Chemistry 2, Mid Year Exam
1/35
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Aliphatic
Without Benzene Rings
Homologous Series
Similar structure but different formulas
Highest Priority In Order PLZ
Carboxylic Acids (-oic acid)
Esters (-oate)
Amide (-amide)
Aldehyde (-al)
Ketone (-one)
Alcohol (-ol)
Thiol (-thiol)
Amine (-amine)
Alkene (-ene)
Alkyne (-yne)
Alkane (-ane)
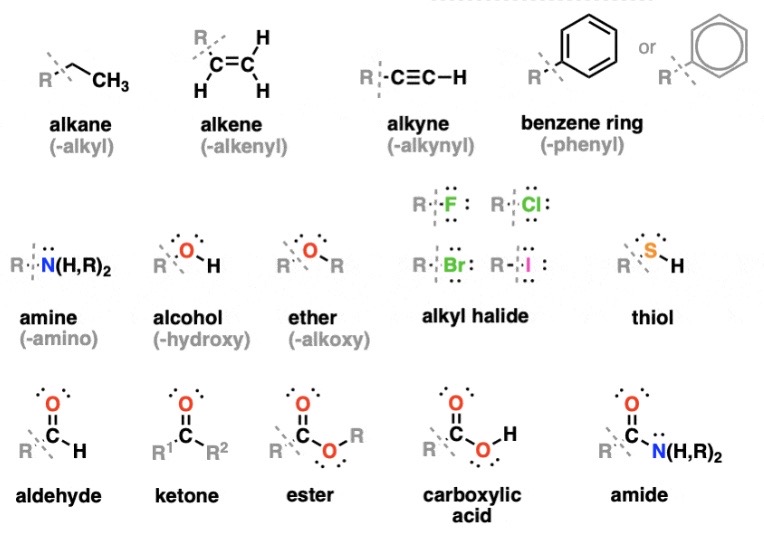
Draw All of the General Structures (11)
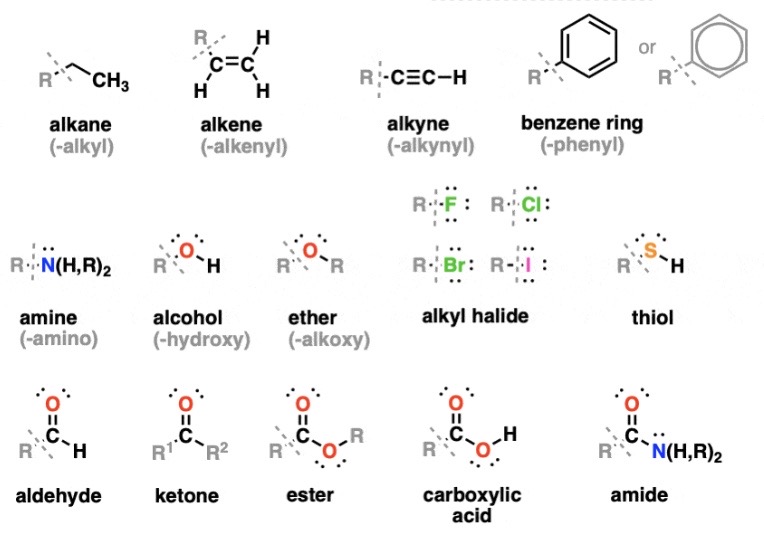
General Formula for Alkane/ Cycloalkanes
CnH2n + 2
General Formula for an Alkyl Group
CnH2n + 1 (but we still use ‘-ane’)
