Organic Chem Test 2
1/20
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
Brief review on nomenclature: meth, eth, prop, but, pent, hex, hept, oct, non, dec, undec, Dedec, icosane, triacontane
1,2,3,4,5,6,7,8,9,10,11,12,20,30

Alkyl groups
CH3 Methyl
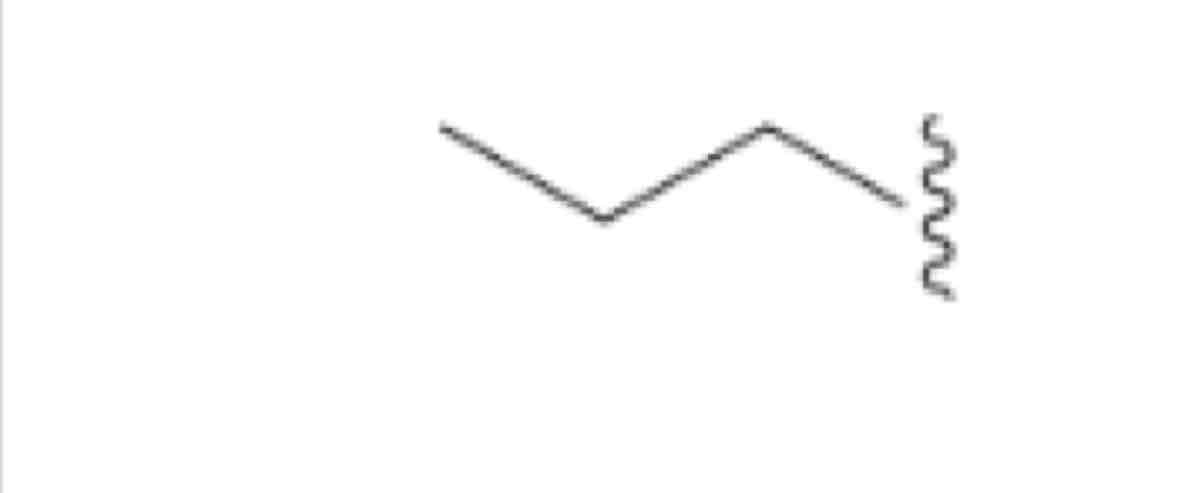
Propyl, ch3ch2ch2
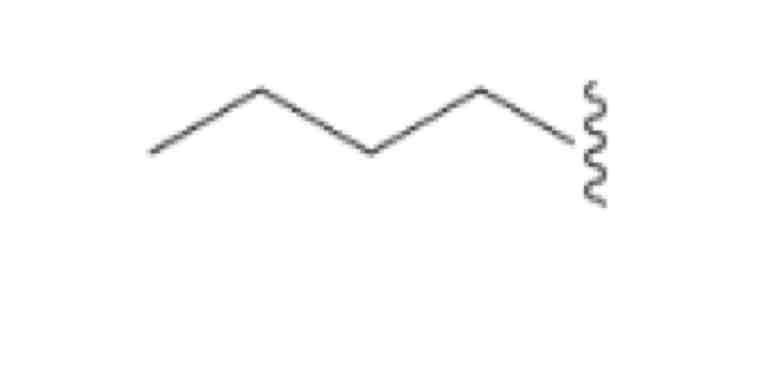
Butyl, CH3CH2Ch2Ch2
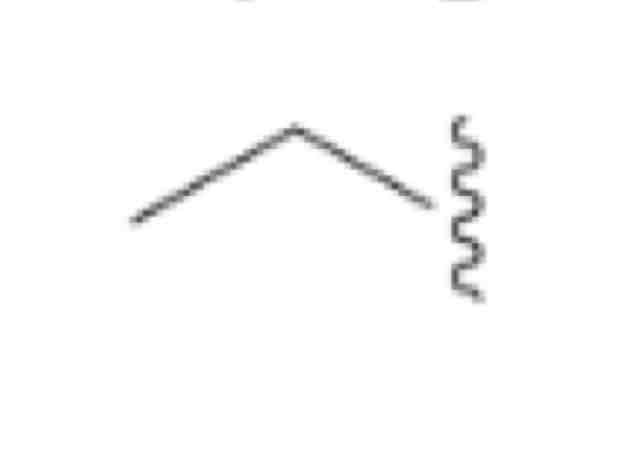
Ethyl, CH3Ch2
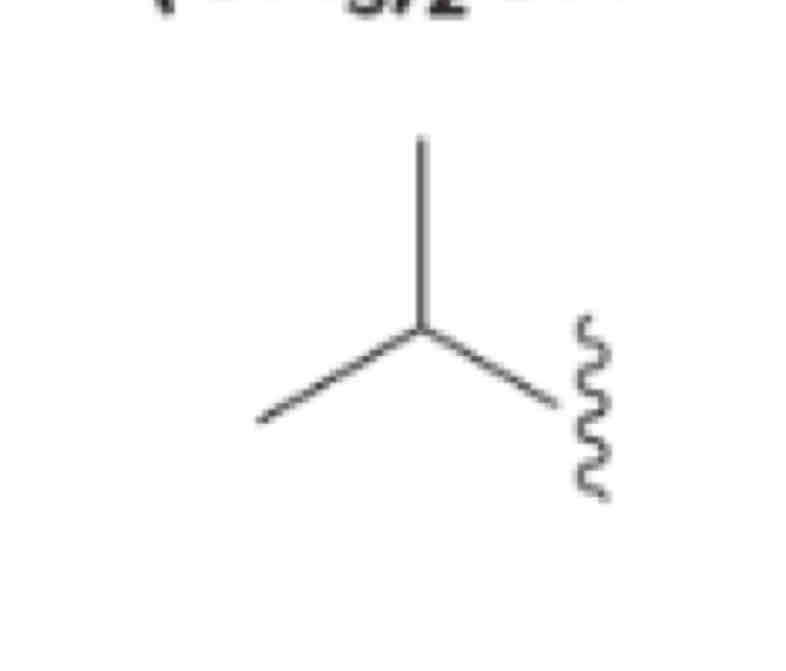
Isopropyl, (CH3)2CH [iso included in alphabetizing]
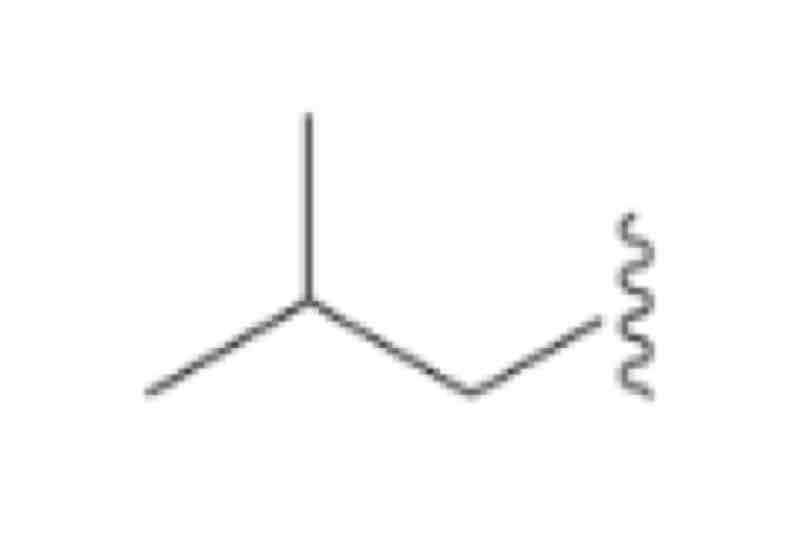
Isobutyl, (CH3)2CHCH2
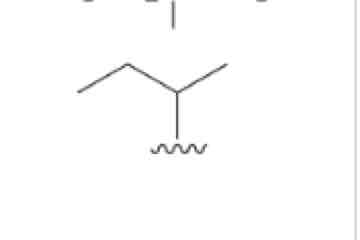
Sec-Butyl, CH3CH2CHCh3 [IGNORE THE SEC WHEN ALPHABETIZING]
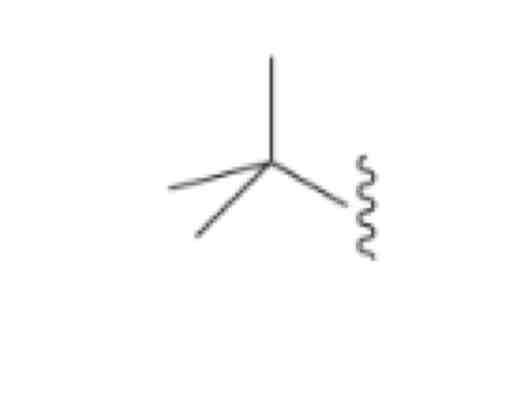
Text-Butyl (ch3)3C- [IGNORE THE TERT WHEN ALPHABETIZING]
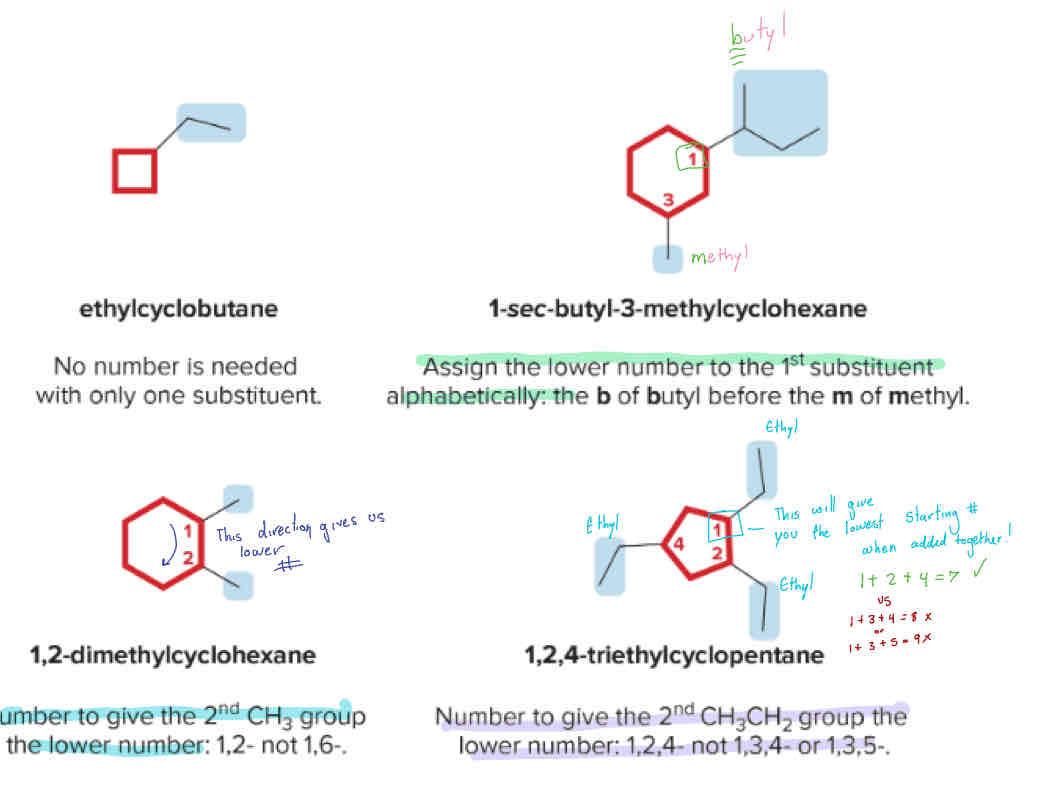
Naming Cycloalkanes
Assign the lower # to the first branch alphabetically
