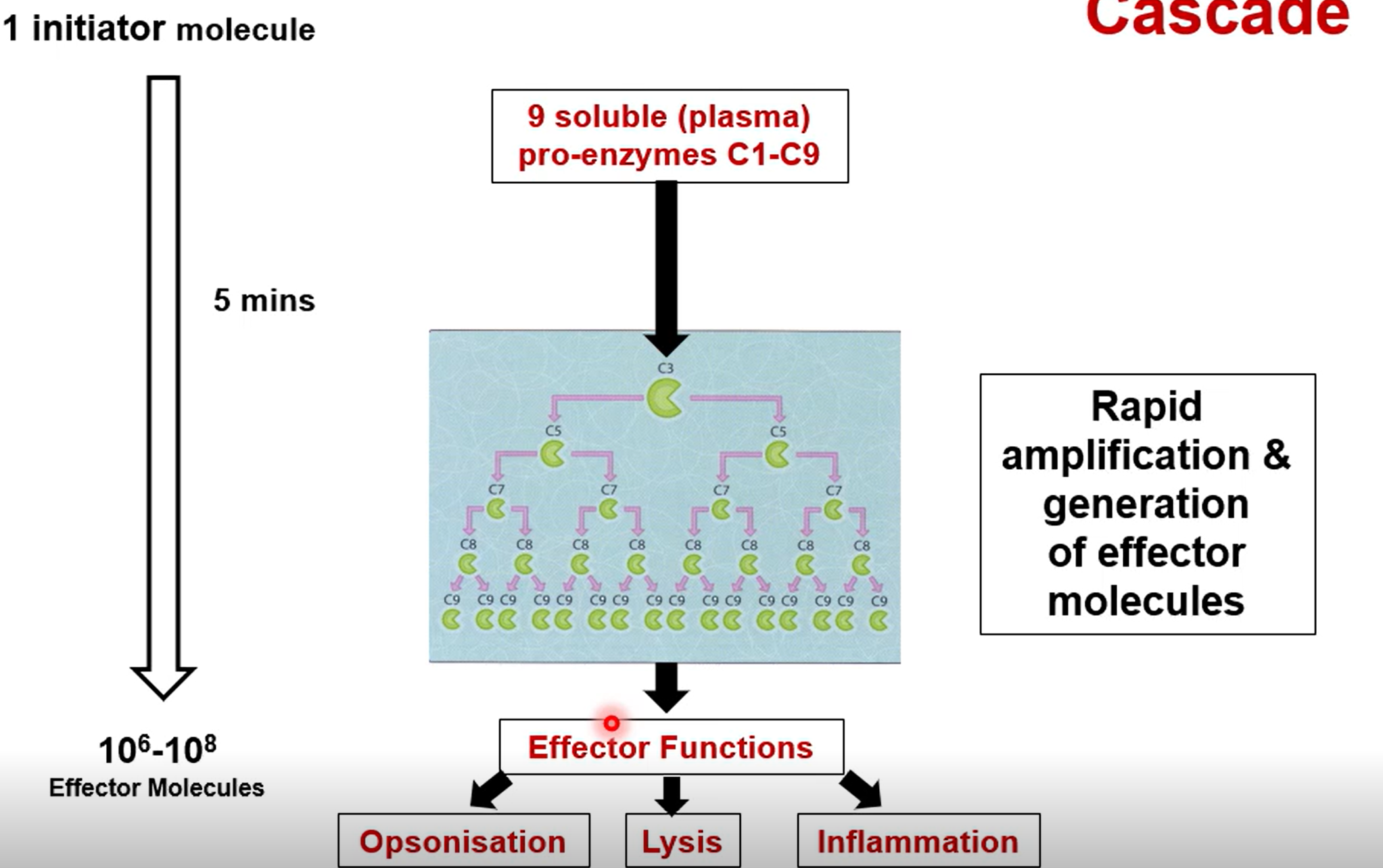
What is the enzyme cascade
A small number of enzymes triggering a large scale response via amplification
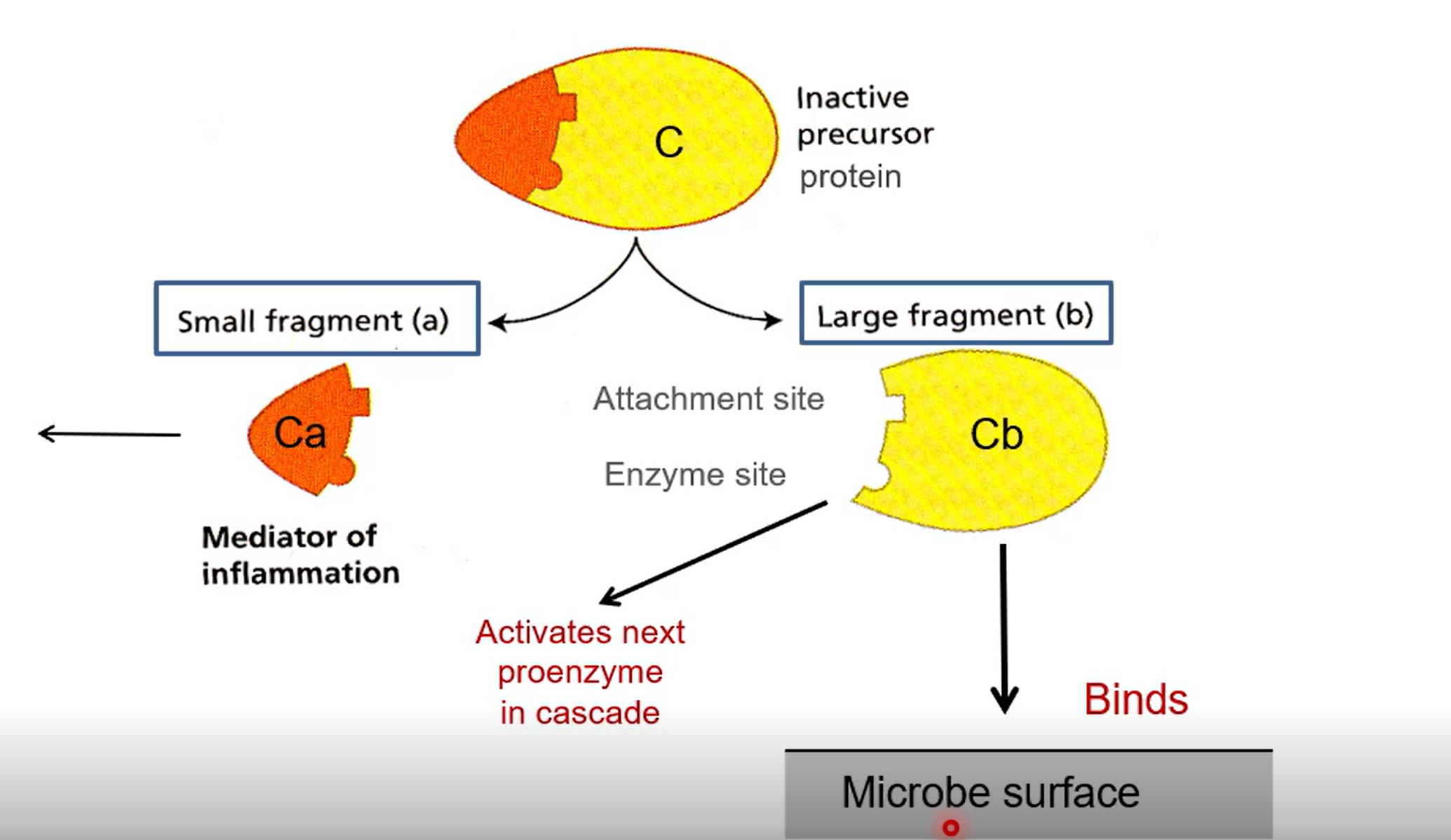
How does the enzyme cascade occur from the perspective of an individual enzyme
Start off as inactive pro-enzymes made of two parts (a and b)
A causes inflammation by attracting innate cells like neutrophils
B sets off the next enzyme in the cascade, or binds to the surface of the microbe
3 causes of complement cascade
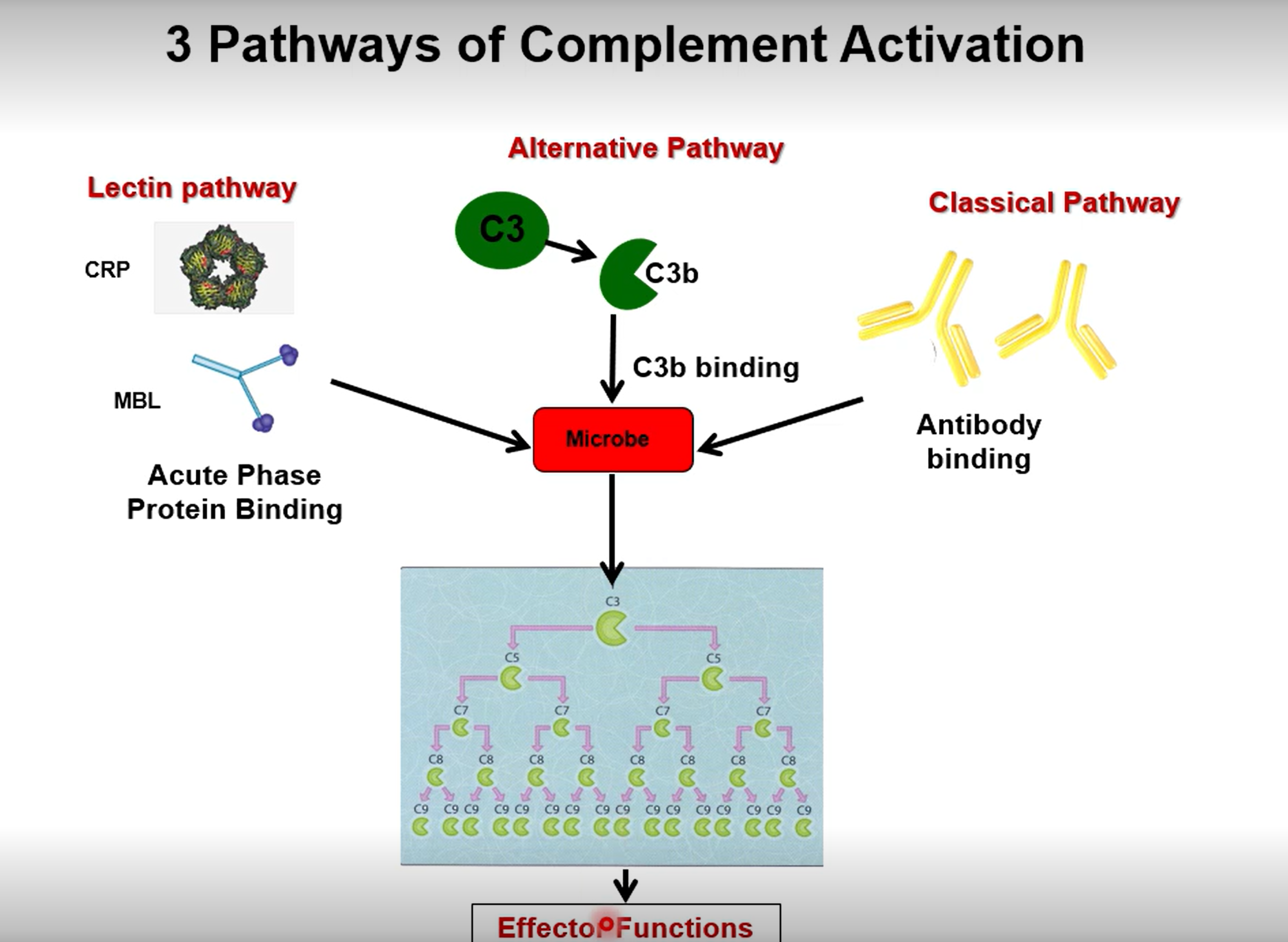
What does the complement protein C3b do
