CHM2211 IR Spectroscopy
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
70 Terms
What is IR Spectroscopy used to identify?
The functional groups present in a compound.
How does IR spectroscopy work?
Infrared light is shot at a sample of a compound. The compound is able to absorb the energy from the IR light (IR photon) which causes the bonds to vibrate in a stretching manner. The frequency that the bonds vibrate (stretch) at is what is measured.
What does IR Spectroscopy measure?
IR spectroscopy measures the frequency at which a bond stretches when it absorbs the energy from infrared light.
What is the unit of IR spectroscopy?
Wavenumbers, denoted by symbol ν̃, measured in units of cm-1 (inverse centimeters).
How are different functional groups distinguished by IR spectroscopy?
Each different bond absorbs a characteristic IR frequency, and the bond will vibrate when it is exposure to IR light (IR photons) at that frequency.
No two molecules are have the same IR spectrum, unless they are enantiomers. Each molecule has a unique IR spectrum.
What is the fingerprint region in an IR spectrum?
Between 400 and 1500 cm-1. This region is useful for diagnosing functional groups on a compound.
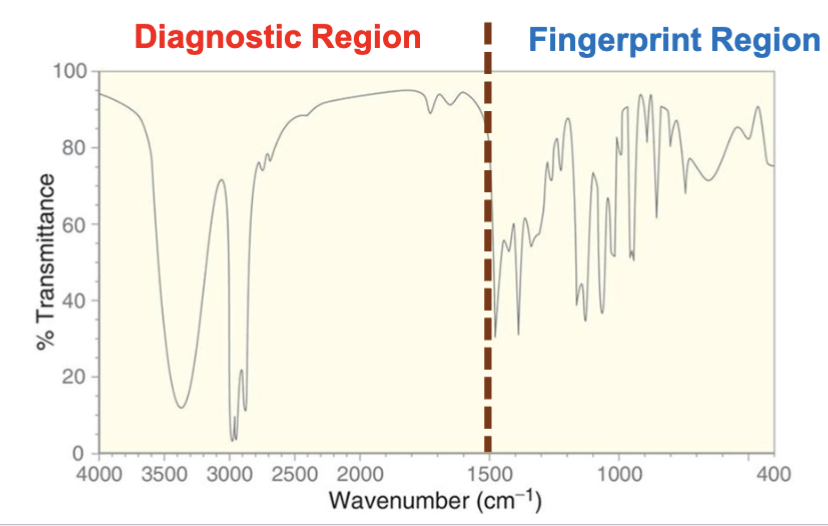
What is the diagnostic region in an IR spectrum?
Between ~1500 and 4000 cm-1. This is the region we use to find out which functional groups a compound has.
What three things characterize an IR signal?
Intensity (strength), shape of the signal, and wavenumber the signal is at (approximately).
What does signal intensity of an IR signal depend on?
The strength of the bonds dipole moment.
Bonds with larger dipole moments have a more intense (stronger) signal.
Bonds with smaller dipole moments have a smaller weaker signal.
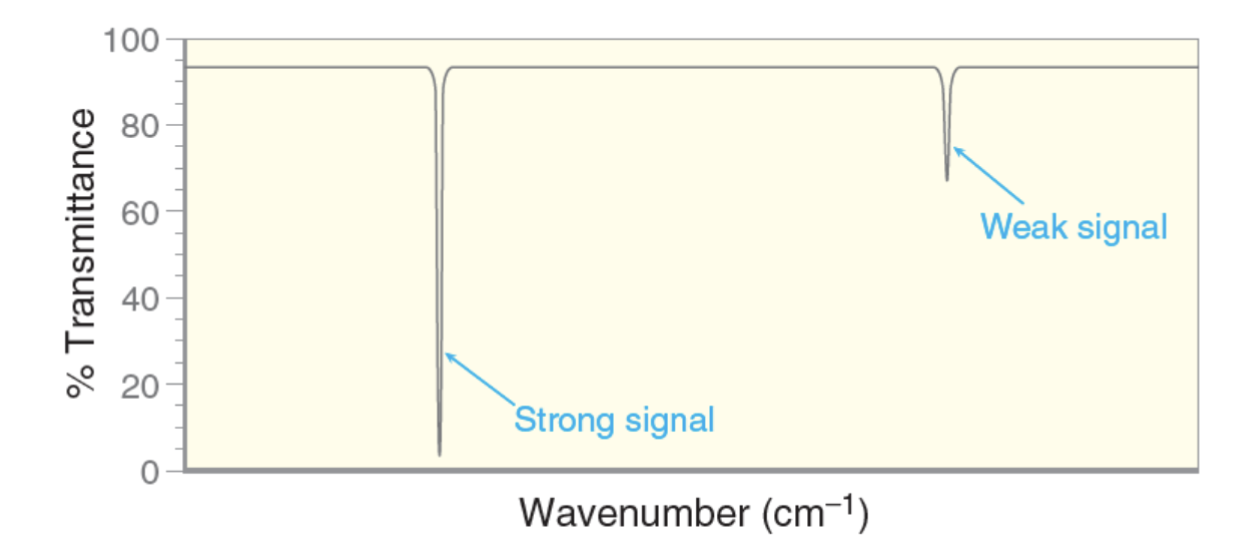
A bond with a smaller dipole moment will have a ____ signal?
A weaker (smaller, less intense) signal.
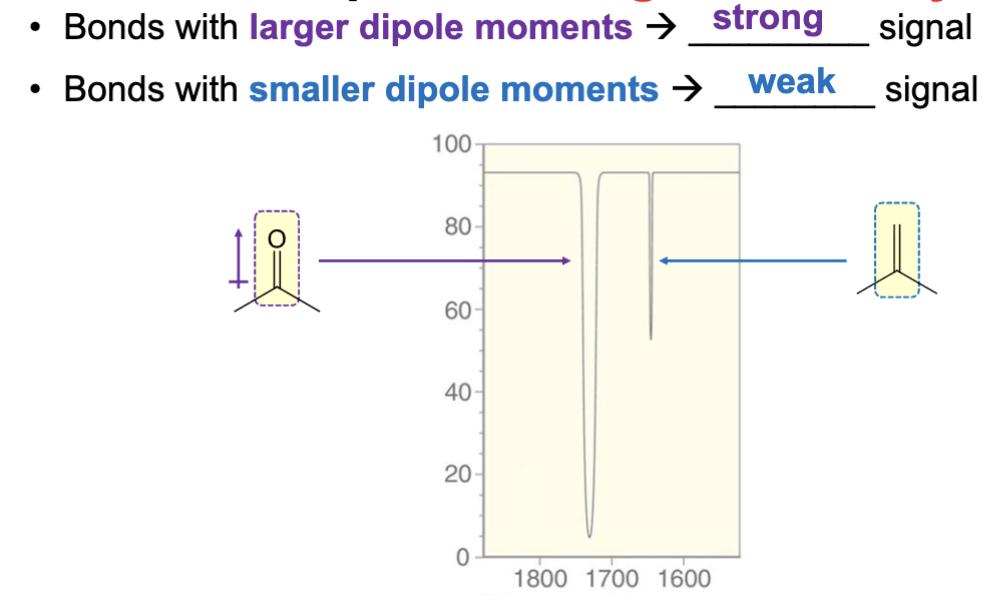
A bond with a larger dipole moment will have a ____ signal?
A stronger (larger, more intense) signal.
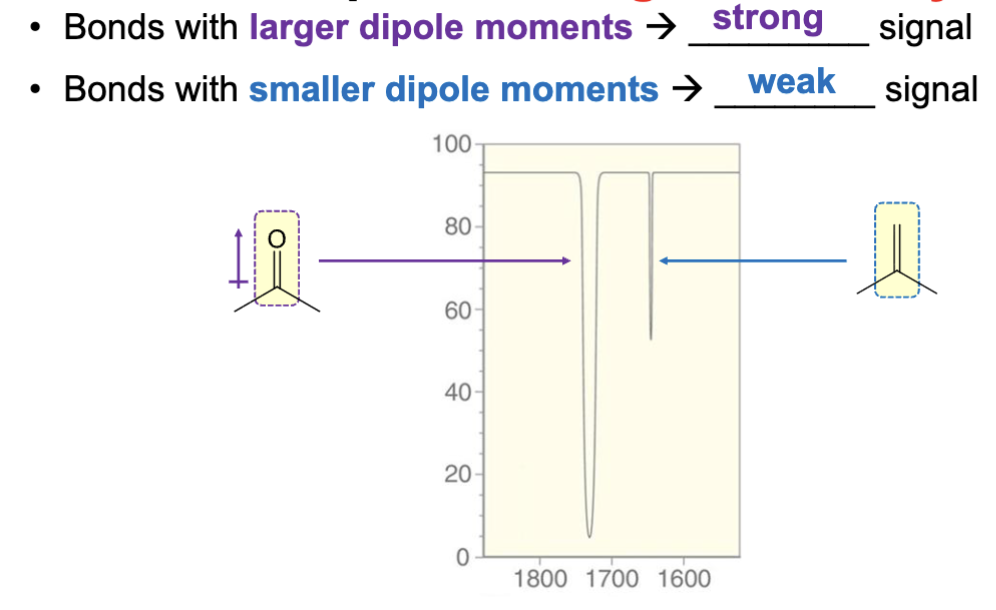
What property must a bond in a compound have in order to produce an IR signal?
A dipole moment.
An IR-inactive bond is unable to produce an IR signal because.
It has no dipole moment, and thus no dipole moment change can occur, rendering it unable to produce an IR signal.
