Gas Laws and Stoichiometry Unit Set
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
law 1: particles in a gas will move..
in straight lines called translational motion until they collide elastically (with no energy or momentum lost) with other particles or sides of the container
law 2: temperature is a measure of..
the average kinetic energy (Ek = ½ mv2) of particles in a sample of matter
law 3: in an ideal gas, the volume of particles
is taken to be 0, they are modelled as a mathematical point.
law 4: in an ideal gas, there are..
no forces acting between particles, the kinetic energy of the particles overwhelms attraction, so attraction does not greatly affect particles
how do you increase kinetic energy?
add more kinetic energy or heat
what do the approximations of ideal gas laws break down?
these approximations will break down for real gasses at very low temperatures or at very high compression (pressure)
particles exert force on the sides of a container when they collide leading to this formula
pressure exerted by a gas = force / area or
newtons (N) / m2 = Pascal (Pa)
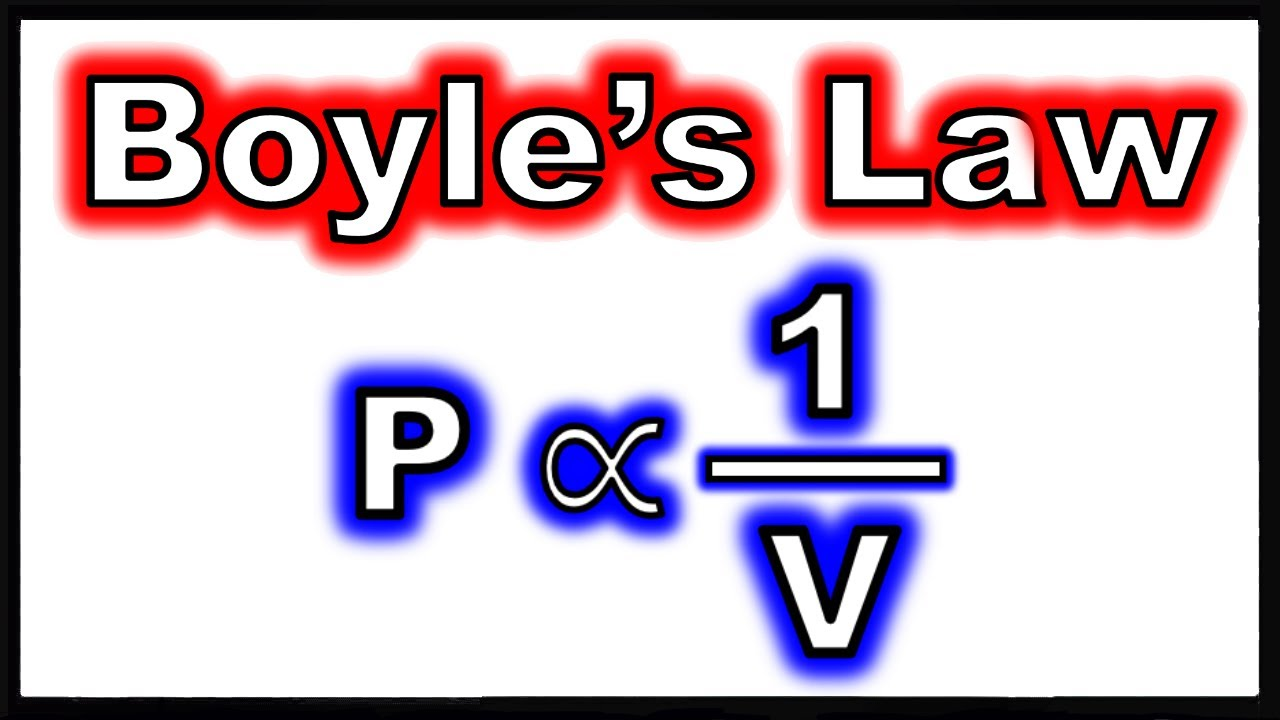
P = k (1/v) or
PV = K
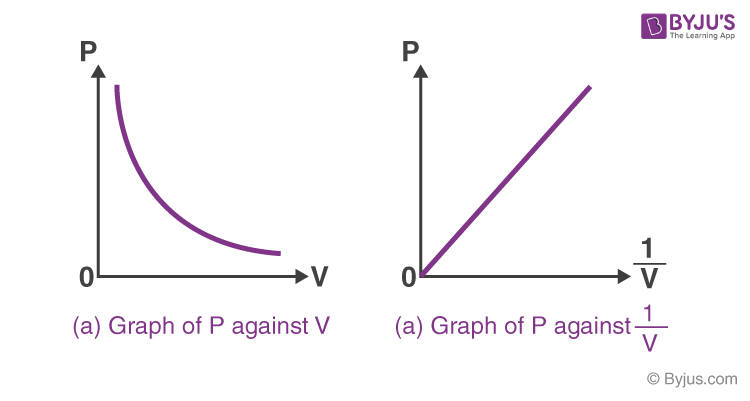
pressure is ________ proportional to
inversely proportional to volume of an ideal gas
increase in temperature (to V)
increase in collision frequency and collision energy, increase in volume
decrease temperature (to V)
decrease collision frequency and collision energy, decreased volume
temperature and volume have a
direct relationship at constant n/m and P
convert °C to K
add 273.15
convert K to °C
subtract 273.15
law of combining volumes (gay-lussac)
gasses react in specific whole number ratios by volume at constant T and P
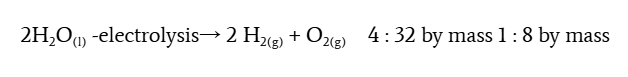
condition of volume ratio
ONLY for gasses, does not work for solids or liquid
definition of mV
We define Molar Volume as the volume of gas at a particular temperature and pressure consisting of one mole of gas