C3b coats the surface of a bacterium, tagging it as a target for immune cells. Gives a target for phagocytes to bind to
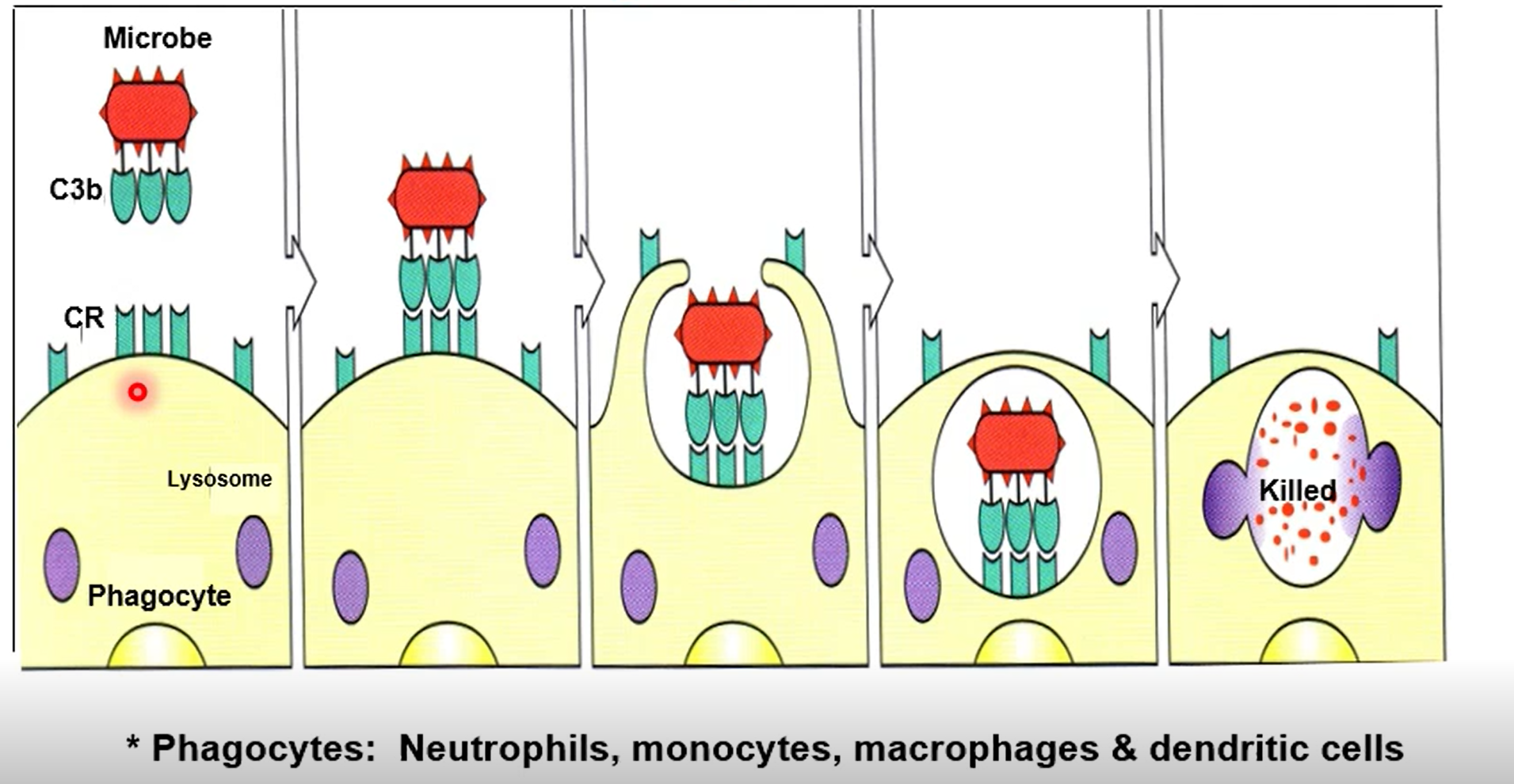
What does the complement protein C3a do
Degranulate mast cells, promoting inflammation and leukocyte recruitment
How does the membrane attack complex (MAC) form? and what is it
A combination of C5b, C6, C7, C8 and C9 enzymes form a barrel shaped transporter on a microbe surface, causing its destruction
What is a PAMP?
Pathogen associated molecular patterns (molecular patterns usually found of pathogens)
What are PRRs
Pattern Recognition Receptors - molecules expressed by innate immune cells that recognize common molecular structures found on pathogens or damaged cells
