EX2 Beta Agonists (MC)
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
Beta agonists SAR

No COMT metabolism and increased chemical stability

Summary
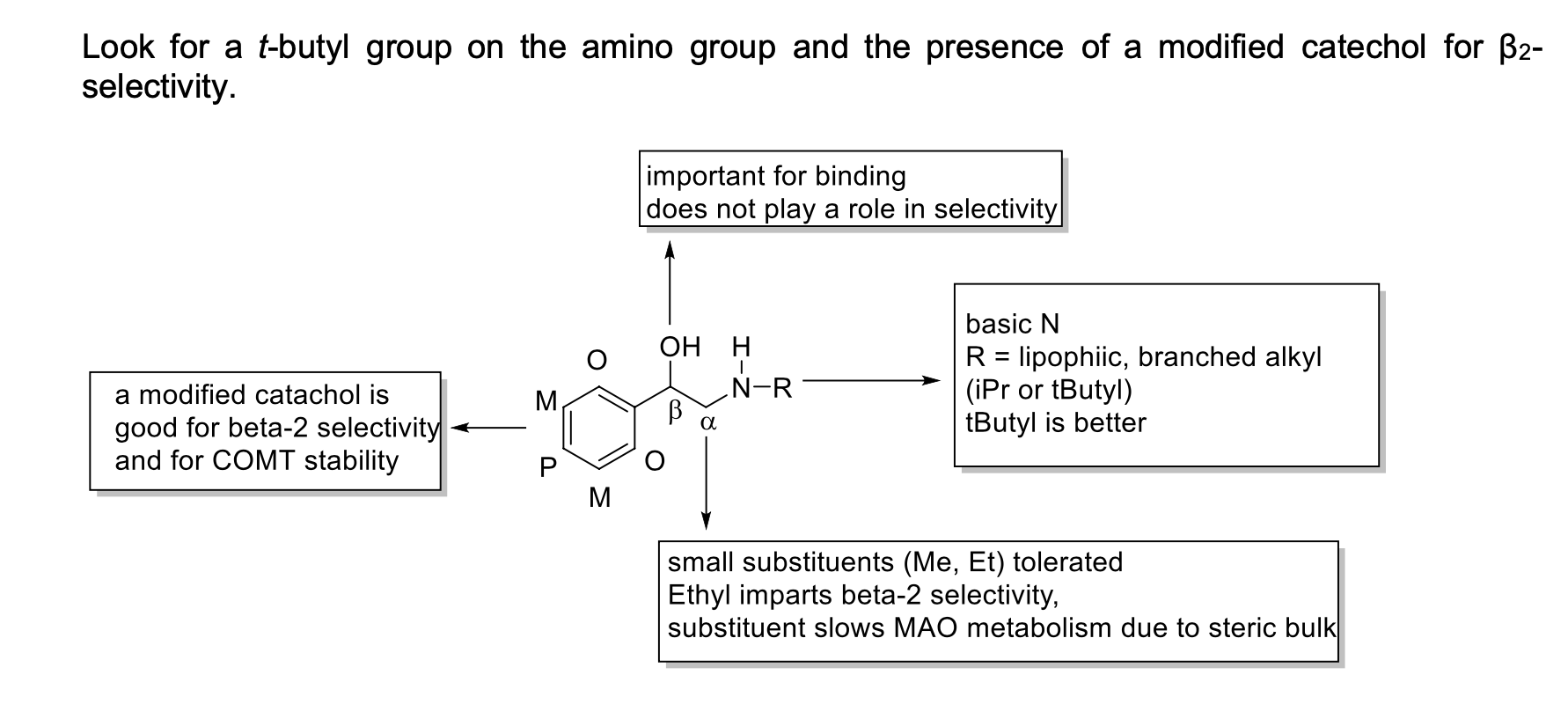
Which alkyl substituent on the N atom produces β2-selectivity
t-butyl
Phenethanolamine Derivatives
Agonists at the B2 receptor generally produce relaxation of the smooth muscle
Agents that relax the bronchii (by agonism at the B2 receptor in the bronchial smooth muscle) are useful in the treatment of pulmonary conditions such as asthma.
Agents that relax the uterus (by agonism at the B2 receptor in the uterine smooth muscle) are useful in the treatment of premature labor.
