Chem 101: Unit 1 Atoms
1/216
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
217 Terms
What formula is used to calculate the energy of stationary states?
En=-RH(Z²/n²)
What does Z indicate?
Atomic number
What does n mean (stationary state)
n is equal to the number of stationary state (1,2,3... to infinity)
What is RH
The Rhydberg constant 2.179 x 10^-18 J
The formula En= -RH(Z²/n²) is only applicable to what type of species?
One-electron
Examples of one-electron species include;
H, He+, Li2+
What is the stationary state of an electron?
Electrons do not radiate energy as they orbit or circle the nucleus, instead they exist in stationary states- a state of constant energy.
What do stationary states imply?
That electrons orbit at a fixed distance from the nucleus
Ground state
n=1
First excited state
n=2
second excited state
n=3
How does energy change from one state to another?
The n=1 state has the lowest energy, the energy increases by a big gap to the n=2 state. The n=2 to n=3 transition is also an increase, but with a smaller gap this time, the energy continuosly increases by progressively smaller gaps
n=infinity
The electron has become free and the hydrogen-like species is now ionized
When will a photon of energy be emitted?
When an atom in an excited state (n=2 - n=infinity) relax to a lower energy state
Energy of a photon is equal to
the difference in energy between the higher and lower states of the electron
The formula Ephoton(or delta E)=hc/λ is used to calculate
the energy of a photon of the difference of energy between stationary states
The planck constant (h) is equal to
6.626 x 10^-34 Js
The speed of light constant (c) is equal to
2.998 x 10^8 m/s
An electron undergoing a transition with a large energy difference produces a photon with a __________ wavelength
short
An electron undergoing a transition with a small energy difference produces a photon with a __________ wavelength
long
The longest wavelength emitted is from the transition between
n=2 to n=1
the shortest wavelength emitted is from the transition between
n=infinity to n=1
wavelength (λ) and energy are ___________ related
inversely
The balmer series has an n final of
2
the lyman sereies has an n final of
1
Since the smallest energy corresponds to the largest wavelength the same energy transition will be seen as a line on the far _____ of the plot.
right
Since the largest energy corresponds to the shortest wavelength the same energy transition will be seen as a line on the far _____ of the plot
left
The Bohr's model is limited to describing the spectra of
Hydrogen like species (one-electron species)
To find a hydrogen-like species, use the expression
E^ +(Z-1), ie. He--> Z-1=2-1= He+
Be Z=4 so Be^(4-1)= Be3+
How do you find the change in energy (delta E)
Use the Rhydberg equation: (delta E) = -RH x Z² ((1-nf²)-(1/ni²))
In the Rhydberg equation, if yoou want to find Z instead of Delta E, what should you do?
Replace Delta E with the energy, if you are provided with a wavelength instead of energy use the formula Delta E=hc/λ to find it then substitute
When calculating make sure that the
units line up. (all lengths are in metres, energy in J or KJ)
In an emission transition the energy is
negative
In an absorption transition the energy is
positive
Wavefunction is symbolized by
𝚿 (psi)
What is the wavefunction?
3D coordinates that have been obtained by solving the Schrodinger equation
What are the two parts of the wavefunction?
Radial (r) and Angular (Y), wavefunctions for singular electrons can be referred to as orbitals
Each solution of the Schrodinger equation yields
the energy state associated with a given atomic orbital
Probability Density is represented by
𝚿²
Probability density is
the probability of finding an electron in some tiny volume of the atom
In a probability density DOT diagram, gretaer density represents
a higher probability of finding the electron in that volume
Radial probability distribution
represents the total probability density at some distance (r) from the nucleus
Total electron density peaks _____ but not _____ the nucleus
near, at
Probability contour
the shape that defines the volume around the nucleus within which an electron spends a given percentage of its time
The 1s orbital has ___ nodes
no nodes
A node on a probability density plot is represented by
a zero (the y-coordinate touches the x-axis)
On a density probability plot, a maxiumum represents
A distance at which the probability of finding an electron is high
In a 3D distribution plot, the white rings represent
nodes
Because a 2s orbital is bigger, an electron spends more time ______ from the nucleus than in a 1s orbital
away
# of radial nodes=
n-ℓ-1
Quantum numbers
specify the properties of atomic orbitals and the properties of electrons in orbitals
Principal quantum number
symbolized by n, indicates the main energy level occupied by the electron and the size of the orbital
angular momentum quantum number
symbolized by ℓ, indicates the shape of the orbital
Magnetic Quantum number
symbolized by mℓ, indicates the orientation of an orbital around the nucleus (spacial orientation)
n= a level so ℓ=
a sublevel
ℓ=0
s orbital
ℓ=1
p orbital
ℓ=2
d orbital
ℓ=3
f orbital
ℓ is an integer from 0 to n-1
The number of possible values of ℓ=n
mℓ is an integer from
-ℓ through zero to +ℓ
-ℓ,(-ℓ+1),0,....ℓ
total number of mℓ values =
2ℓ +1
If n=1 and ℓ=0 then mℓ has to be
equal to zero
if n=2 then ℓ can equal:____, ____,
0,1
If n=2 and ℓ=1 then mℓ can equal: ____, ____,____.