What are toll like receptors (TLRs)
key family of pattern recognition receptors. Transmembrane proteins found on the surface or inside immune and other cells.
What is the function or interferon and when is it released
Virus infected cells release interferon, which binds to uninfected cells.
Interferon stimulates production of :
RNAase
Protein Kinase-R
Which degrade RNA and block protein translation respectively, stopping virus replication.
Two types of T cell
T Helper cells - Enhance immune response of other immune cells
Cytotoxic T cells - Destroy pathogens
What innate immune cell stimulates T-cells?
Dendritic cellsW
What is Ig short for?
Immunoglobulin (aka antibody)
What is clonal selection theory, briefly
The selective breeding of lymphocytes, only allowing the lymphocytes that bind to the pathogenic antigens to proliferate
(billions of random lymphocytes are produced)
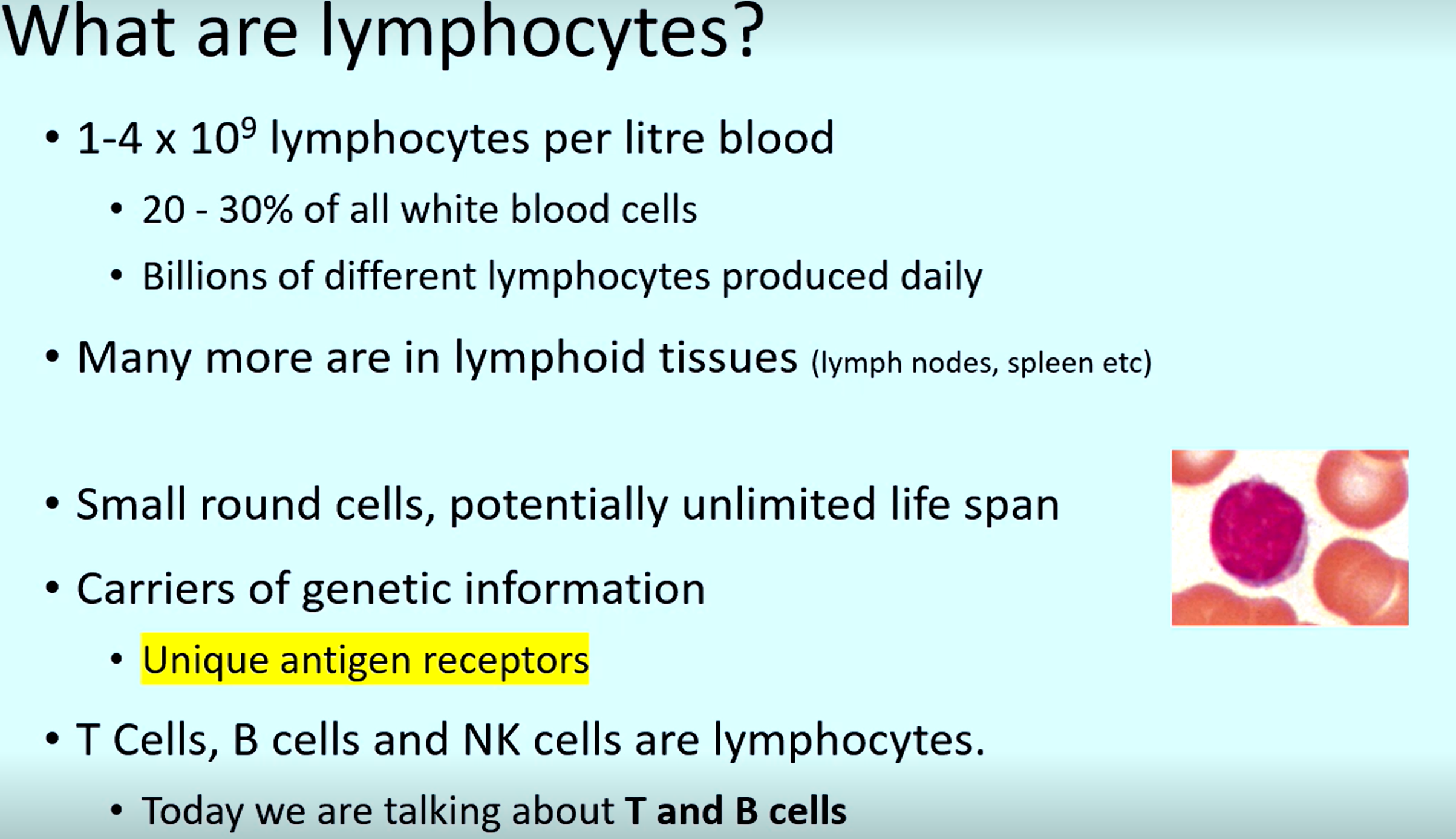
3 types of cells that are lymphocytes
T cells, B cells, NK cells
What cells are carriers of the genetic information required to code for previously used unique antigen receptors
Lymphocytes
What are B-cell receptors and what do they do