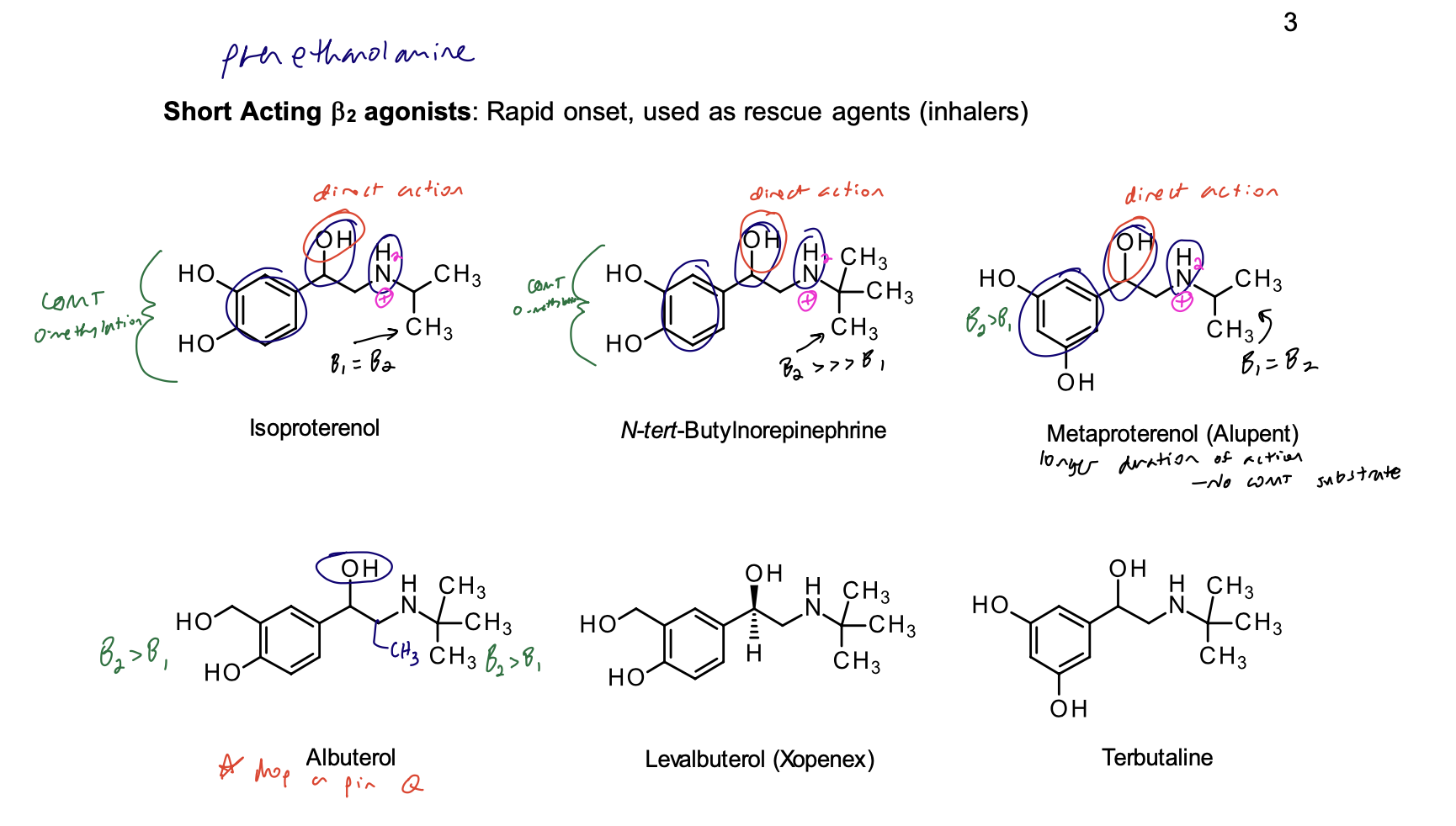
Short Acting B2 agonists: Rapid onset, used as rescue agents (inhalers)
When the catechol is modified, what does that do for selectivity and stability?
increased selectivity and longer lasting
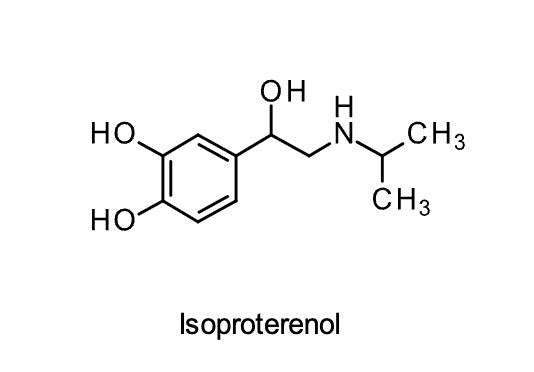
Isoproterenol
Structural Evaluation:
• N-isopropyl group makes it selective for the B-receptor.
• The intact catechol makes it an agonist
• No selectivity between B1 and B2 receptors and acts as an agonist at both
• Presence of the catechol results in poor bioavailability and chemical/metabolic stability.