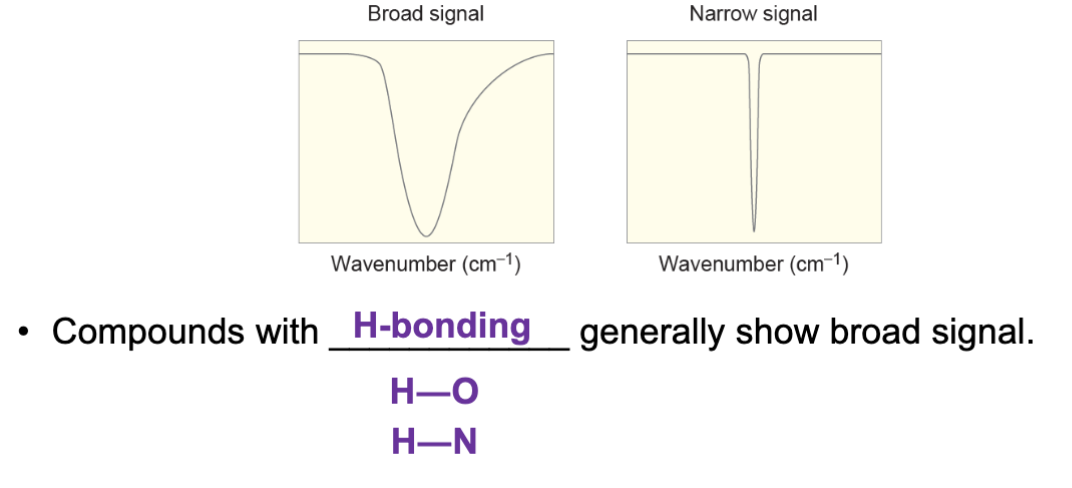
Compounds with ____ typically produce broad signals?
Hydrogen bonding.
Compounds with O-H or O-N bonding typically produce broad signals.
Compounds without hydrogen bonding typically produce narrow signals.
The wavenumber of the stretching vibration of a bond when it is exposed to IR light depends upon which two properties?
The bond strength.
The masses of the atoms sharing the bond.
A stronger bond produces a ____ IR frequency (wavenumber) signal in an IR spectrum?
Higher
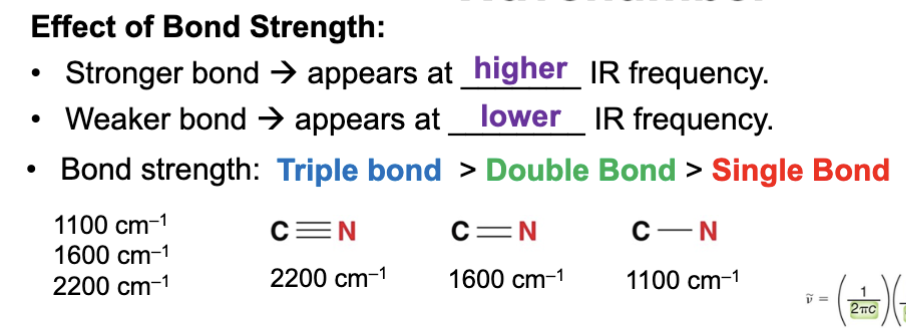
A weaker bond produces a ____ IR frequency (wavenumber) signal in an IR spectrum?
Lower
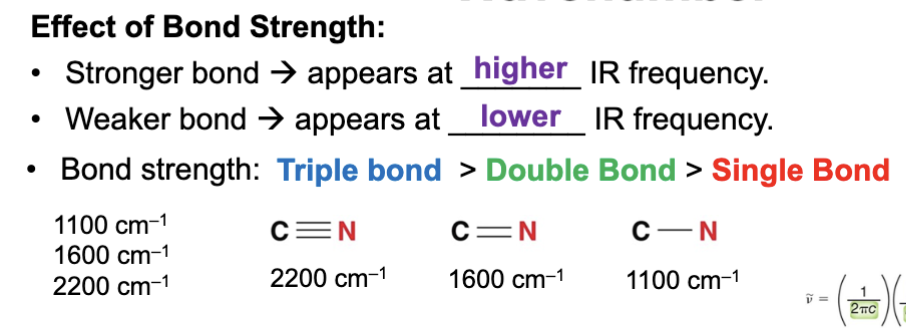
Is bond strength directly proportional to the IR wavenumber of a bond or inversely proportional to IR wavenumber of a bond?
Directly proportional
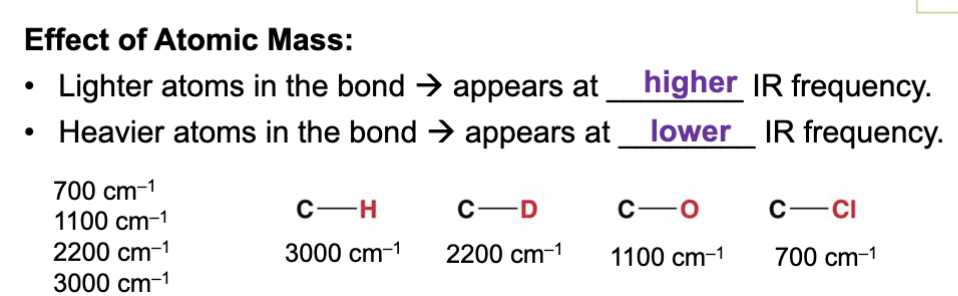
Lighter atoms in a bond produce a ____ IR frequency (wavenumber) ?
Higher
This is because lighter atoms can vibrate faster with less energy because they have less mass.
Less mass = less energy required to move it faster.
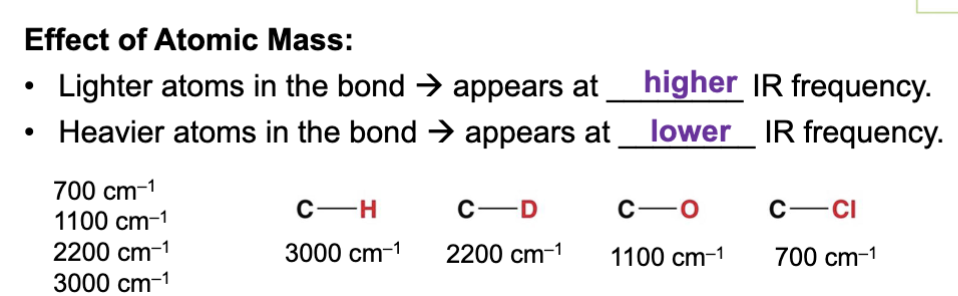
Heavier atoms in a bond produce a ____ IR frequency (wavenumber)?
Lower
Stronger bonds absorb at _____ IR frequencies?
Higher, the stronger the bond the higher the IR frequency it will absorb, which is why stronger bonds have higher wavenumbers.

Conjugation and Resonance structures do what to the wavenumber (frequency) signal of a particular bond?
Lower the wavenumber (frequency) of the IR signal.
Why are single bonds, except to hydrogen, not useful in diagnosing functional groups?
Because they fall below the 1500 cm-1 mark in the fingerprint region, which is not useful in an IR spectrum.
What is the general double bond range in an IR spectrum?
Around 1600 cm-1 to 1800 cm-1.
What is the general triple bond range in an IR spectrum?
Around 2000 cm-1 to 2260 cm-1.
What is the IR range of an isolated (meaning un-conjugated) carbon to carbon double bond (alkene DB)?
Around 1600 to 1690 cm-1.
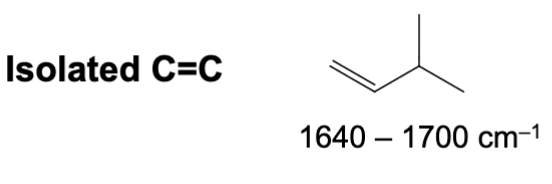
What is the IR range for a conjugated (i.e. resonance capable) carbon to carbon double bond (alkene DB)?
Around 1600 to 1640 cm-1. It lowers in frequency due to conjugation.
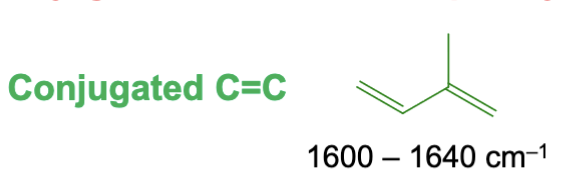
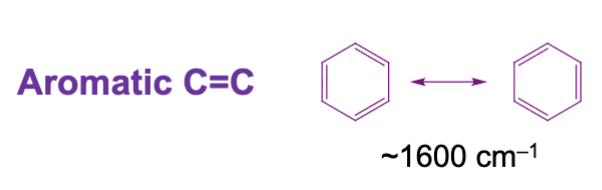
What is the approximate IR range for an aromatic compound’s carbon to carbon double bond?
Approximately at or below 1600 cm-1. It lowers in frequency due to conjugation.
Which type of alkyne bond (triple bond) would show a signal on an IR spectrum?
A terminal alkyne. This is because a terminal alkyne has a dipole moment, whereas an internal alkyne either has no dipole moment or has a dipole moment thats to small and only produces a very weak C to C triple bond signal.