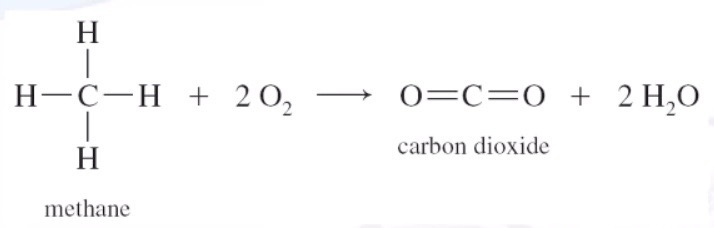
Oxidation
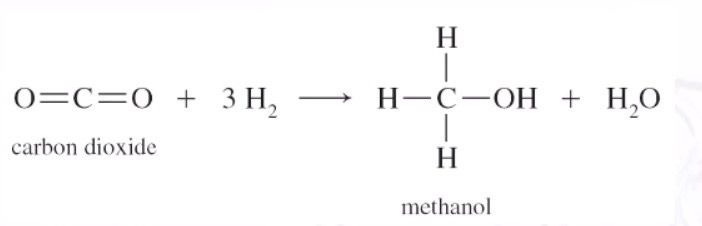
Reduction
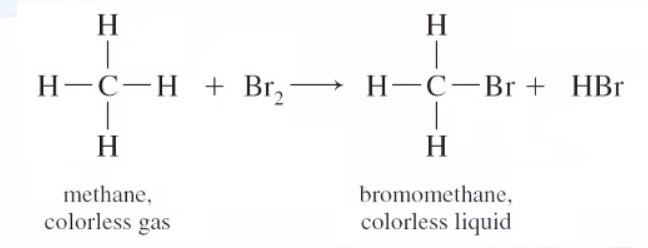
Substitution
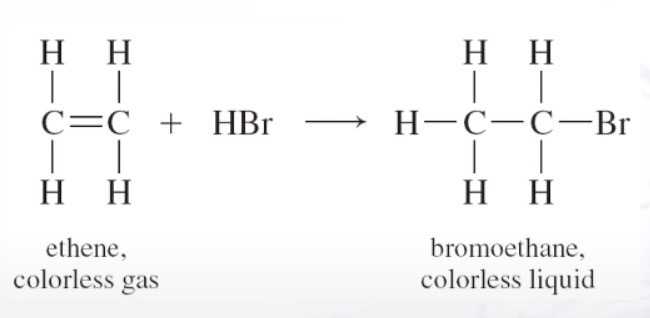
Addion
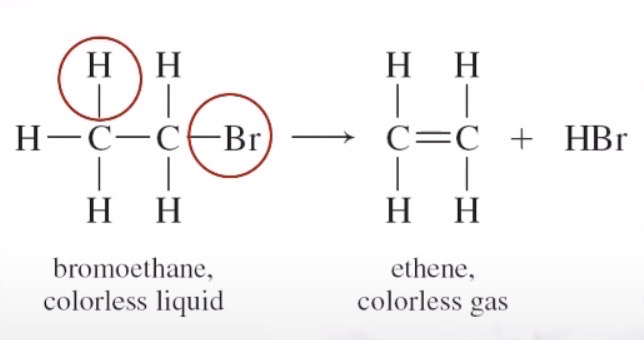
Elimination
Becoming a Smaller Version of Itself
Cracking/ Decomposition
Rearrangement
Isomerisation
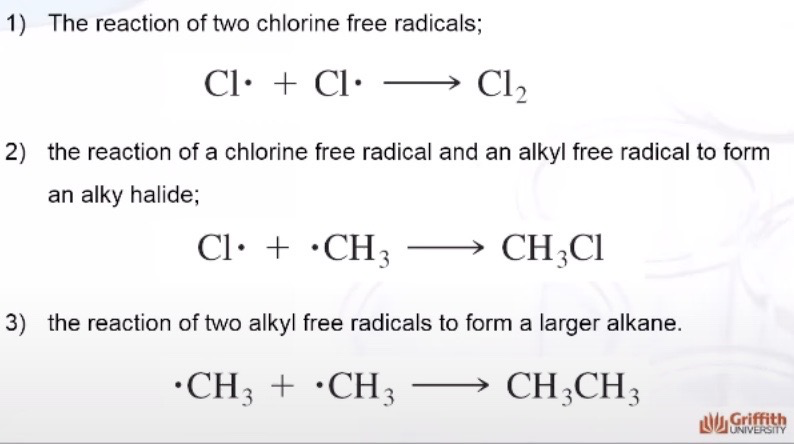
Initiation
Formation of free radicals
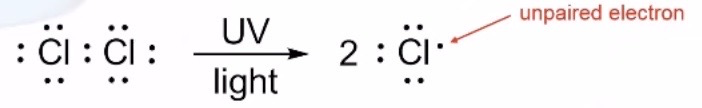
Propagation
The chlorine free radical reacts with the alkane to form hydrogen chloride and an alkyl free radical