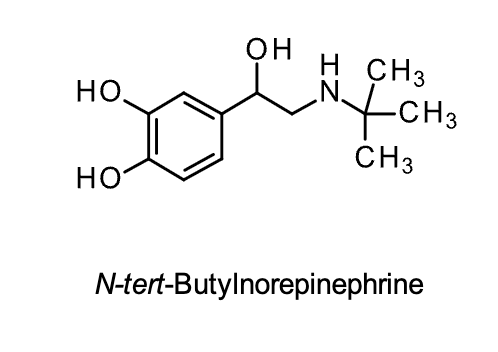
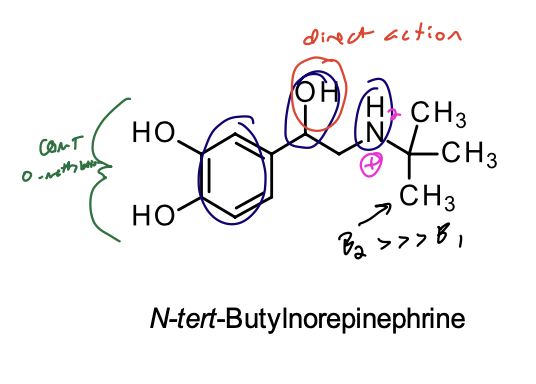
N-tert-butyl Norepinephrine
Structural Evaluation:
• t-Butyl substituent on the nitrogen gives it a slight selectivity for the B2 receptor.
• Catechol prevents it from being highly selective for the B2 receptor.
• Presence of the catechol results in poor bioavailability and metabolic stability
Albuterol, Levalbuterol, and Terbutaline
Structural Evaluation:
• Modified catechol gives them higher selectivity for the B2 receptor.
• Presence of the t-butyl group on the nitrogen makes them B2 selective.
Long Acting B2 agonists
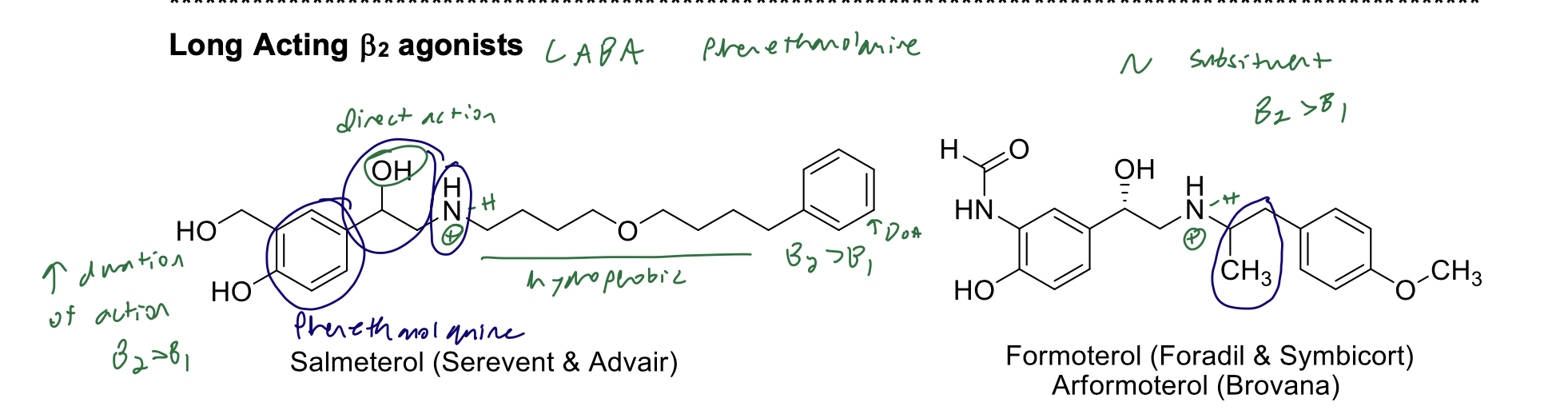
What is the structural rationale for these agents being categorized as long-acting?