Rotate around to give the branches the lowest possible numbers
If branches are the same, start where the branches will add to have the lowest number possible
Confirmations of acyclic alkanes! Double and triple bonds do not ___ single bonds do. They can be __(line up) or__(bisecting)
Spin, eclipsed, staggered (more stable)
Cyclohexanes: when the larger molecule is ___ it is more stable
Equatorial.[put bulky groups on equitorial]
Wedges and dashes when putting into chair notation
Wedges mean up and dashes mean down. [in normal notation dashes are going into the page away from you, and wedges are coming towards you but this is for putting them into a chair :]
![<p>Wedges mean up and dashes mean down. [in normal notation dashes are going into the page away from you, and wedges are coming towards you but this is for putting them into a chair :]</p>](https://knowt-user-attachments.s3.amazonaws.com/f72d8831-31c7-42af-8565-b5724c6d02e6.jpg)
Oxidation
Increasing number of C-O bonds / decreasing number of C-H bonds
Reduction
Decreasing number of C-O bonds, Increasing number of C-H bonds
Constitutional isomers
Same molecular formula, different name. [life hack! Drop this class:) also if it’s an alkane hydrocarbon you can quickly figure out the # of hydrogens by doing, (Carbon x 2 )+2 =hydrogen . So like here it’s C6 X2 = 12+2=14]
![<p>Same molecular formula, different name. [life hack! Drop this class:) also if it’s an alkane hydrocarbon you can quickly figure out the # of hydrogens by doing, (Carbon x 2 )+2 =hydrogen . So like here it’s C6 X2 = 12+2=14]</p>](https://knowt-user-attachments.s3.amazonaws.com/a2fdbaca-15f9-4303-8a09-ada4f131ed14.jpg)
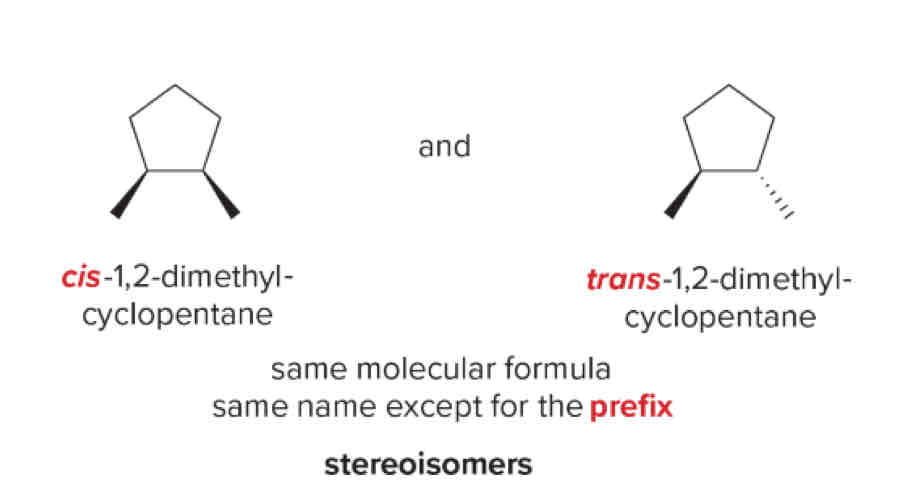
Stereoisomers
Same molecular formula, same name, different prefix Possible prefixes include: R,S,Cis,Trans
Hands 🖐🤚
Not superimposable, Chiral, one stereocenter, enantiomers
Marker 🖋✒
Superimposable, achiral, no stereocenter
Naming enantiomers R or S
assign priorities based on atomic #, most protons=lowest number
If 2 atoms are the same, assign based on the atomic # of the atoms bonded to it
-its that if 2 isotopes are bonded to the sterogenic center, assign based on decreasing mass number like deuterium is heavier than just H so higher priority but she will not test this one-
Treat atoms with double or triple bonds as them having another atom attached (treat bonds like another atom) this one does get tested

Naming R-S steps
Assign priority
(If lowest priority is in the back) draw an arrow going from 1 → 3 “clocks are Right” clockwise is R-configuration and counterclockwise is S-configuration (flip these rules id lowest priority is up front)