The alky free radical keeps reacting with molecules of chlorine to form another chlorine free radical and an alkyl halide
Termination
Making Alkenes
Dehydration and cracking (making a smaller molecules) from alcohol
Getting Rid of Alkenes
Hydrogenation (adding H)
Halogenation (adding F, Cl, Br, I)
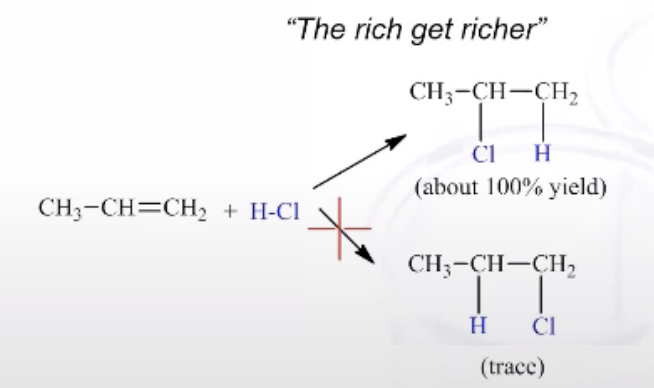
Unsymmetrical added to Alkene
2 Produces are theoretically possible (Markovnikov’s Rule= The rich get richer)
Baeyer Test
Look for double or triple bonds in an unknown sample
(Should turn brown)
Boiling Points
Amount of carbons in the chain (more=higher boiling) (branched will be lower)
Type of forces