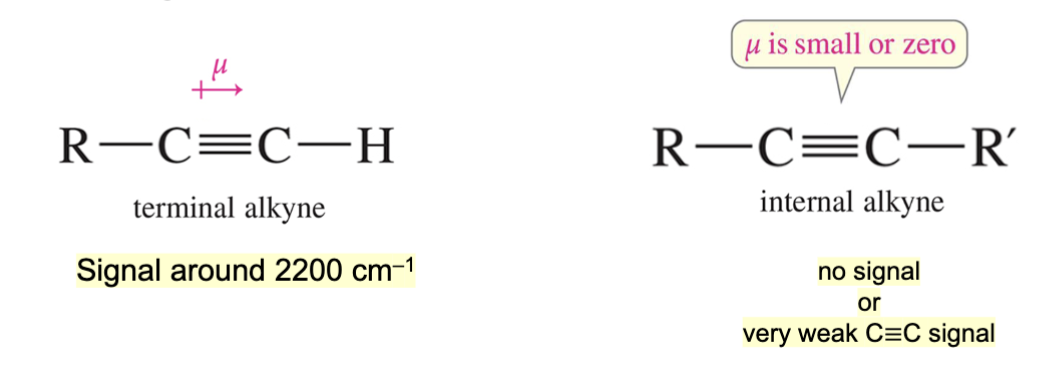
What is the approximate IR range for a terminal alkyne triple bond?
Below 2200 cm-1.
Does a higher s-character in the hybrid orbitals of a carbon atom increase or decrease the strength of a C-H bond?
Increases. A higher s-character increases the strength of a carbon to hydrogen bond.
sp3 < sp2 < sp
Will a stronger C-H bond result in a higher IR frequency (wavenumber) for that bond or a lower frequency?
A higher frequency. Stronger bond = higher frequency (wavenumber).
Alkyne C-H < Alkene C-H < Alkane C-H
Analogous to sp3 < sp2 < sp
What is the approximate range IR range for an sp3 hybridized carbon to hydrogen single bond (alkane C-H bond)?
A somewhat broad signal ranging from 2850 to 3000 cm-1.
Broad with multiple peaks.
Always below 3000 cm-1.
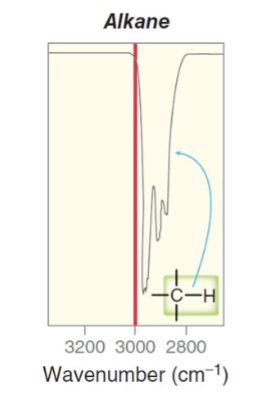
What is the approximate IR range for an sp2 hybridized carbon to hydrogen single bond (alkene C-H bond)?
A medium strength pinpoint signal from ranging from 3000 to 3100 cm-1.
Single pinpoint peak
Just above 3000 cm-1.
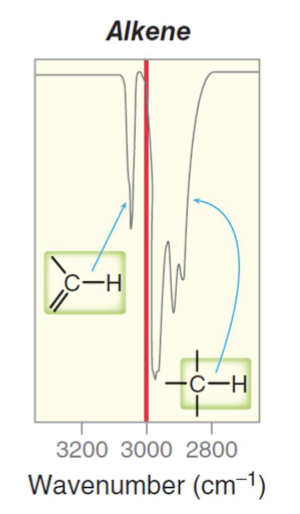
What is the approximate IR range for an sp hybridized carbon to hydrogen bond (alkyne C-H bond)?
A usually strong pinpoint signal around 3300 cm-1.
Usually strong and pinpoint.
Around or above 3300 cm-1.
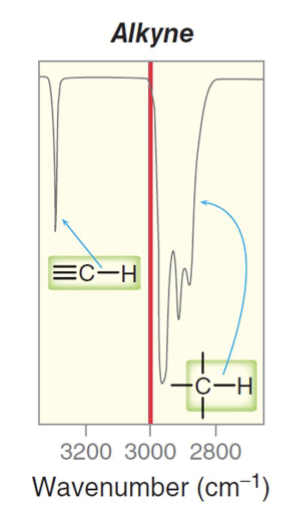
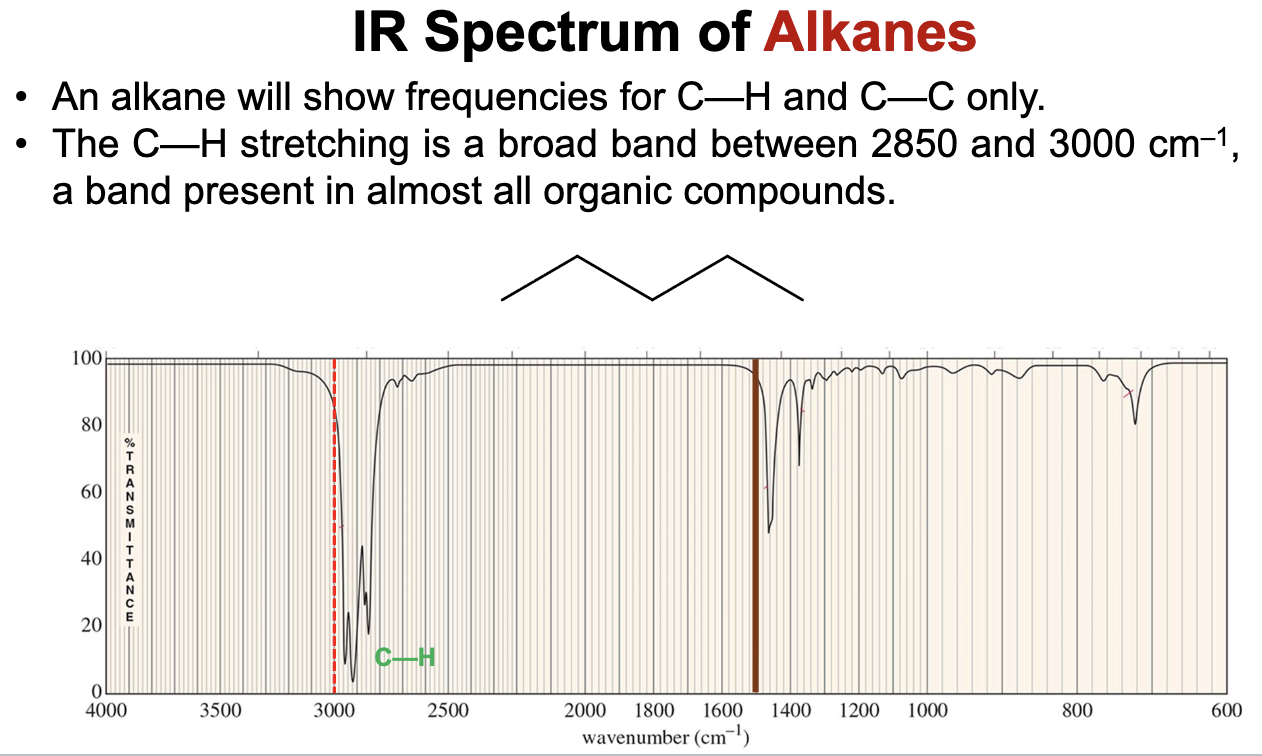
What bonds and frequencies will an alkane compound demonstrate signals for on an IR spectrum?
Will only show IR signals for the C-C and C-H bond stretches.
C-C bond falls in the fingerprint region and is thus not useful for diagnosis.
C-H bond falls between 2850 and 3000 cm-1 and can be used for diagnostic purposes.
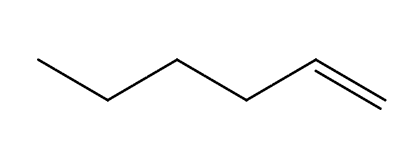
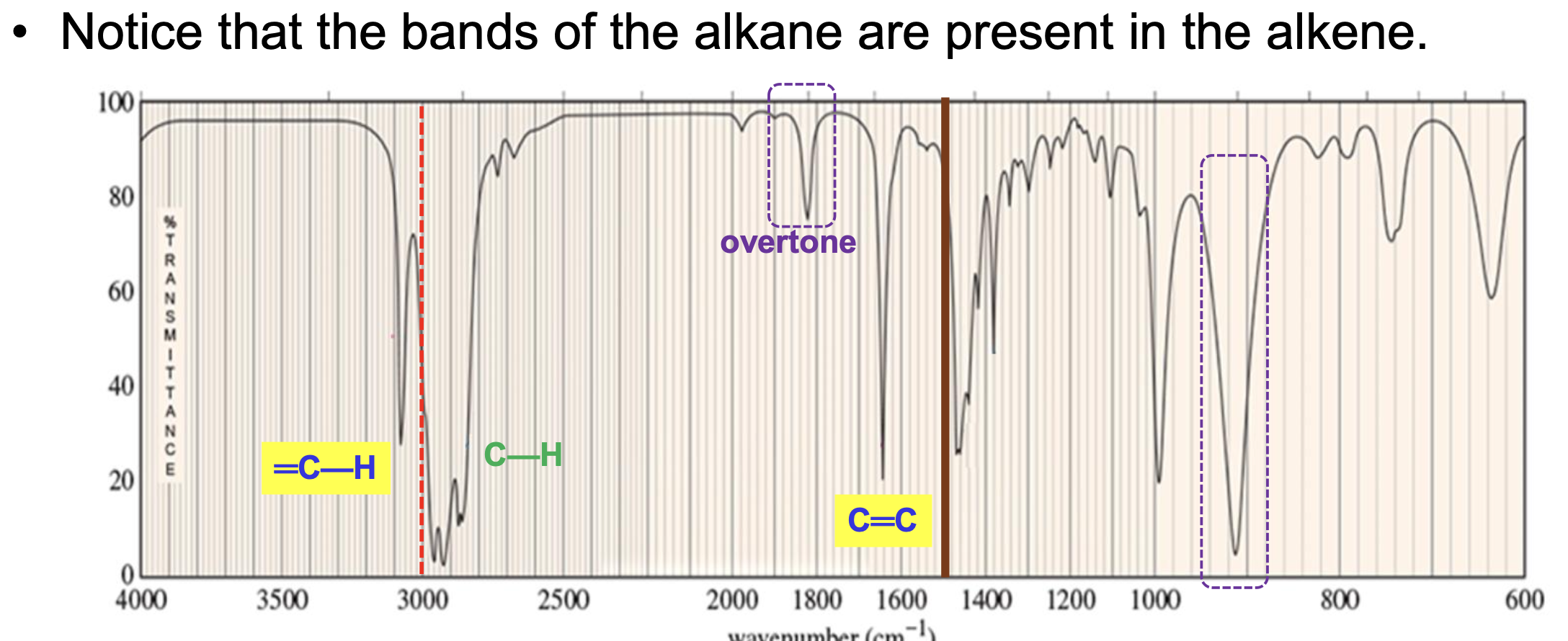
What bonds and frequencies will an alkene compound demonstrate signals for on an IR spectrum?