Receptors on the surface of B-cells that bind to free antigens (e.g. antigens on a pathogenic cell). This means they can bind to pathogens and toxins directly and release BCR(antibody).
What is secreted BCR
A BCR that is secreted from a B-cell is an antibody, which binds to the pathogen
What do T-lymphocytes recognise to bind to
Processed peptides presented on an MHC
Difference between how TCRs and BCRs bind to a pathogen
B-cells bind to a pathogen directly, T-cell are fussy and require a different immune cell (e.g. dendritic cell) to present a pathogen antigen to it.
what are the 4 chains in a B cell receptor
2 x Heavy
2 x Light
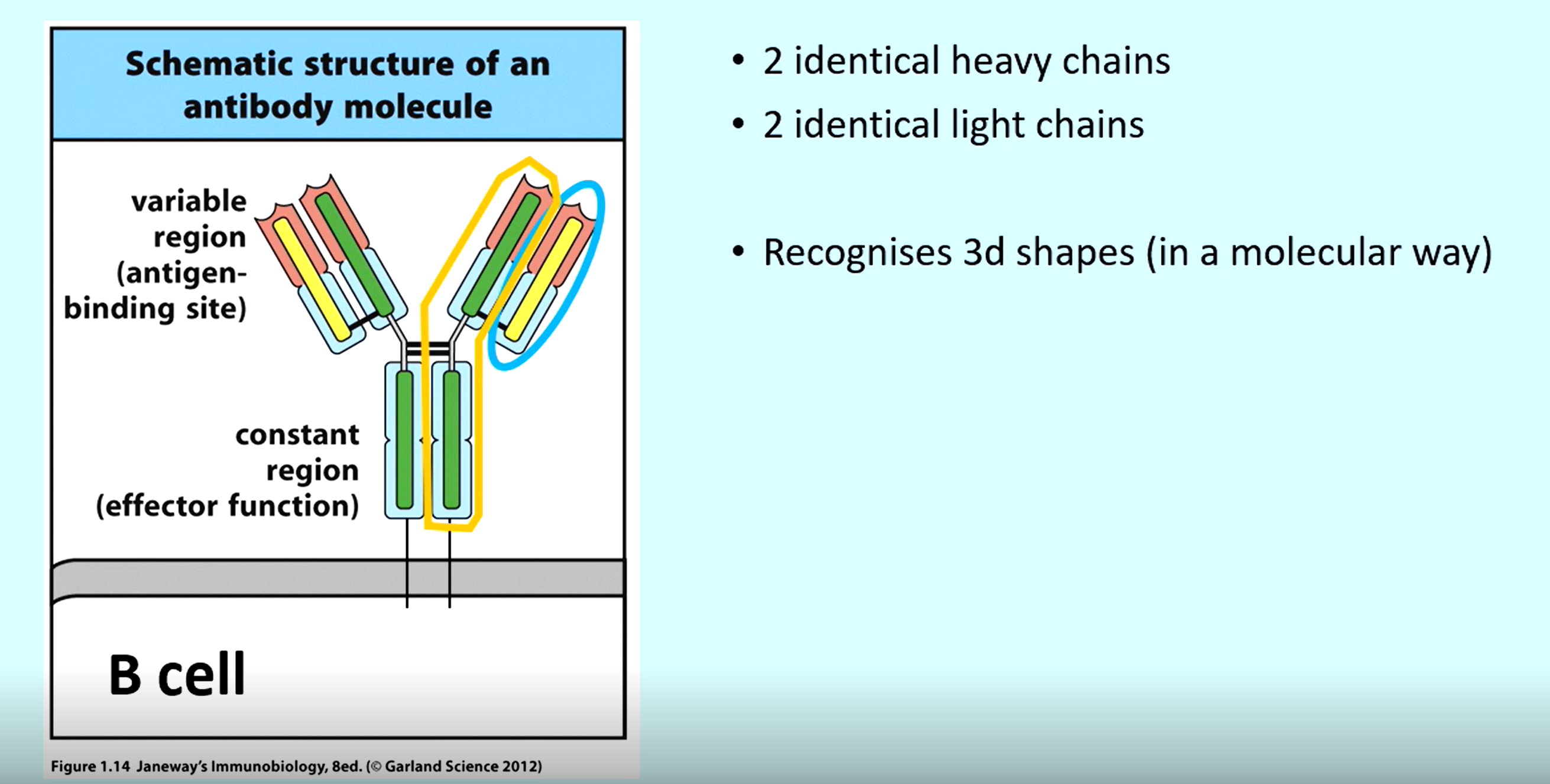
What is an epitope?
