ATOMS
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
22 Terms
Atom
The atom is the smallest part of an element. They make up everything.
Charge of Proton
Positive
Mass of Proton
1 a.m.u.
Location of Proton
Nucleus
Change of Neutron
Neutral
Mass of Neutron
1 a.m.u.
Location of Neutron
Nucleus
Charge of Electron
Negative
Mass of Electron
1/1840 a.m.u. (negligible)
Location of Electron
Shells/Orbits
A.M.U.
Atomic Mass Unit
Electronic Configuration
How electrons are arranged
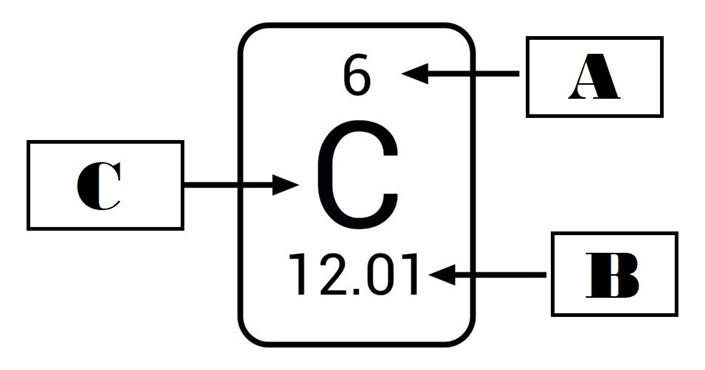
Element in Periodic Table
A - Atomic Number, B - Mass Number, C - Chemical Symbol
The smaller number
Is always the atomic number
The number of protons is the same as the
Number of electrons
Maximum in first shell
2
Maximum in second shell
8
Maximum in third shell
18
Maximum in fourth shell
32
Maximum in fifth shell
50
Atomic Number
Number of protons in an atom
Atomic Mass Number
Number of protons + neutrons in atom