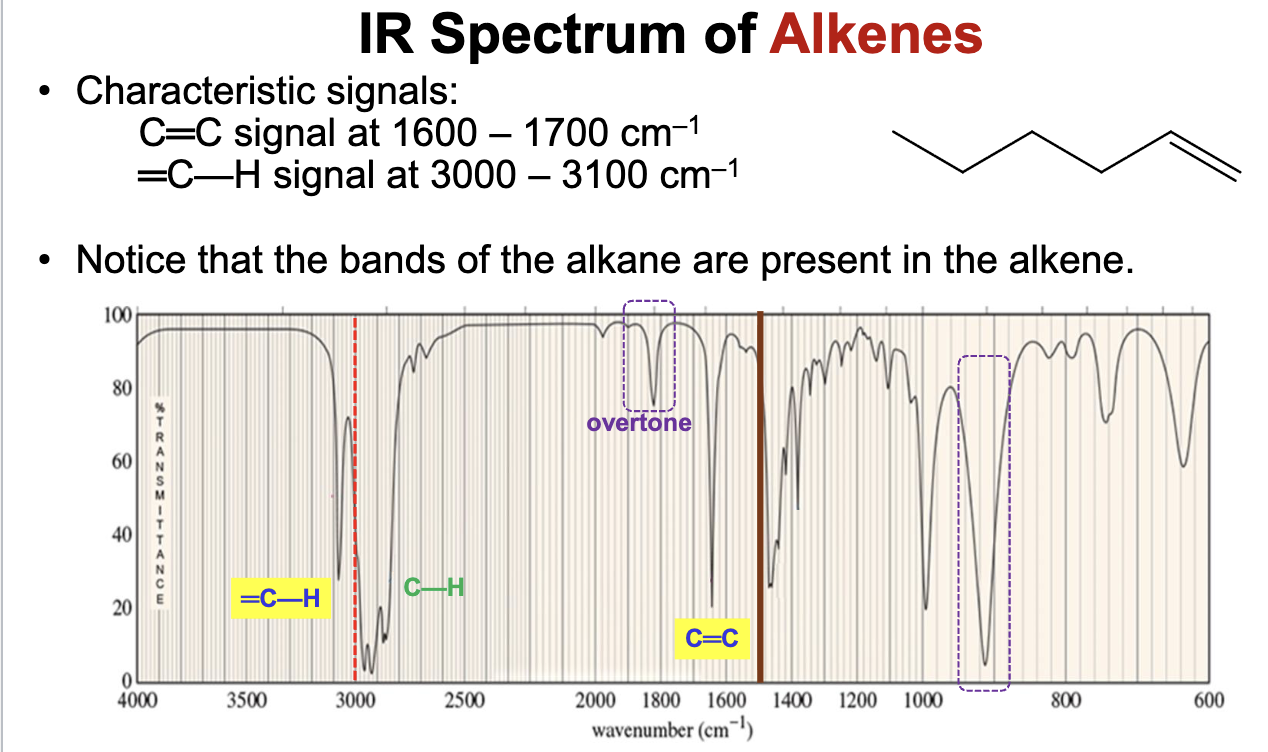
Will show characteristic IR signals for the C=C bond and the =C-H bond stretches.
The C=C bond falls between the 1600 to 1690 range.
The =C-H bond falls above 3000 cm-1 between 3000 to 3100 cm-1.
Will also have the same bands present in alkanes, but the C=C and =C-H bonds are what distinguish and alkene functional group from an alkane.
Also has a distinct overtone past the C=C signal around 1800 to 1900 cm-1.
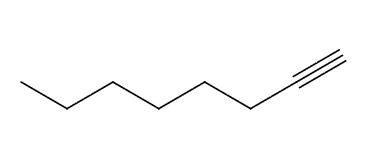
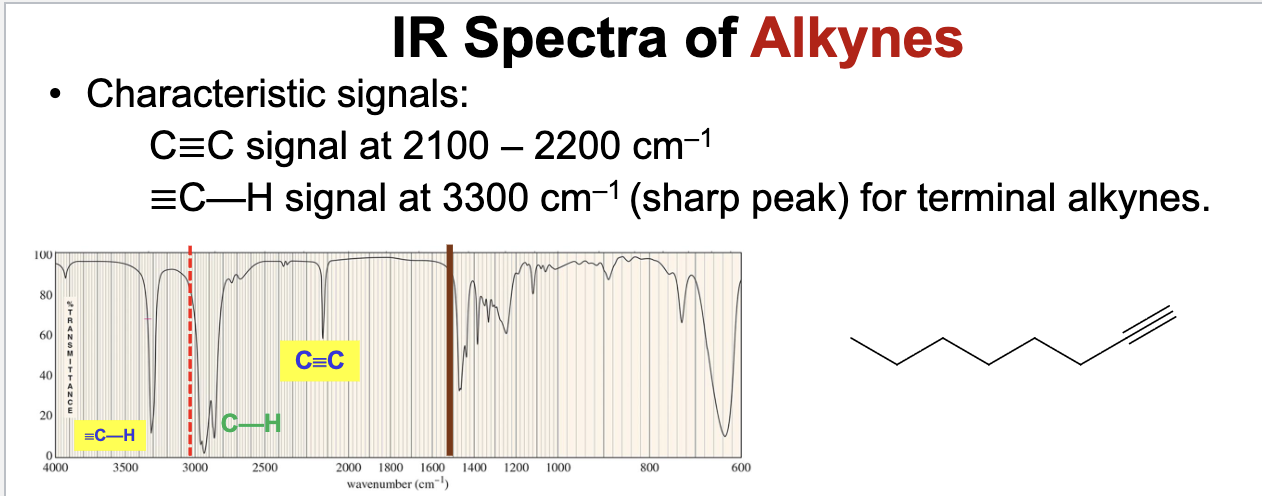
What bonds and frequencies will a terminal alkyne compound demonstrate signals for on an IR spectrum?
Will show characteristic IR signals for the C≡C and ≡C-H bond stretches.
The C≡C bond stretches falls below 2200 cm-1, ranging from 2100 to 2200 cm-1.
The ≡C-H bond stretches falls around ~3300 cm-1 with a sharp strong peak for terminal alkynes.
Remember an internal alkyne wont show either of these signals.
For which compound is this IR spectrum most likely for?
An alkane compound. The IR spectrum shows signals for only C-H bonds in the diagnostic range just below 3000 in the 2850 to 3000 cm-1 range, indicating it can only be an alkane with no other functional groups.