Solubility
Amount of carbon chain (more=lower solubility)
polarity
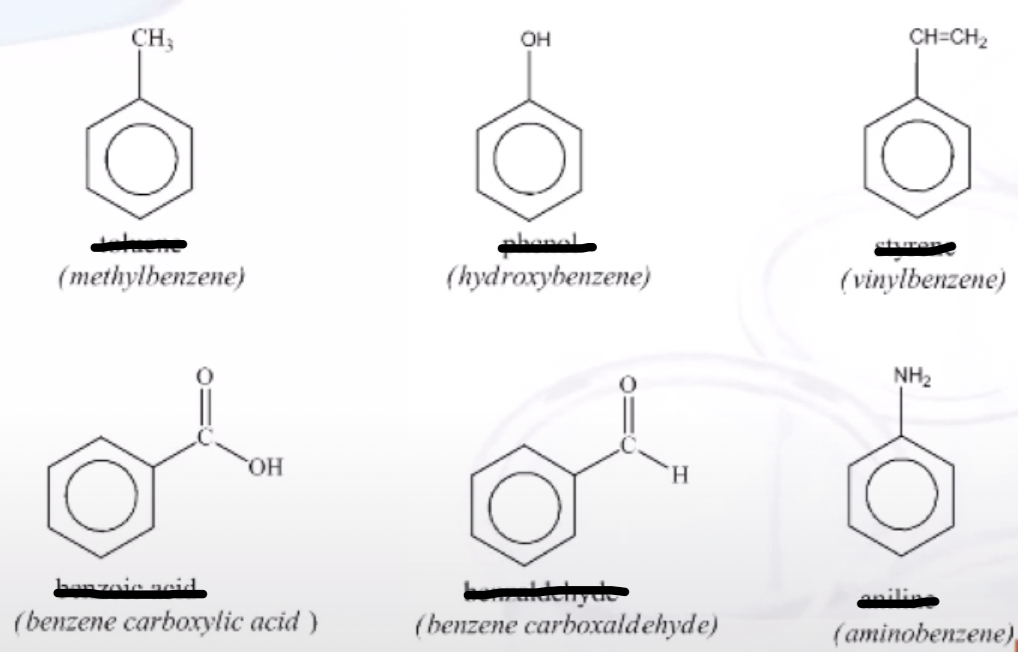
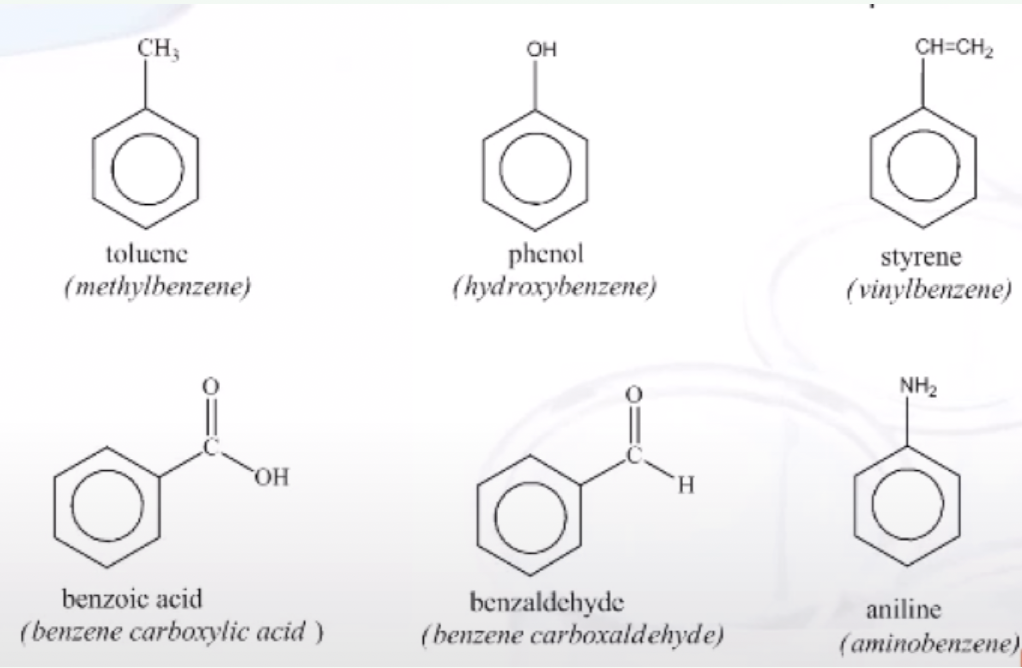
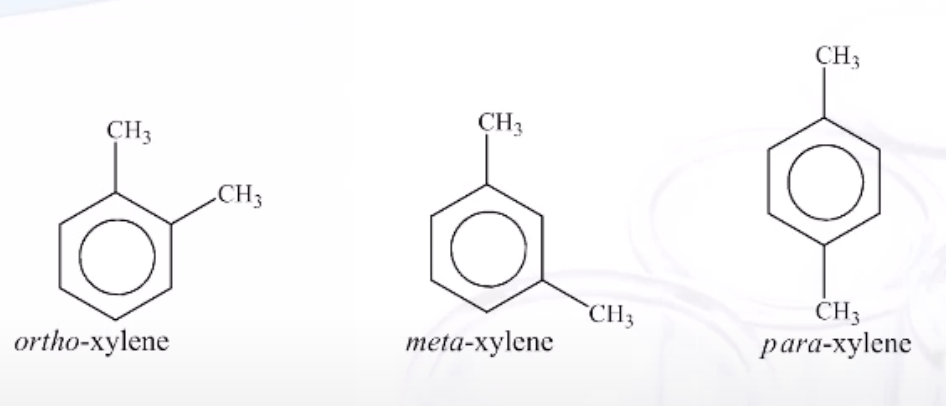
Dimethyl Benzenes Special Name
Oxidation of Alcohols
Alkanes → Primary Alcohols → Aldehydes → Carboxylic Acid
Secondary Alcohols → Ketones
Ester + Water = ?
Ester + Water = = Alcohol + Carboxylic Acid
Alkyl Halide + Water = ?
Alkyl Halide + Water = Alcohol + Salt
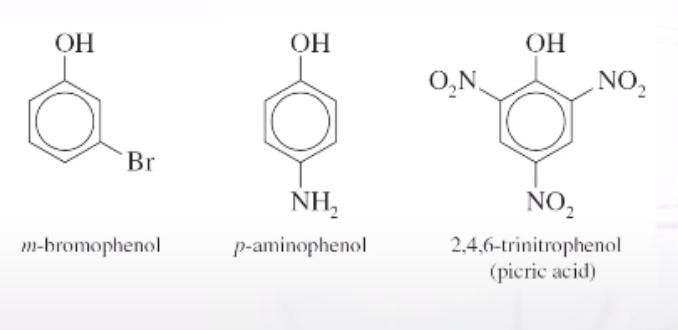
Phenol
Tollens, Fehings, and Benedicts tests
Positive for Aldehydes and Negative for Ketones
Ester + Base (NaOH) = ?
