Lecture 12- Allosteric Models
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
What is Allostery?
A way of regulating the activity of proteins
Cooperativity is a type of allostery
The binding of a molecule at one site on a protein, that’s going to affect change at distinct/diff site on that same protein
(change will affect activity binding of protein)
In hemoglobin it affects the binding
Hemoglobin subunits and how they’re subunits go from tightly coupled to bonded with oxygen
In the T-state the individual hemoglobin subunits are tightly conformationally coupled to each other. (alpha 1 binds to alpha 2 and Beta 2) (beta 2 binds to Beta 1 and alpha 1 ) (beta 1 binds to alpha 2 and beta 2)
Oxygen binding induces conformational transition that breaks ionic interactions with neighbors
Breaking of ionic bonds reduces conformational restraint (further relaxes state) and allows neighbors to adopt high affinity state
Oxygen can bind to any of the subunits
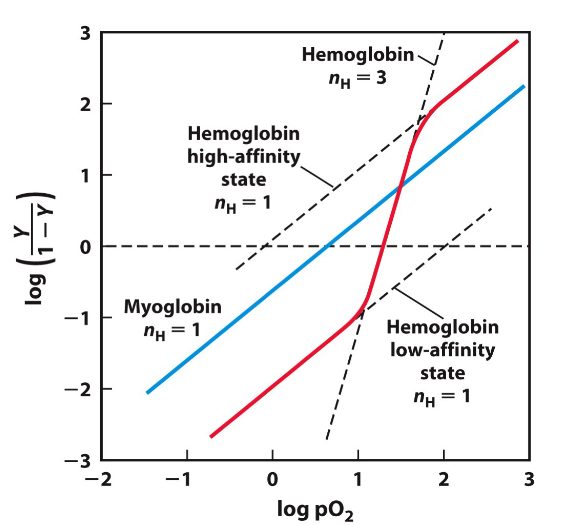
What does a sigmoidal binding (ss shaped curve) curve indicate, explain.
It indicates cooperative binding
Thes first O2 molecules binds weakly to T state subunit
T to R transition is easier for second binding
Last molecule binds to a subunit that is already in the R state
Left on graph is low affinity and right on graph is high affinity
Experimental indicator of Allostery
Cooperative binding ( if you see an SS shaped curve)
what is the hill equation
Shows the fraction of cooperative binding
Tells us if the binding is cooperative or nah (NH )= slope
If nH = 1 No cooperativity
If nH >1 Positive Cooperativity
If nH <1 Negative Cooperativity
For myoglobin: Slope never changes so it is =1
A Hill coefficient of 1 means no cooperative ligand binding is occurring in this particular protein. True for myoglobin
nh cannot exceed n
What is the Monod, Wyman and changeux (MWC) model (concerted Model)
The MWC model explains cooperativity by saying all subunits switch between low- and high affinity states together, and ligand binding shifts the balance toward the high-affinity state (R).
only 2 conformations of the tetramer exists (T and R)
T and R conformations are in equilibrium (can go back and forth)
All subunits undergo transition simultaneously
ligand can bind to both T and R states but has a higher affinity for R
Each successive ligand binding increase likelihood of T to R transition (shifts equilibrium so it lies more in the R state as more oxygens bind)
What is the Koshland Nemethy and Klmer (KNF) Sequential model?
The KNF model says binding happens one site at a time, and each binding slightly changes the shape of the protein — making the next site more likely to bind.
In reality, hemoglobin behavior fits both models partly/
it shows concerted-like switching overall (MWC) but also local stepwise changes between subunits (KNF).
Ligand can bind both T and R state
Ligand binding induces T to R in single subunit
Transition in one subunit increases likelihood of transition in adjacent subunit
Requires existence of many mixed tetramer conformations
Concerted (MWC) vs. Sequential (KNF) Models
Sequential works in showing positive cooperativity in hemoglobin since it shows local stepwise changes between subunits (KNF). But usually compatible with negative cooperativity.
Concerted Model: If we follow 2 parameters below we can come up w/ a predicted way the , hemoglobin will bind and plot data points of fractional binding of hemoglobin at diff conc.
Controlled by: (1) affinity difference (T vs R), (2) equilibrium position without ligand.
All subunits switch together → only T or R states, no intermediates.
Pulmanory Respiration
1 of the 2 types of respiration:
We breathe oxygen from lungs and we exhale CO₂ out.
Cellular respiration
1 of the 2 respirations:
Occurs in tissue
Uses oxygen and produces CO₂
How to transport CO₂ safely back to the lungs from tissue?
CO₂ is not very soluble; it will form bubbles in blood and kill you, bad'
We must do this rxn:
CO2 + H2O <=> H++ HCO3 -
This reaction gets catalyzed by Carbonic anhydrase to make
HCO3 - which is much more soluble and can be transported back
It also releases more protons, which raises the PH→ Ph in tissues will be slightly more acidic
Carbonic anhydrase
Enzyme that reacts w/ co2 and H2o to produce bicarbonate (HCO3)
Bohr effect
How pH and CO2 concentratration will affect O2 affinity
High Ph= High O2 affinity→lungs
Low Ph=Low O2 affinity—→tissues
High CO2 =Low affinity
Low CO2= High affinity