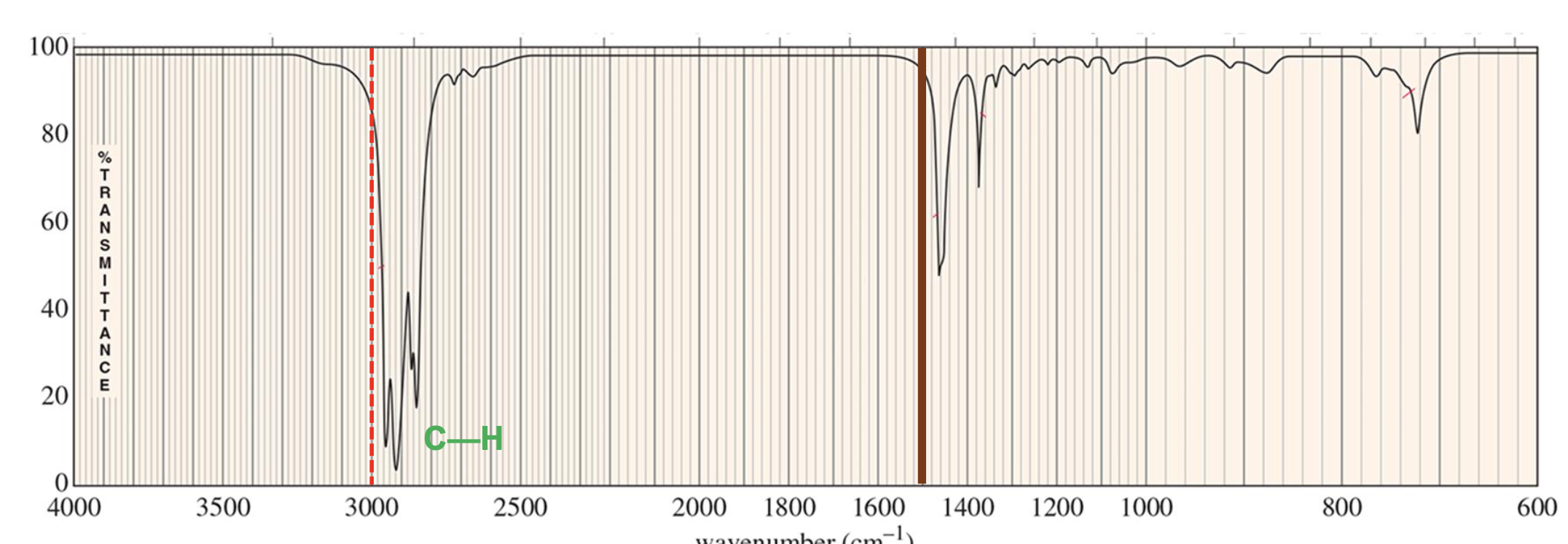
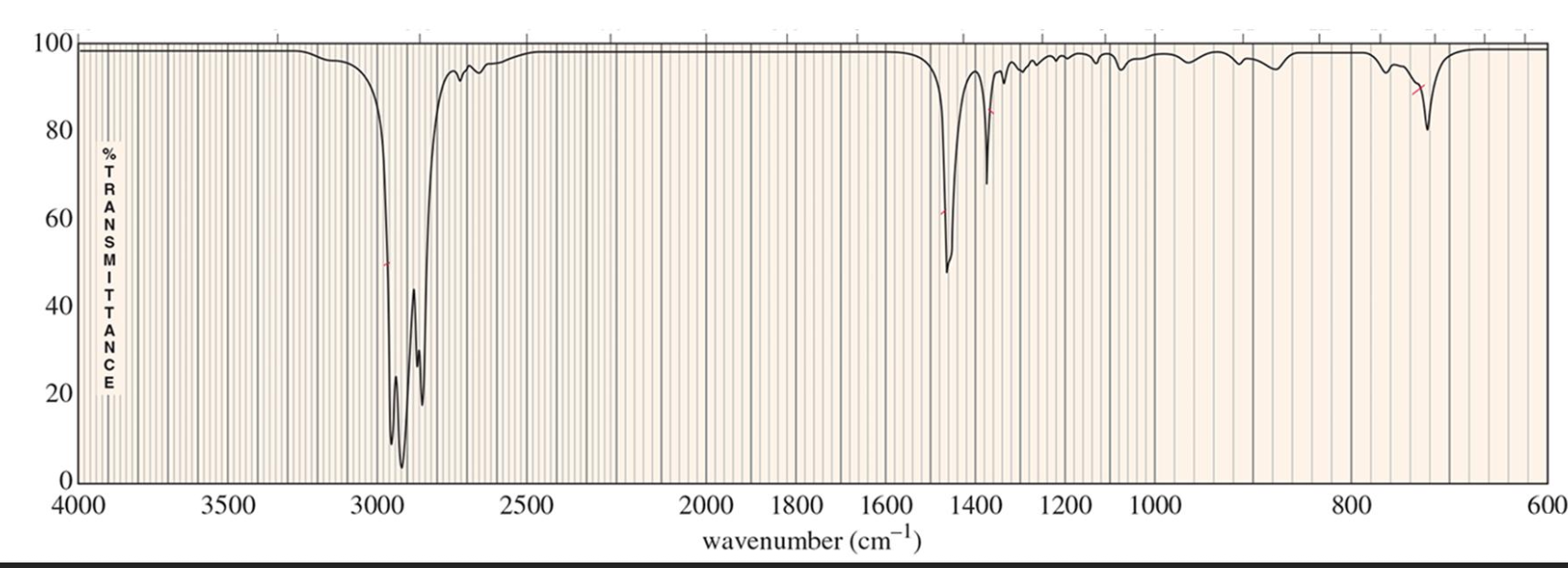
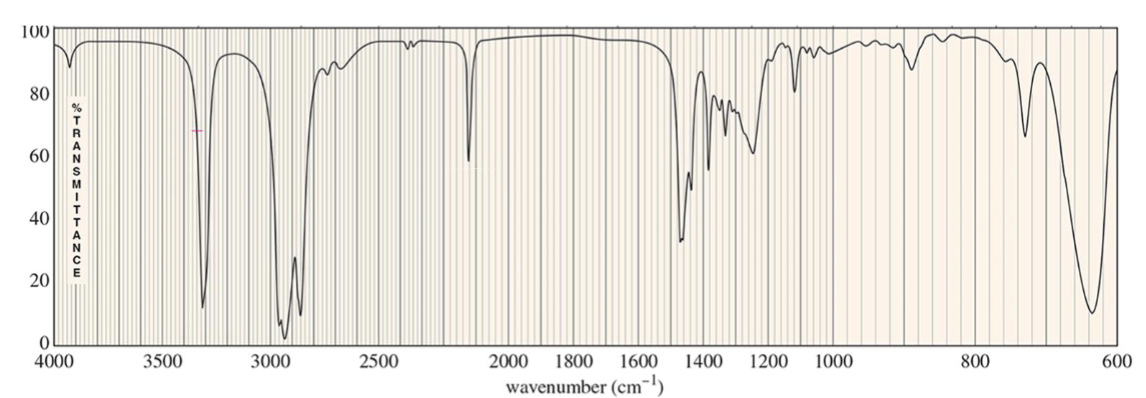
For which compound is this IR spectrum likely for?
An alkyne compound. The IR spectrum shows signals for the C=C double bond stretches around 1600 to 1690 cm-1 (closer to 1690 cm-1), and =C-H bond stretches just above 3000 cm-1.
It also demonstrates the distinct alkene compound overtone around 1800 to 1900 cm-1.
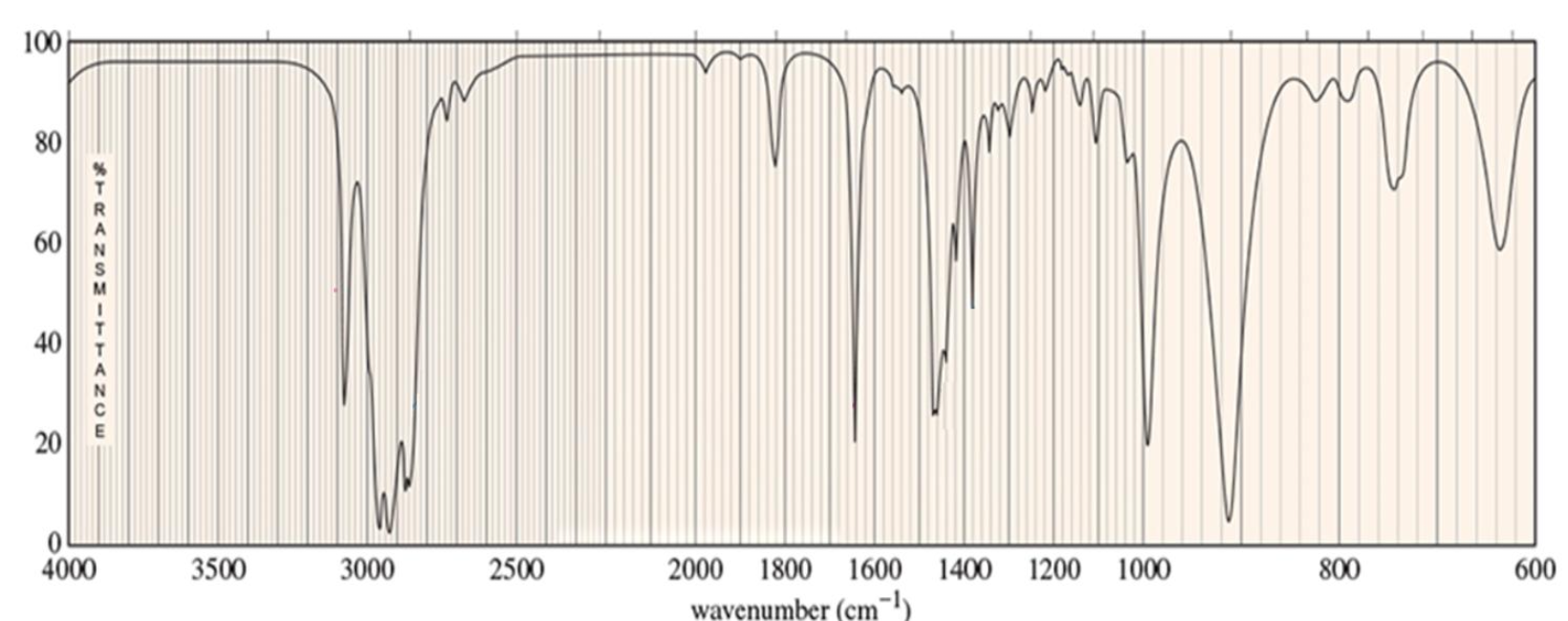
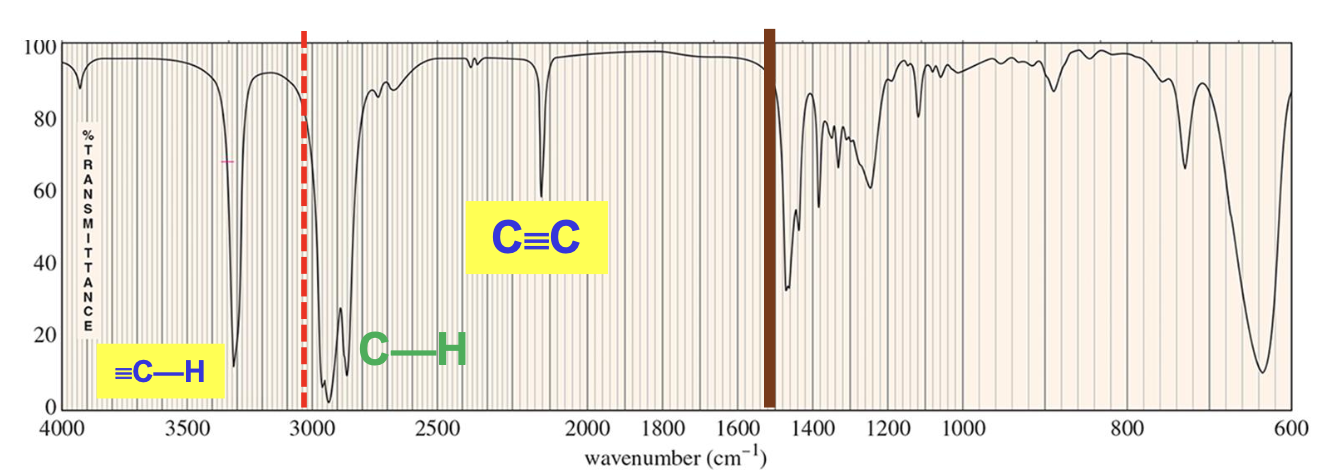
For which compound is this IR spectrum most likely for?
