Physics
1/41
Earn XP
Description and Tags
Assessment Revision
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
What are the three states of matter?
Solid, liquid and gas
What is the process called when a solid turns into a gas or a gas turns into a liquid?
Sublimation
1.What is the process called when a liquid turns into a gas
2.What is the process called when a gas turns into a liquid?
Evaporation
Condensation
What are the movement of particles in a solid like?
The particles vibrate in a fixed position
What happens to the particles when the temperature increases?
As the temperature increases the vibrations of the particles get bigger therefore the particles move further and the solid expands.
What happens in liquids and gases as they are heated?
They melt
What is the movement in a particle like in liquids?
They vibrate in a fixed position, packed closely together and able to move past another
What is the forces of attraction like in solids?
The forces between particles are very strong this is why they are so close together
What is the forces of attraction like in Liquids?
The forces of attraction are strong
What are the forces of attraction like in a gas?
The force of attraction in a gas is very weak
Which state of matter is the only one that doesn’t have a fixed volume?
Gases and they have definite volume
Which state or matter is the only one that will not fill the size of the container
Solid
What is the compressibility in gases like?
Very easy to squash
Evaporation only occurs from the what of the liquid?
The surface of the liquid
When ice melts what is taken in?
The heat is taken in
What is density?
Density is the measure of how much is packed at a specific volume
What are the units for density
What is the symbol for density
Kgcm3 or gcm3
A curly P though D is acceptable
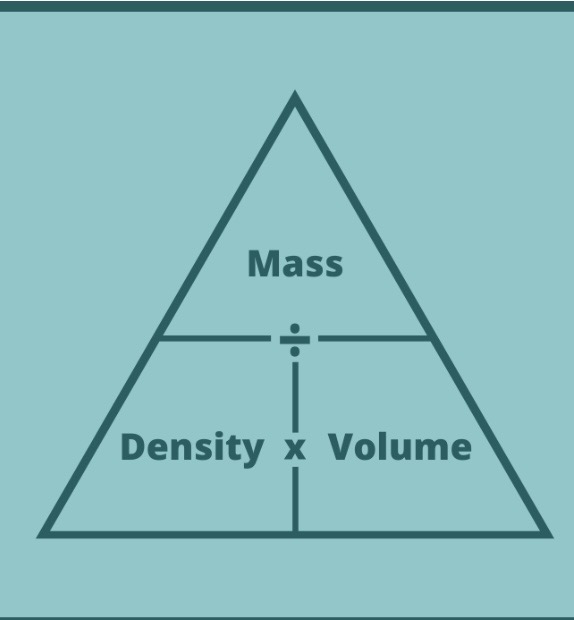
What is the formulae for density?
Density= Mass/Volume
How do we find out the mass or the volume if we are given the density
The formulae triangle
The formulae triangle tells you what to do multiply or subtract
For example to find out the mass you multiply the density and the volume
What apparatus would you use to record the mass of an object
What apparatus would you use to find the volume
1.Top pan balance
2. Measuring cylinder
What does the density of solids depend on?
The mass of individual particles and molecules
The way the particles are arranged
What is the formulae for weight?
Weight=Mass x Gravitational field strength
Would it be easier to move a hammer on the moon or on earth and which direction?
On the moon and down
What is mass the measure off
Mass is the measure of how much mass is in an object
What is mass measured in?
Does the mass change
Kilograms
No the mass is the same anywhere in the universe
What is weight measured in?
Why is the weight the force of an object?
Newtons
This is Because of the gravity acting on it
Why do astronauts weigh less on the moon?
This is because the gravitational a force acting upon them is much smaller
What are the ways to not make any mistakes during calculations?
Unit conversions
Make sure if you need to substitute or rearrange
The common sense check ( make sure answer is not completely ridiculous)
Any special requests (does the question ask for anything like SF or DP)
What makes an object float?
An object will float if it is less dense than it
When and why does pressure occur?
pressure occurs when a solid is in contact with a surface and exerts a force on the surface
What is the formulae for pressure?
Pressure=force/Area
What is the N/m2 called
A pascal which is the unit of pressure
What happens to the pressure when a force is concentrated on a small area
The pressure increases
What is the formulae for area and force
area= force/pressure and force=areaXpressure
Why is the atmospheric effect much greater in water than in air?
This is because water is much denser than in air so it has a greater mass for the same volume and therefore exerts a greater force (pressure)
What does the pressure exerted by fluid get increased by?
Depth
How can the depth increase the pressure be tested?
This can be demonstrated by making holes in a container which contains water at different levels.
Why are dams thicker at the bottom?
This is because the pressure increases as the water gets deeper
How does the pressure act?
It acts equally in all directions
What are the units for Gravity?
N/m2
What is the second formulae to work out the pressure?
Pressure= Height x Density x Gravitational field strength