Hydrophobic N substituent → increased duration of action; modified catechol
Ultra-Long Acting B2 agonists

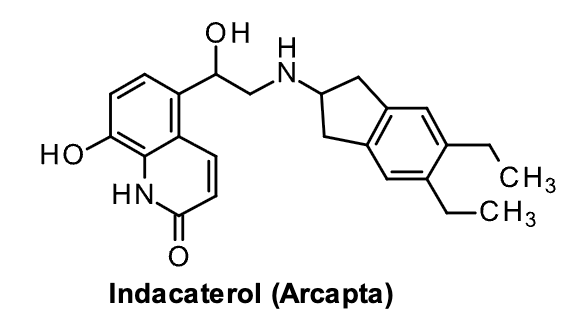
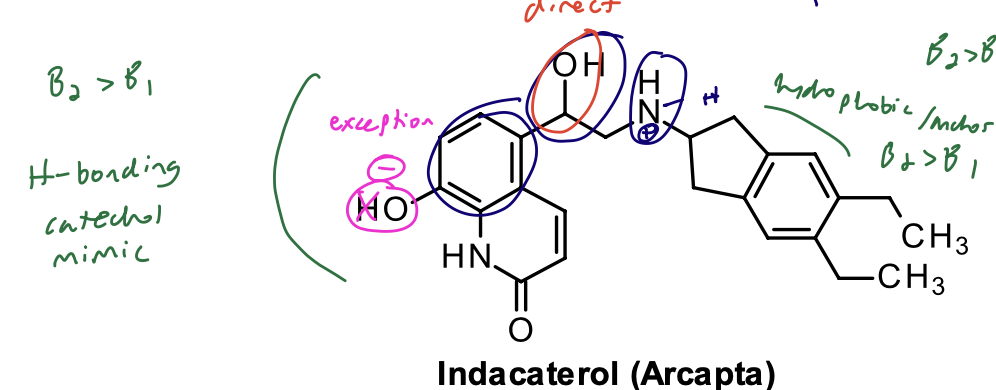
Indacaterol
Structural Evaluation:
Phenol has a pKa of 6.7 and is ionized at physiologic pH. Forms zwitterion with the basic amino group.
Zwitterionic form binds to the B2 receptor with very high affinity, contributes to long duration. Its onset of action is similar to salmeterol
B1 Agonists, dobutamine SAR
Structural Evaluation:
• Dopamine analog with a bulky aralkyl substituent (B>a) on the nitrogen.
• Lacks the beta hydroxyl group and is relatively weak in its agonist actions. decreases affinity
• Racemic dobutamine has direct activity on both B1 and the B1 receptors. Overall effect B1 agonist.
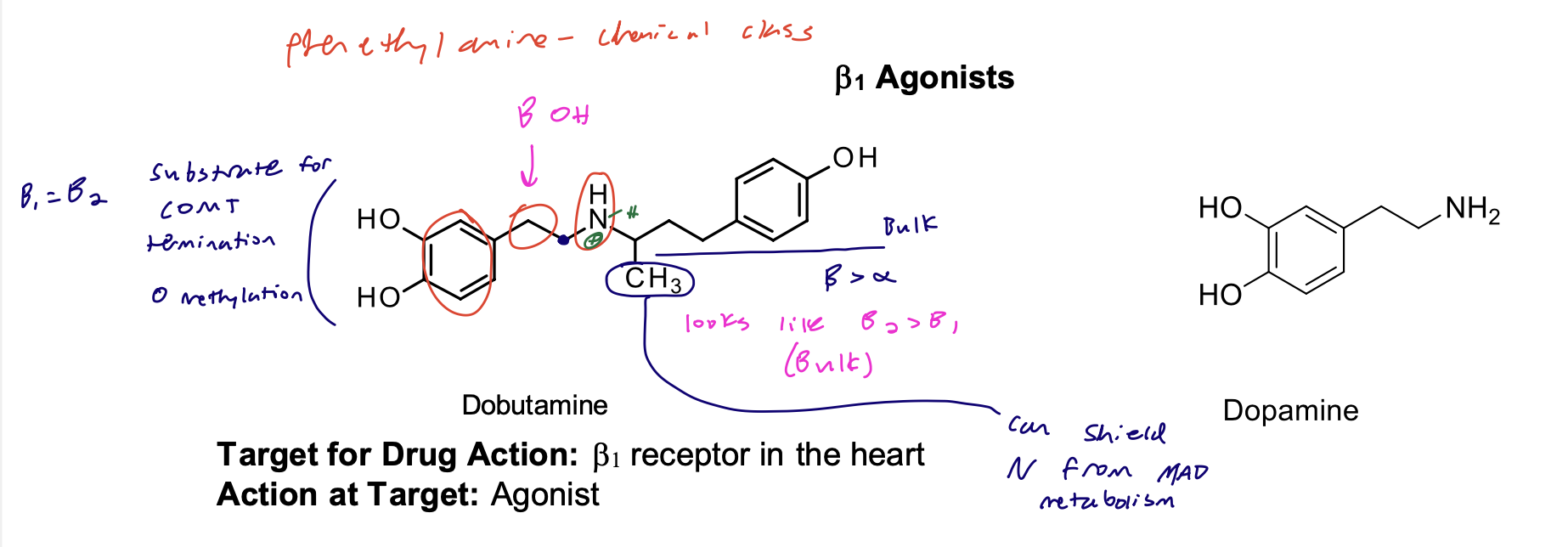
How do the catechol and secondary amine contribute to the duration of action?
Catechol → available for termination (COMT substrate)
amine - no a substituent → MAO substrate