Concentration of solutions
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Solution
solution is a mixture of two or more substances in a single liquid phase. one constituent is regarded the solvent and the others as solutes. Solute + Solvent = Solution
Solute
the part of the solution that is being dissolved (usually the lesser amount)
Solvent
the part of the solution that dissolves the solute (usually the greater amount)
What is solution concentration?
Indicates the amount of solute in a given amount of solution/solvent.
Types of concentrations - percent concentration
weight/weight percent concentration
weight/volume percent concentration
volume/volume percent concentration
weight/weight percent concentration is defined by the following equation
shows the percent of the solute

weight/volume percent concentration
shows the weight of the solute in the total volume of solution

volume/volume percent concentration
used with liquid solutes
volume is expressed in millilitres (mL)

Solution density
Refers to the ratio between the weight and volume of a give solution. Two types of density are used: relative density, mass density
Relative density (d)
a digit that shows how much the weight of a given solution is bigger than the weight of an equal vol of water
Mass density (ρ)
ρ (density) = m (mass solution)/ v (vol solution)
expressed as kg/cm3 or g/mL
Molarity
It is the number of moles of solute pre litre of solution (mol/L or M)
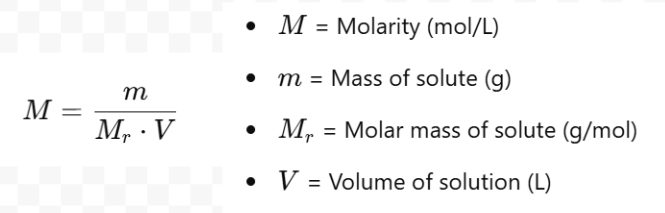
How to calculate moles?
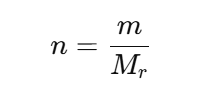
How to calculate concentration?
Conc = moles/vol
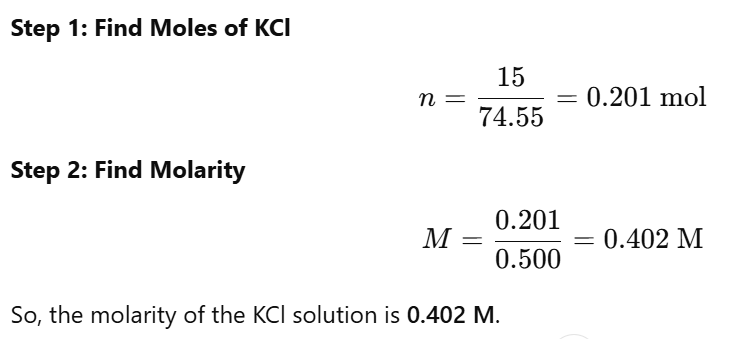
How to calculate molarity as two equations?
first determine the moles of solute (n=m/Mr)
once you have n substitute in the molarity equation
Suppose you dissolve 15 g of KCl (Mr=74.55M_r = 74.55Mr=74.55 g/mol) in 500 mL (0.500 L) of solution.
Molal concentration (Cm)
shows the number of moles of a solute in 1000g of solvent
Molality is a measure of concentration that expresses the number of moles of solute per kilogram of solvent.
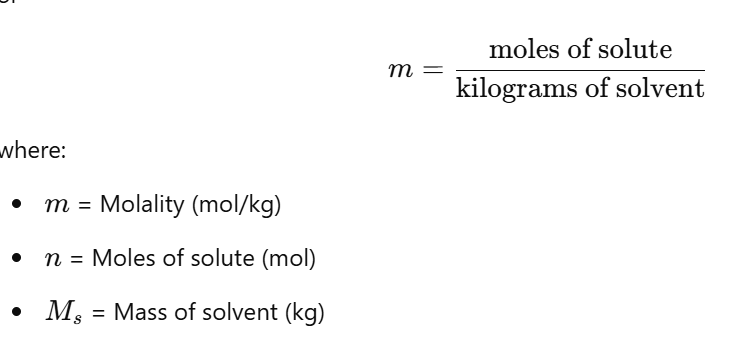
Key differences between molarity and molality