A terminal alkyne compound. The IR spectrum demonstrates signals for the C≡C bond stretches around 2200 cm-1 and ≡C-H bond stretches around 3300 cm-1.
For bonds that permit hydrogen bonding (O-H and N-H), their IR signals are typically broad or pinpoint, in terms of their wavenumber range?
Broad, compounds with hydrogen bonding typically exhibit broad IR siignals.
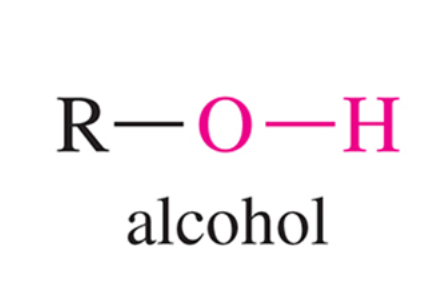
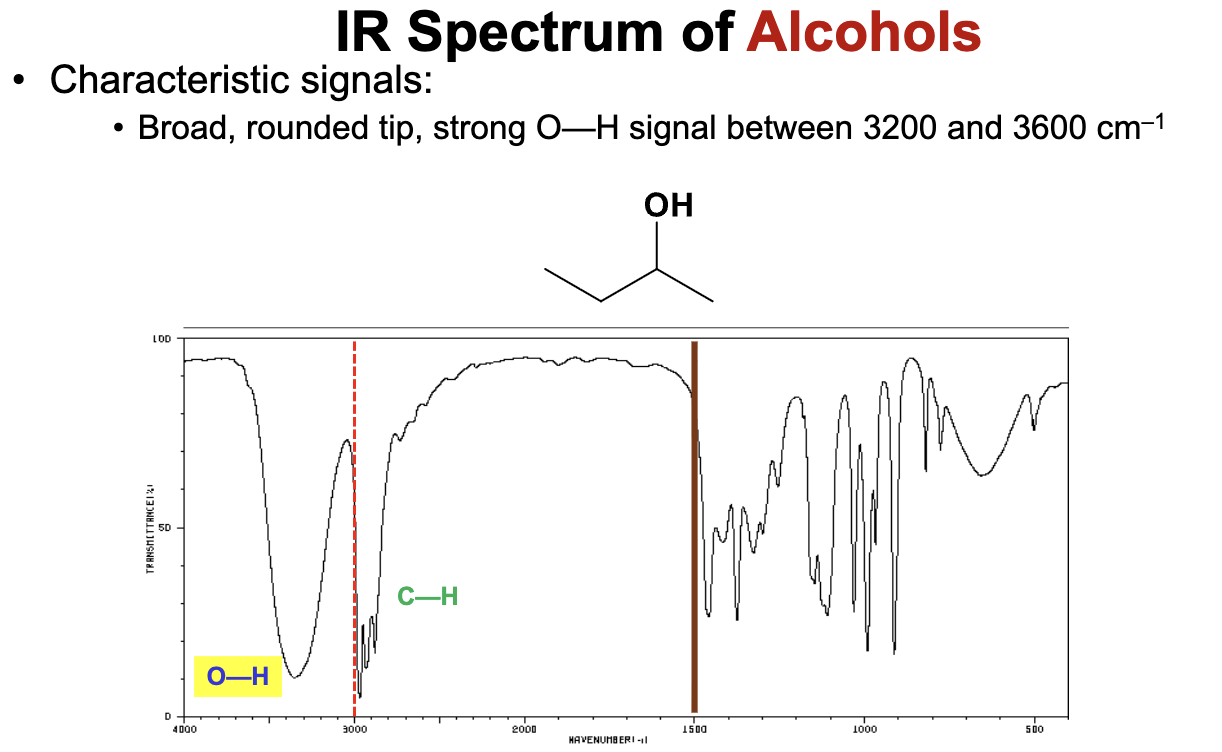
What is the characteristic IR signal for an alcohol group (OH), i.e. the O-H bond stretch?
An alcohol only group will demonstrate a very broad signal ranging from 3200 to 3600 cm-1, with a rounded tip.
What is the characteristic IR signal for amino groups N-H bonds?
Typically demonstrates broad and moderate strength signals between the 3350 to 3500 cm-1, with sharp spikes between these ranges.
Tertiary amines (R3N) groups do not demonstrate any N-H signals because they do not have any hydrogen atoms bound to the nitrogen atom.
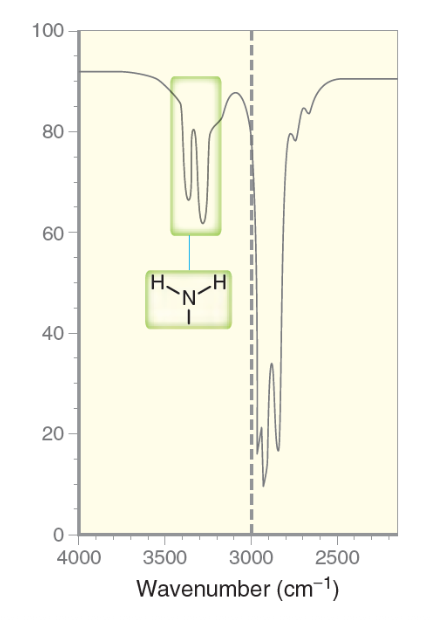
What is the characteristic IR signal for a primary amino group (RNH2)?
Will typically demonstrate a broad but moderate strength signal between the 3350 to 3500 range, with TWO distinct sharp spikes.
Primary amines have TWO hydrogen atoms bound to the nitrogen atom, thus show TWO sharp spikes.
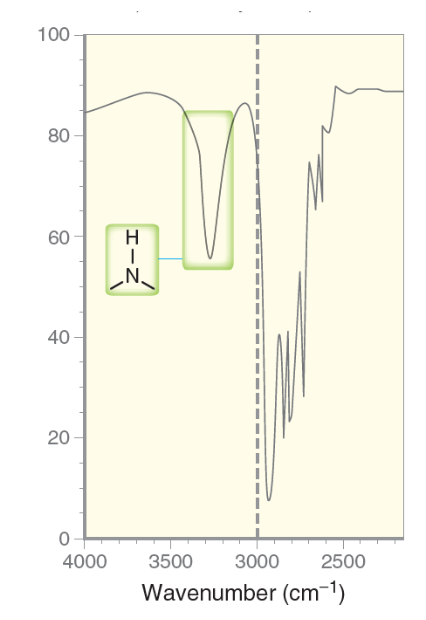
What is the characteristic IR signal for a secondary amino group (R2NH)?
Will typically demonstrate a moderate strength broad signal between the 3350 to 3500 range, with ONE broad sharp spike.
Only shows one sharp spike or peak across the broad signals because only hydrogen atom is bound to the nitrogen atom.
What is the characteristic signal for an imine group (C=N)?
Typically will display a signal around 1650 to 1690 cm-1, typically appearing higher than the C=C bond signals.
Also usually demonstrates a moderate to strong intensity band.
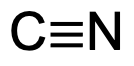
What is the characteristic IR signal of a Nitrile (C≡N) group?
Will typically display an intense strong signal ABOVE 2200 cm-1, around 2200 to 2300 cm-1.