molar volume formula
mV = V/n
Density of an ideal gas
Dgas = mm/mV, this ONLY works for gasses

how to convert g/L to g/mL
divide by 1000 since the conversion is happening in the denominator
universal gas constant
R = 8.314 kPaL/Kmol (kilopascal litres/ Kelvin mol)
ideal gas law
R = PV / nT or PV = nRT
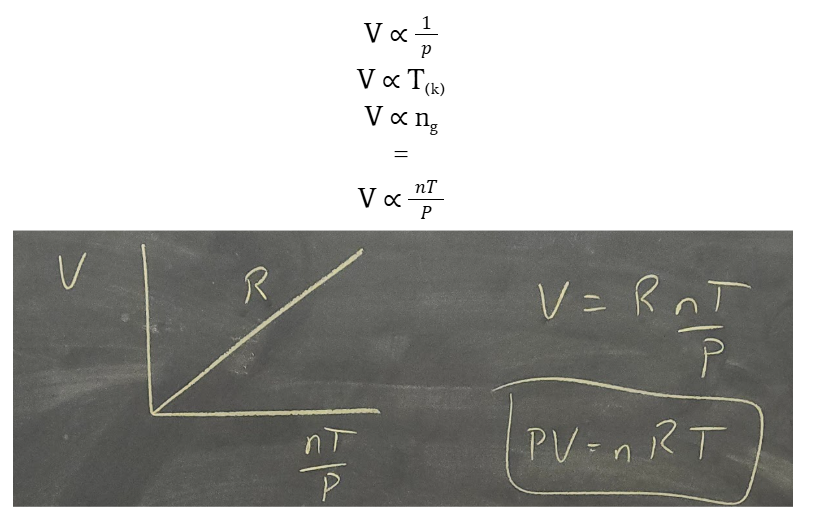
use ideal gas law to find molar volume
PV = nRT
V = nRT/P
V/n = RT/P
since V/n is molar volume, mV = RT / P
use ideal gas law to find density
D = m/V
V = m/D
substitute V = m/D into PV = nRT
Pm/d = nRT
Pm = nRTD
D = Pm/nRT
since m/n is mm, D = mmP/RT
since P/RT is mV, this is the same as D = mm/mV
use ideal gas law to find mm
substitute n = m/mm into PV = nRT
PV = mRT/mm
mmPV = mRT
mm = mRT/PV
boyle’s law
Volume is inversely proportional to pressure at constant T and n
P1V1 = P2V2
charles’ law
volume is directly proportional to temperature in Kelvin at constant P and n (moles per mass)
V1/V2 = T1/T2
Gay-Lussac’s law
pressure is directly proportional to temperature in kelvin at constant V and n
P1/P2 = V1/V2
combined gas law
P1V1/T1 = P2V2/T2 at constant n/m and T in kelvin
Avagadro’s law/hypothesis
number of moles of gas is directly proportional to volume at constant T and P
n1/n2 = V1/V2
Density of a gas
Dgas = mm/mV at specific T and P
Density
D = m/v
use ideal gas law to find density
D = m/V
V = m/D
substitute V = m/D into PV = nRT
Pm/d = nRT
Pm = nRTD
D = Pm/nRT
since m/n is mm, D = mmP/RT
since P/RT is mV, this is the same as D = mm/mV
use ideal gas law to find molar volume
PV = nRT
V = nRT/P
V/n = RT/P
since V/n is molar volume, mV = RT / P
use ideal gas law to find mm
substitute n = m/mm into PV = nRT
PV = mRT/mm
mmPV = mRT
mm = mRT/PV
standard conditions
PSTP = 101.3 kPa
TSTP = 0.00000 degrees C or 273.15 K
mVSTP = 22.4 L/mol
Standard Ambient Temperature and Pressure
PSATP = 100.0 kPa
TSATP = 25.00 degrees C = 298.15 kPa
mVSATP = 24.8 L/mol
universal gas constant
R = 8.314 kPaL/Kmol