The part of an antigen that a receptor binds to
Explanation of how antigens are presented to T-cells
To reveal epitopes of pathogens, it must be broken into peptides
The epitope peptide binds to an MHC molecule on a (e.g. dendrite cell)
MHC molecule binds to T-cell receptor
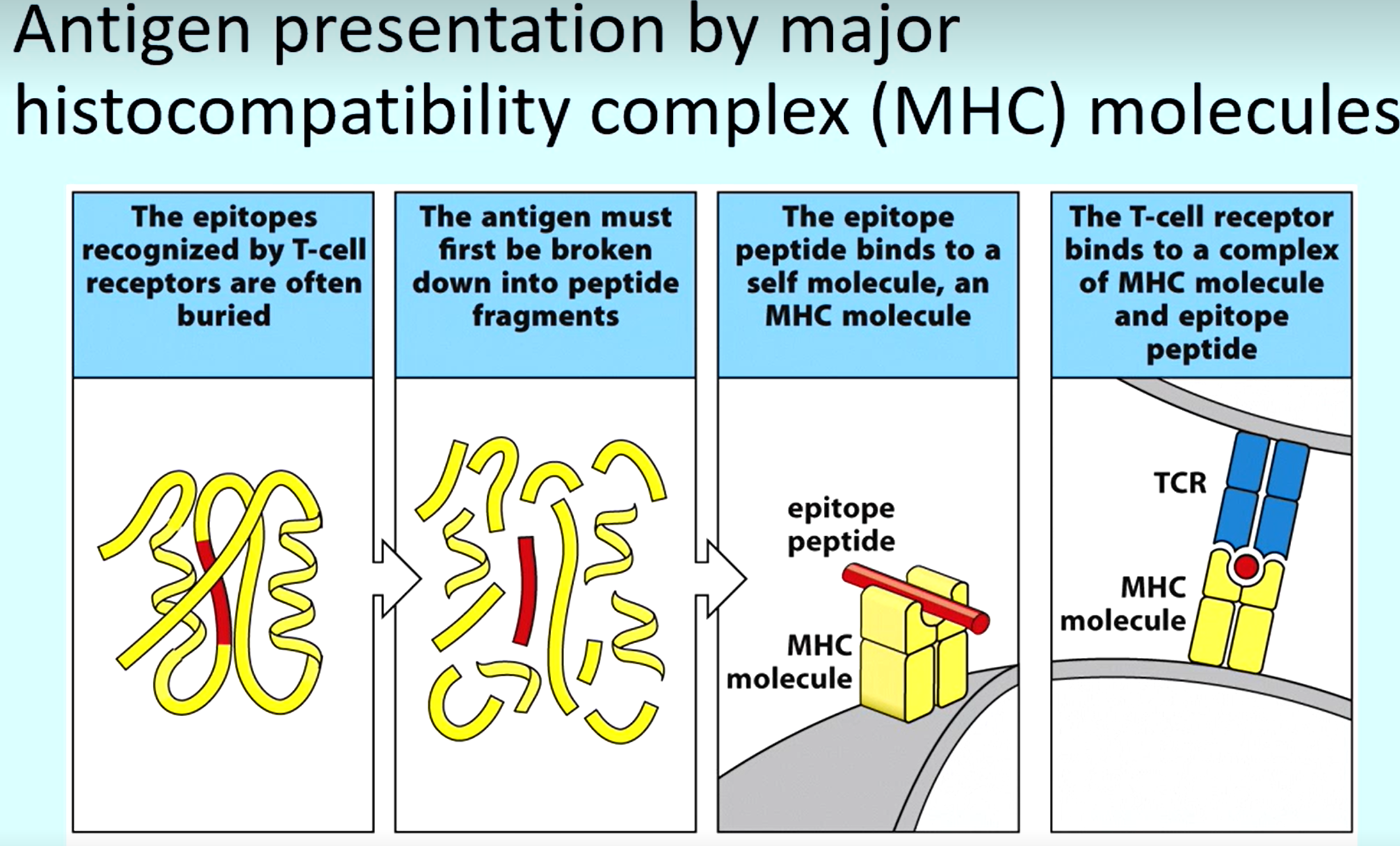
What do MHC molecules do Cytotoxic and T-helper cells bind to respectively
Cytotoxic - MHC I