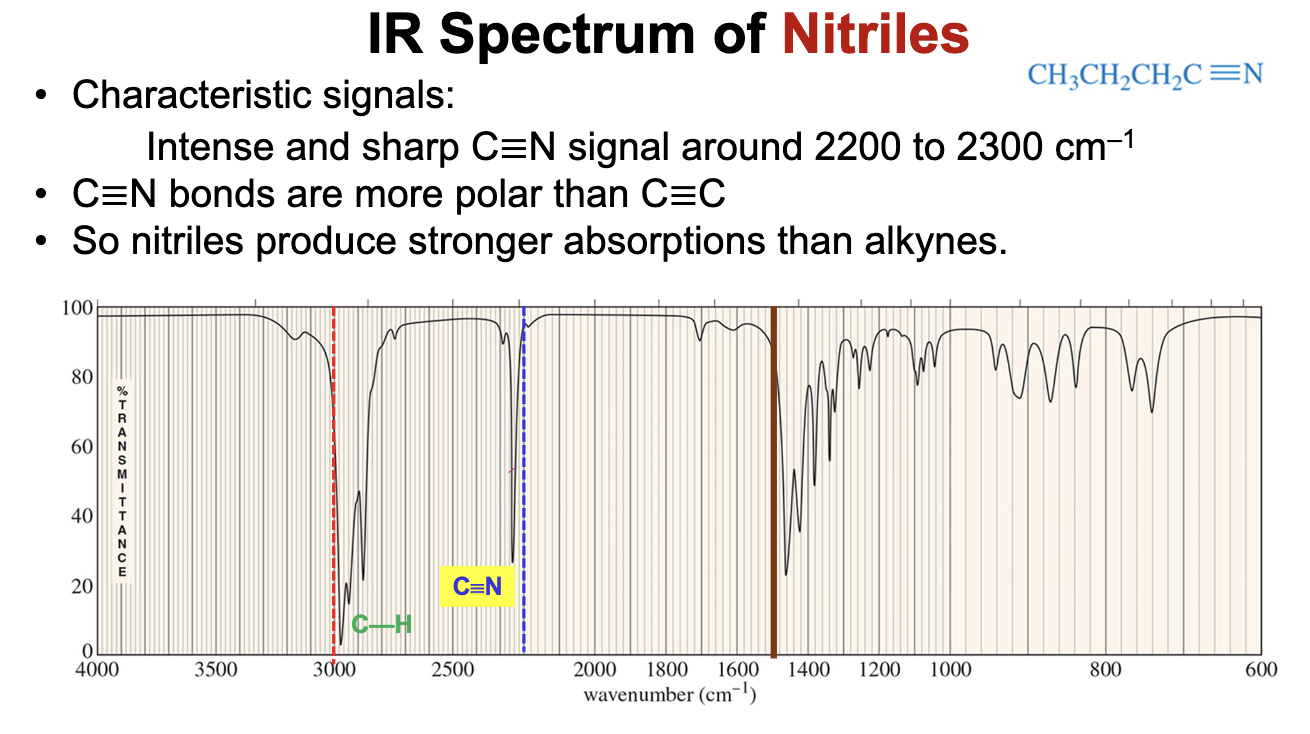
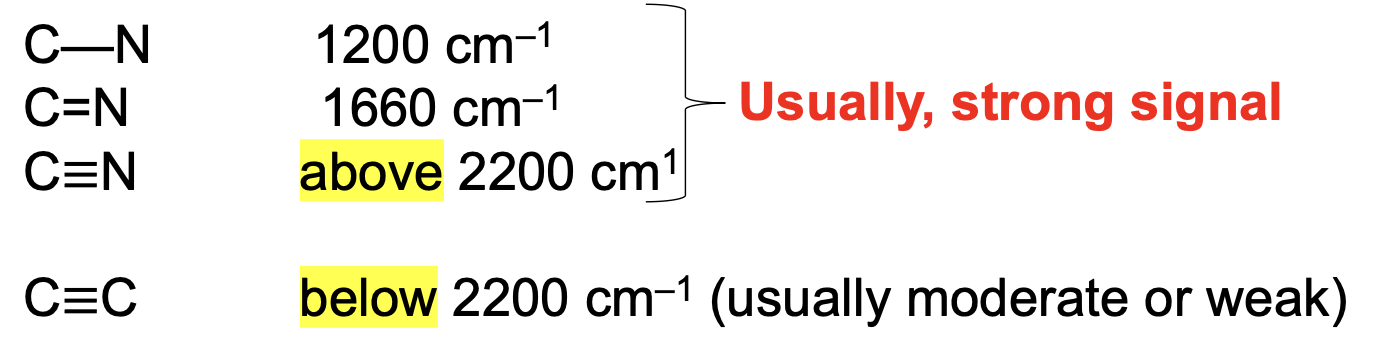
Based upon wavenumber alone, how are you able to distinguish C≡C bonds from C≡N bonds?
C≡C bonds will be below 2200 cm-1, while C≡N bonds will be above 2200 cm-1.
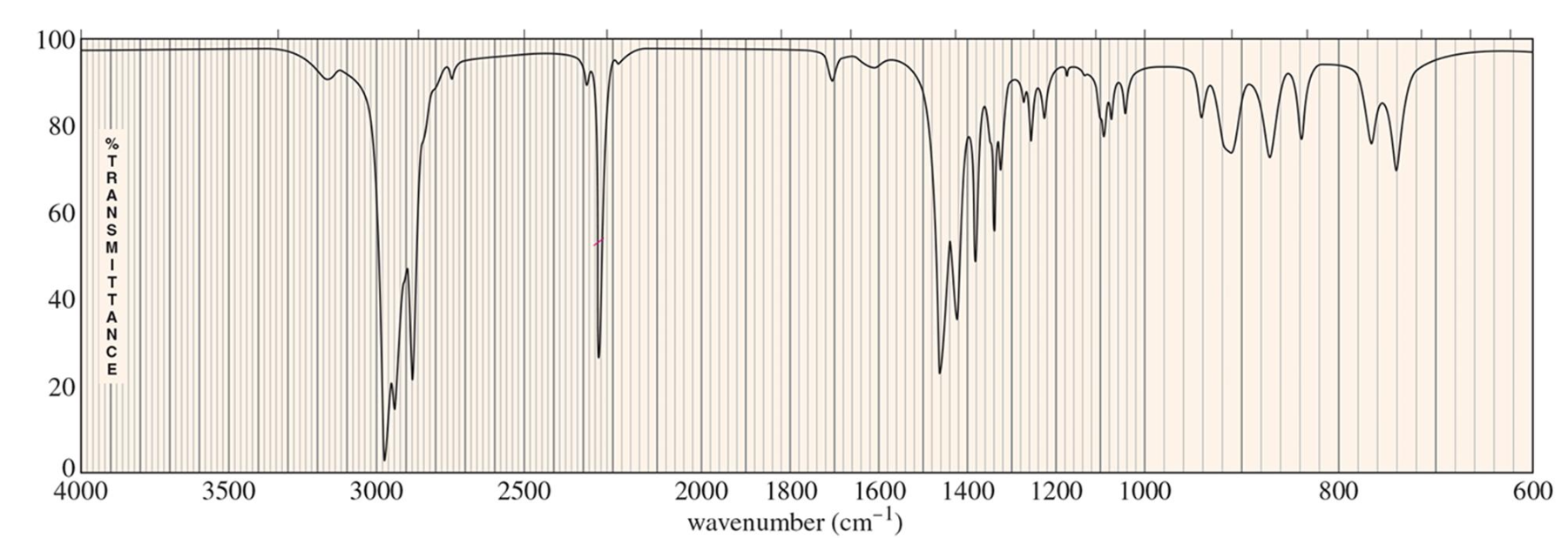
For which compound is this IR spectrum likely for?
A nitrile group (C≡N) compound due to the intense and sharp signal that is above the 2200 cm-1 range, with the signal being around 2200 to 2300 cm-1, and the C-H bond stretches ranging from the 2850 to 3000 cm-1 range (just below 3000 cm-1).
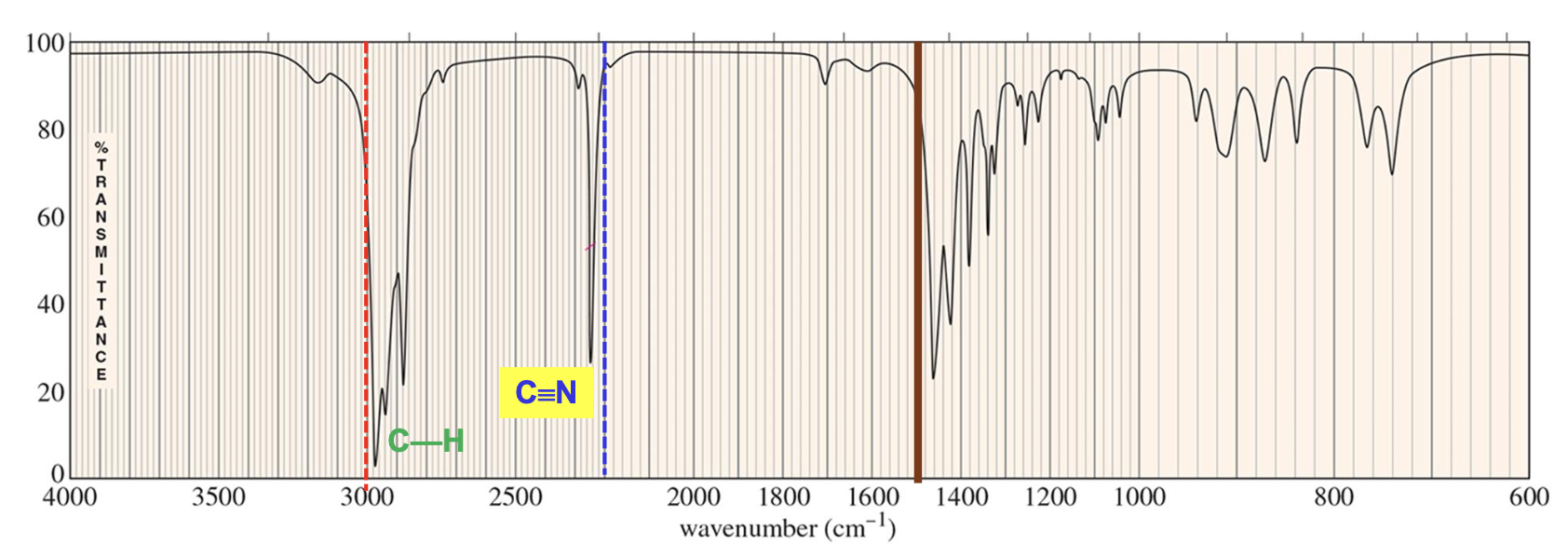
What is the characteristic IR signal for Aromatic Compounds (benzene ring containing compounds)?