-1,0,1
The total number of mℓ values for a given n value=
n², which equals the total number of orbitals in that energy level
When ℓ= 0 there is only one value for mℓ, so that means there is only
one "s" orbital
When l=1 there are three possible values for mℓ, which means there are
3 "p" orbitals
How many d orbitals are there?
five
How many "f" orbitals are there?
7
How many electrons are in the "s" orbital?
2 electrons
How many electrons are in the "p" orbital
6 electrons
Which orbital has 10 electrons
the "d" orbital
How many electrons in the "f" orbital?
14 electrons
How many orbitals in an atom can have the following designation: n=3
To solve this, you want to use the formula to calculate the total number of mℓ values which is n², so in this case 3²=9
In an orbital diagram, two arrows pointing in different directions represent
a pair of electrons
how many electron pairs can fit in the 1s orbital?
1 (up, down)
How many electron pairs can fit in a 2s orbital?
1 (up, down)
How many electron pairs can fit in a 2p orbital?
3 (up down, up down, up down)
Hund's Rule
When orbitals of equal energy are availible, maximize unpaired electrons.
How does Hunds rule apply to an orbital diagram?
When you don't have enough electrons to fill all of the orbitals in pairs, it means that the electrons will spread out over the orbitals before pairing up.
Example of Hund's Rule
Carbon: fill as many orbitals as possible before pairing electrons on the valence orbital
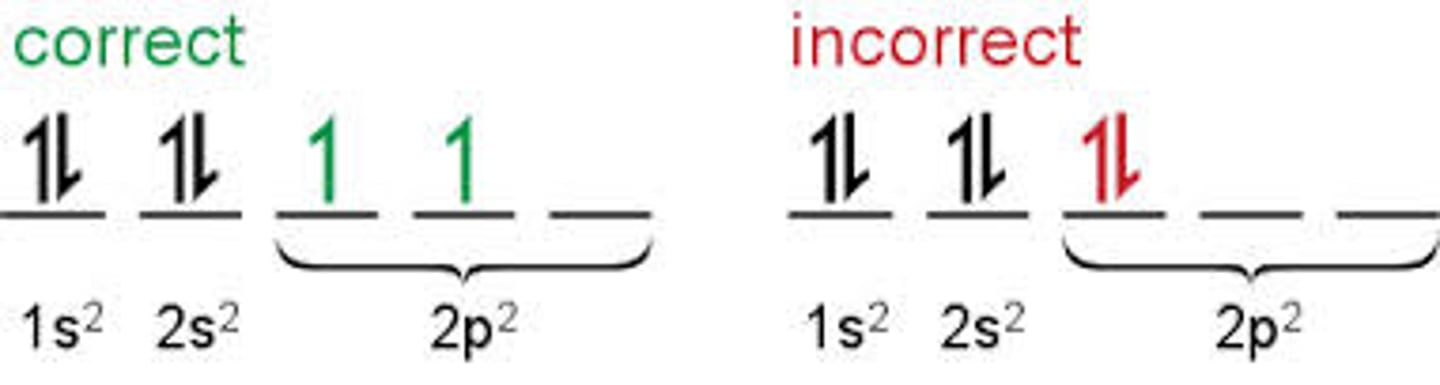
The 2p has 3 orbitals, what subscripts would be used to identify them?
X,Y and Z (2px, 2py, 2pz)
What is the spdf notation for Boron?
1s² 2s² 2p^1
What is the spdf notation for oxygen?
1s² 2s² 2p^4
Core electrons
same as their preceeding noble gas
Valence electrons
electrons that go beyond their preceeding noble gas core
Condensed notation
involves using the noble gas before the element to represent the core, then using regular spdf notation to represent the valence electrons
Condensed notation for Mg
[Ne] 3s²
Condensed notation for S
[Ne] 3s² 3p^4
In condensed notation, n= period numbe, therefore Lithum is
[He] 2s^1 because Li is in the 2nd period
Elements in the same group have the same valence electron configurations, and thus
similar chemical behaviour
For neutral atoms, the 4s orbital is ________ in energy than the 3d orbital
lower
Chromium and Copper Exception
Copper and Chromium do not follow the regular method for detecting configurations
Condensed configuration of Copper (Cu)
[Ar] 4s1 3d10
Condensed configuration of Chromium
[Ar] 4s1 3d5
Why are copper and chromium exceptions?
Because chromium orbital configuration has completely half-filled orbitals, and Copper has all fully filled 3d orbitals, which are more stable than their expected configuration
s block elements include
Groups 1 and 2 +helium
d block elements include
all the transition metals, groups 3-12
p block elements include
the mettaloids and non-metals (groups 13-16 -helium