Helper - MHC II
Where are MHC I molecules expressed
Nearly all body cellsWh
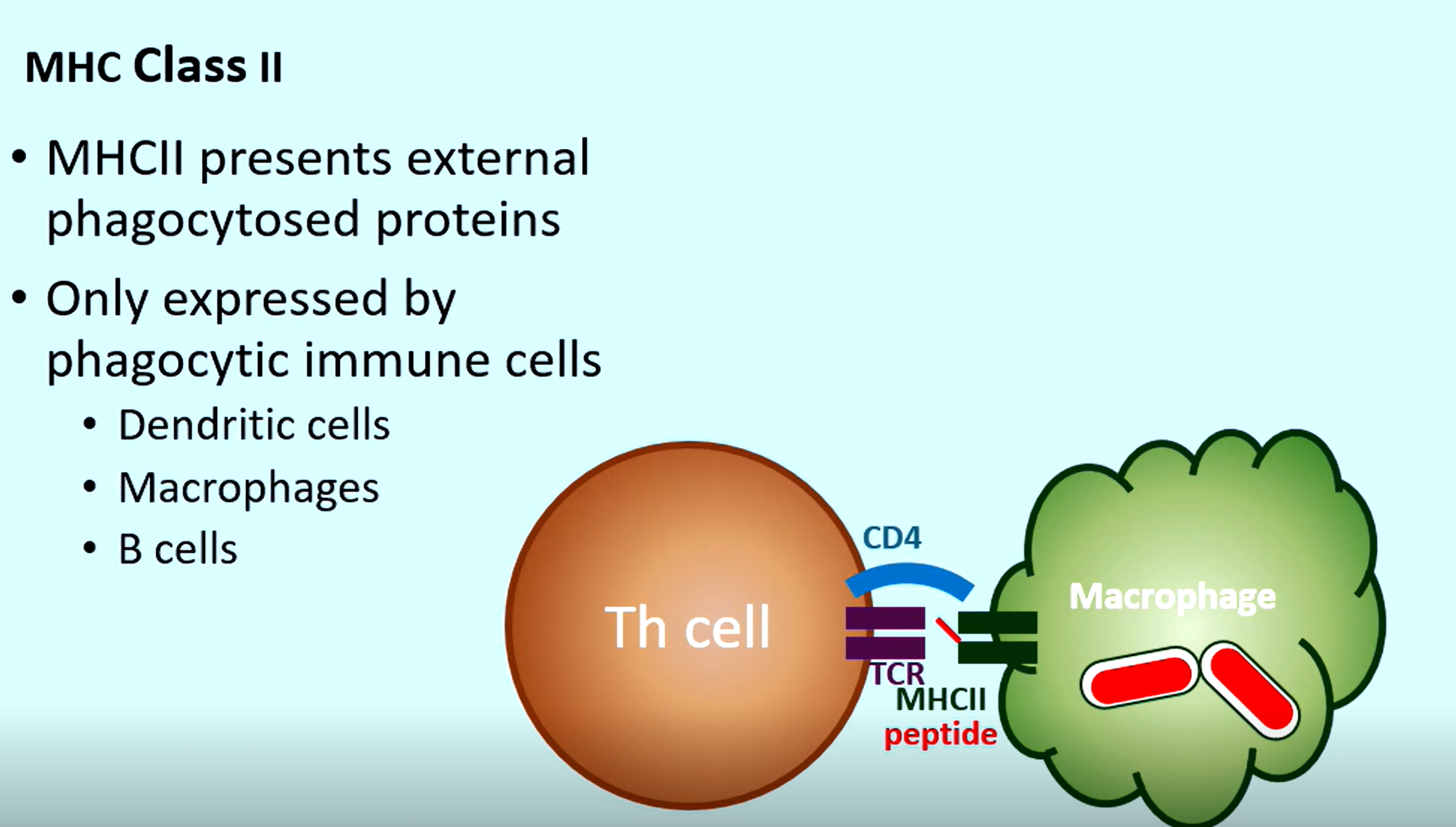
Where are MHC II molecules expressed
Only on Phagocytic immune cells
Dendritic cells
Macrophages
B-cells
What is on a MHC II molecule after the phagocyte has engulfed a protein
Phagocytosed protein of the pathogen (so a T helper cell can bind and boost its power)
What is the purpose of the thymus?