Ester + Base (NaOH) = Carboxylic Acid + Alcohol
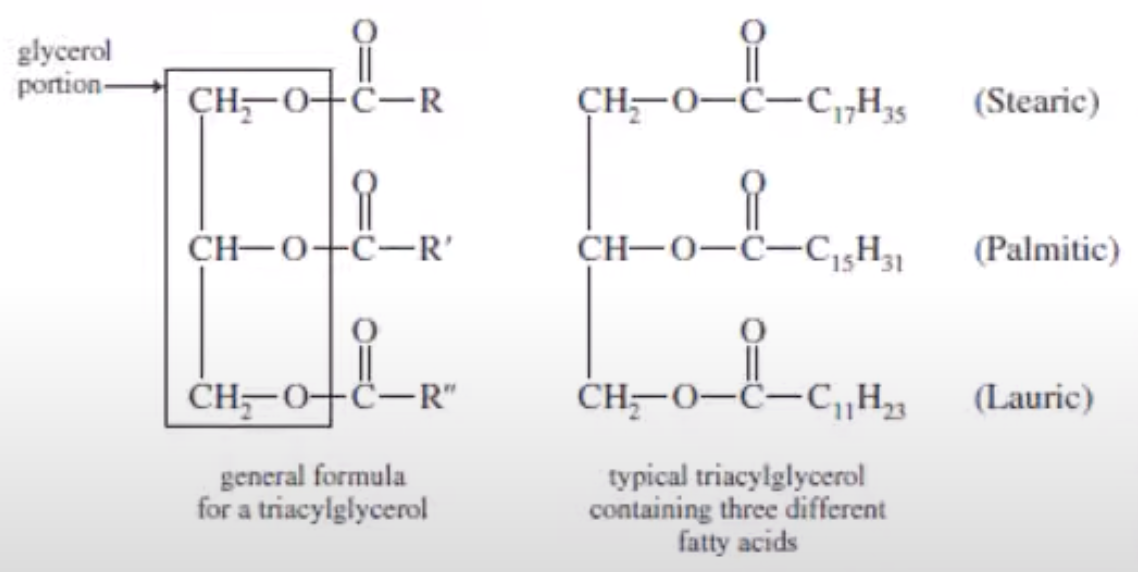
Tricylglcerols
Fats from animals and oils from plants
Saponification
Soap is made from hydrolysis of Tricylglcerols
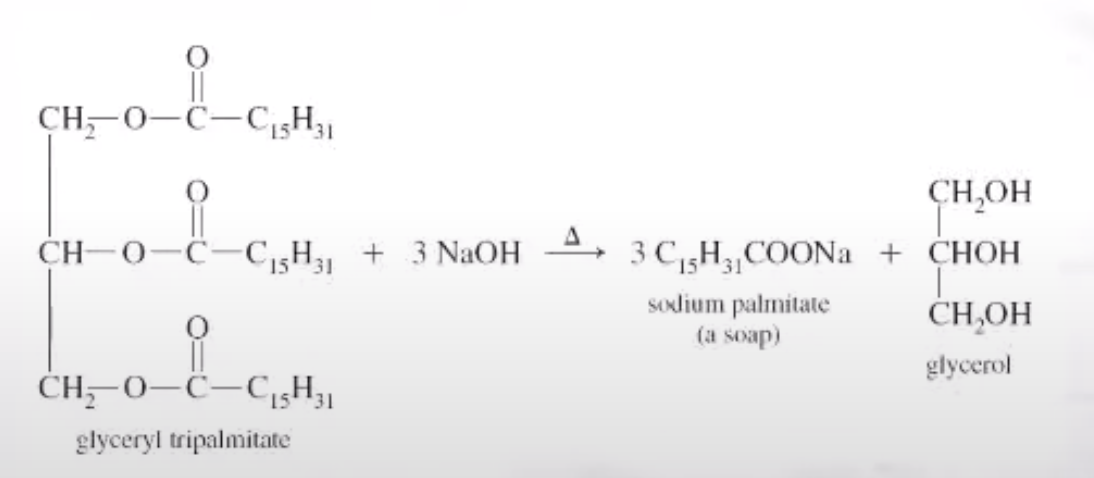
Soap and Detergents
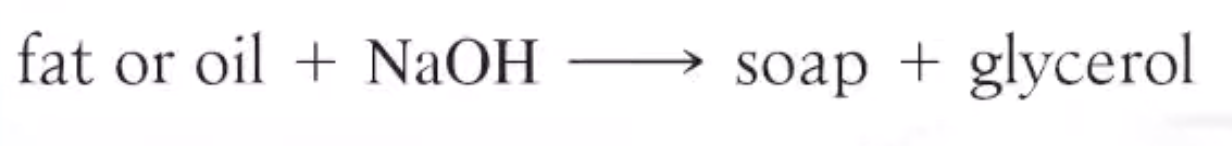
Stearic, Palmitic and Lauric Acids
C17, C15, C11
Carboxylic Acid + Ammonia (NH3) = ?
Carboxylic Acid + Ammonia (NH3) = Amide + Water