Produce and mature T lymphocytes
What are the 3 types of different genes that are randomly recombined in the thymus to form different antigens for the T-cell (the TCRs)
Variable, Diversity and Joining genes
(also don’t forget about random nucleotides between these genes that give even more diversity)
What is negative selection?
Destruction of self-reacting antigens
Where are B-cells made
Bone marrow
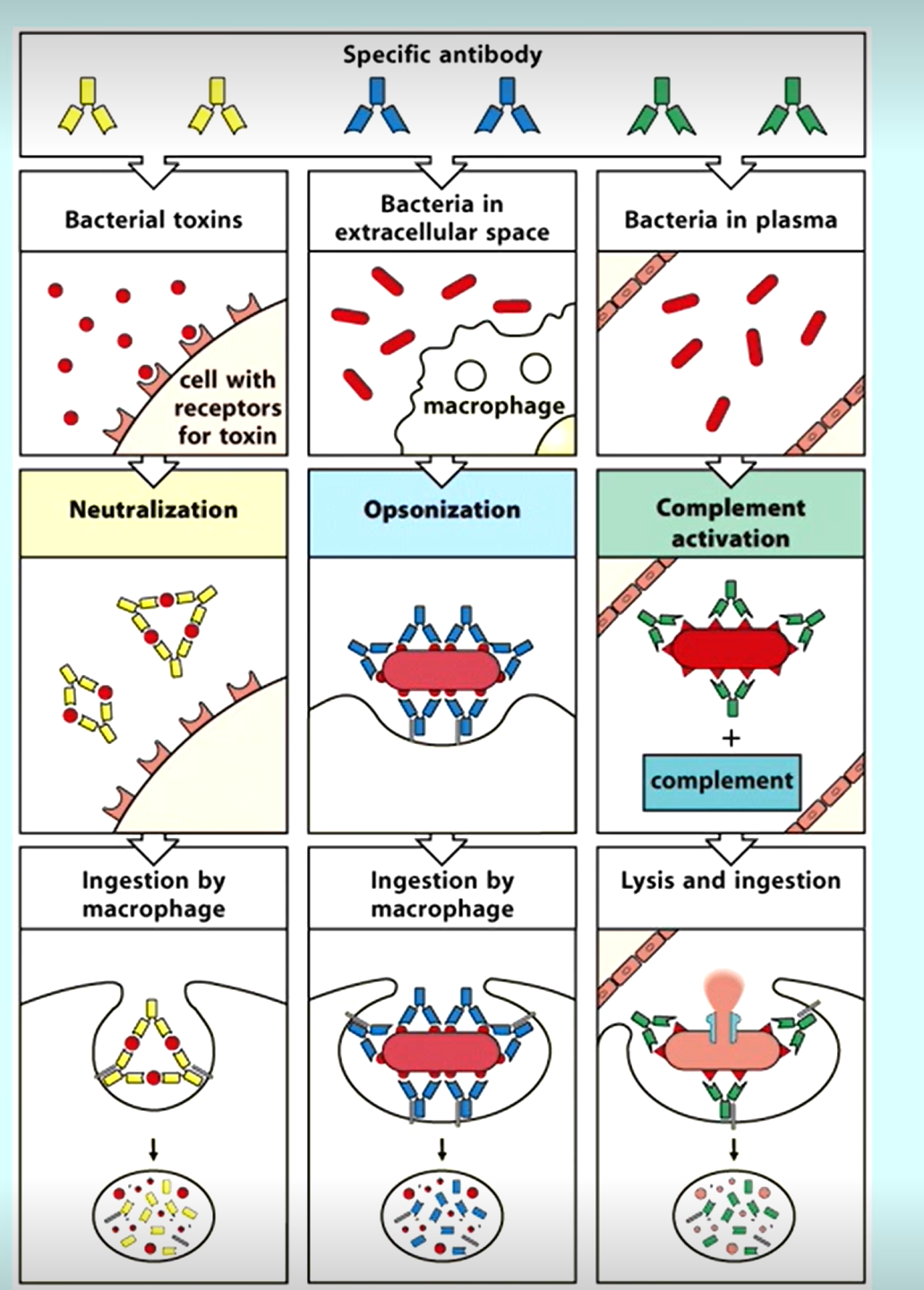
3 Main ways antibodies aid in immune function
What happens in the Germinal Centre
After a B cell is activated by its specific antigen (and receives help from a T helper cell), it migrates to the germinal centre and the following occurs:
Clonal expansion
B-cell proliferate, creating many clones of themselves
Somatic hypermutation
B-cells change their variable region antibody genes, changing their affinity
Affinity maturation
Higher affinity cells survive, lower affinity/self reactive cells apoptose
Class switch recombination
B-cells change their isotype (e.g., from IgM to IgG, IgA, or IgE).
Differentiation
B cells leave germinal centre and differentiate into memory B-cells or plasma cells
What is the function of the plasma cells
Secrete many antibodies
What is the function of B-memory cells
Long-lived cells that reproliferate when a disease reoccurs