Aromatic compounds have large conjugated systems which lowers the C=C bond stretcher frequencies (wave numbers) to around 1600 cm-1, while also demonstrating =C-H bond stretch signals ABOVE 3000 cm-1 from the 3000 to 3100 cm-1 range.
Will also demonstrates characteristic aromatic compound overtones around 1700 to 2000 cm-1.
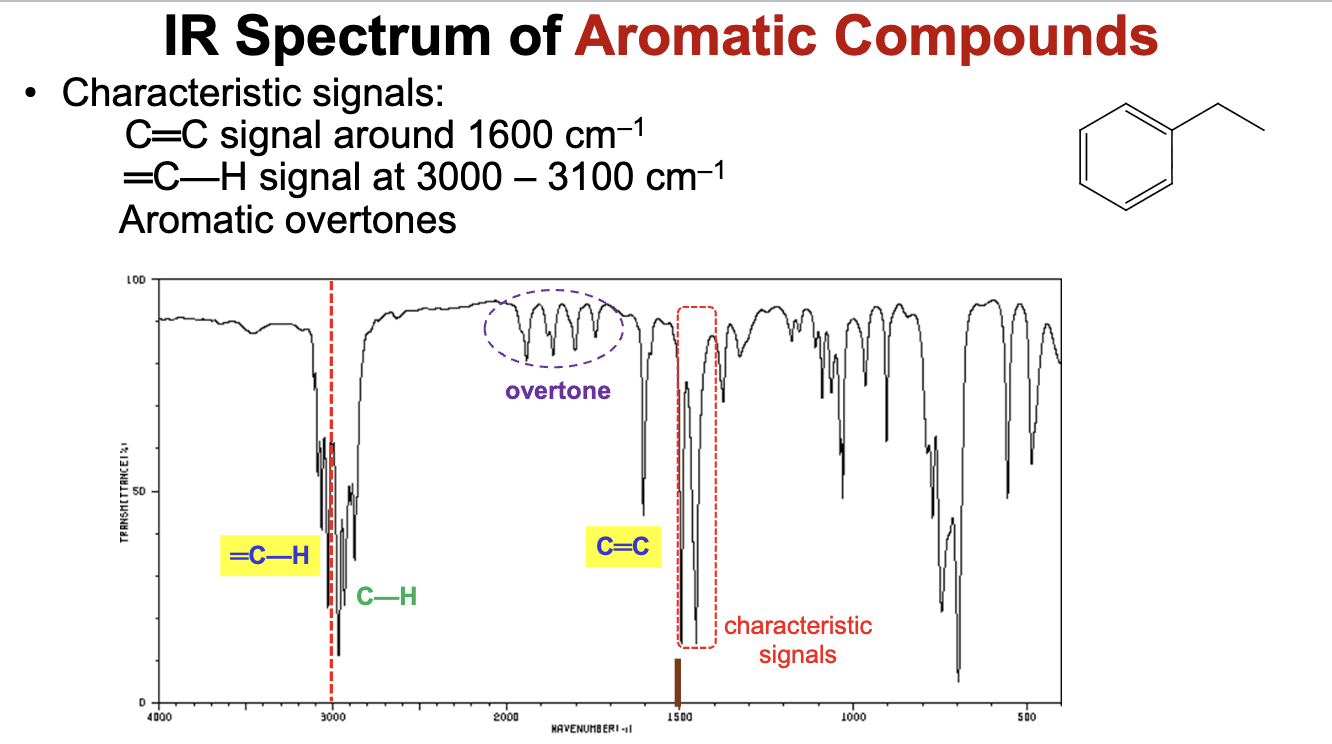
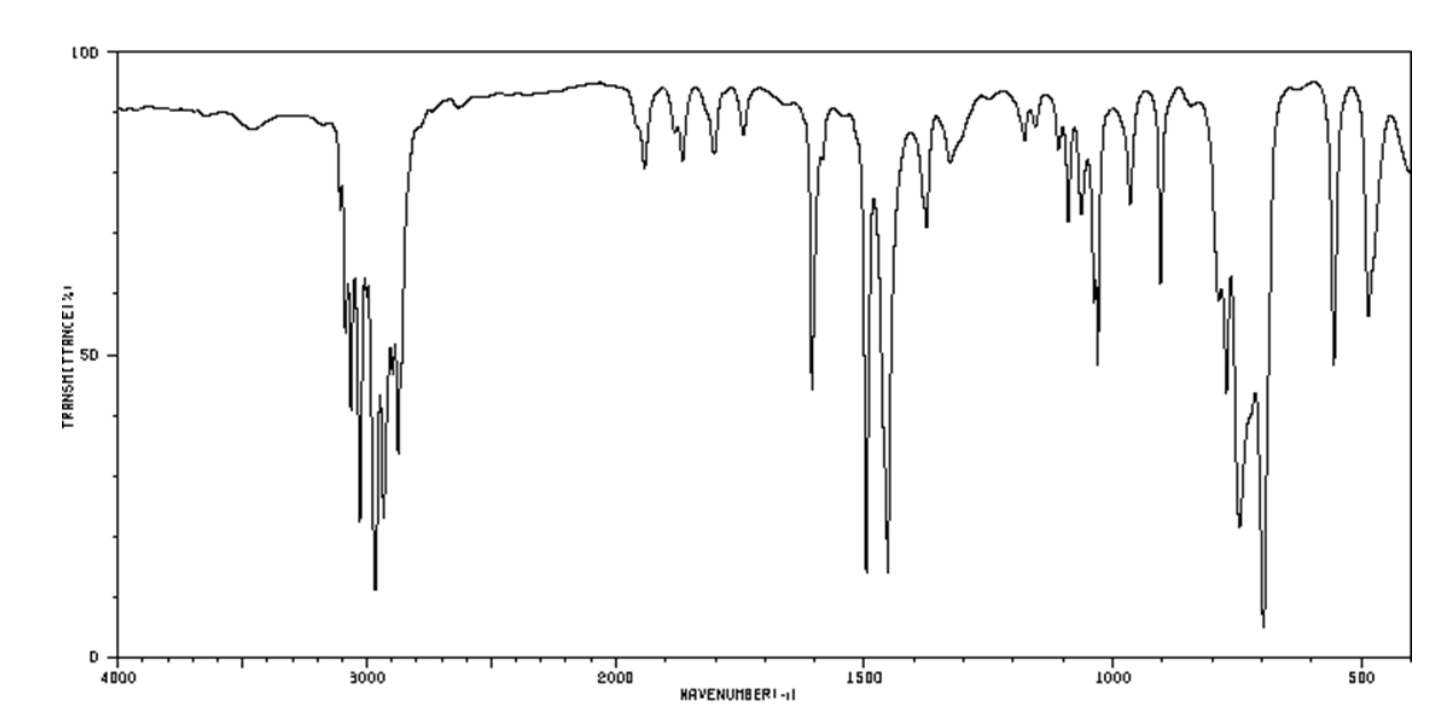
For which compound is this IR spectrum most likely for?
An aromatic compound. The IR spectrum demonstrates a lowered C=C bond signal around the 1600 cm-1 range, =C-H bond signals around the 3000 to 3100 cm-1 range, and characteristic aromatic overtones ranging from 1700 to 2000 cm-1 range, all characteristic of aromatic compounds.
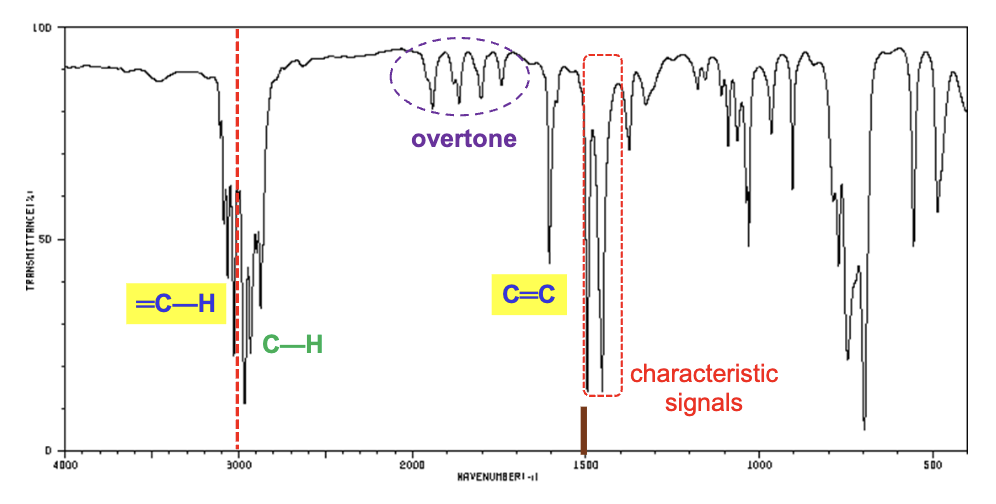
For compounds containing a carbonyl group (C=O), what tends to be the strongest characteristic signal?
The carbonyl group itself is usually the strongest.
What is the characteristic IR signal for a carbonyl groups (in general)?
They tend to show signals around 1700 cm-1 (typically above), and also demonstrate weak overtone bands around 3400 cm-1.
Does conjugation also lower the IR frequency of carbonyl signals?
Yes, conjugation always lowers the frequency as the bonds will have more single bond-character due to resonance structures.
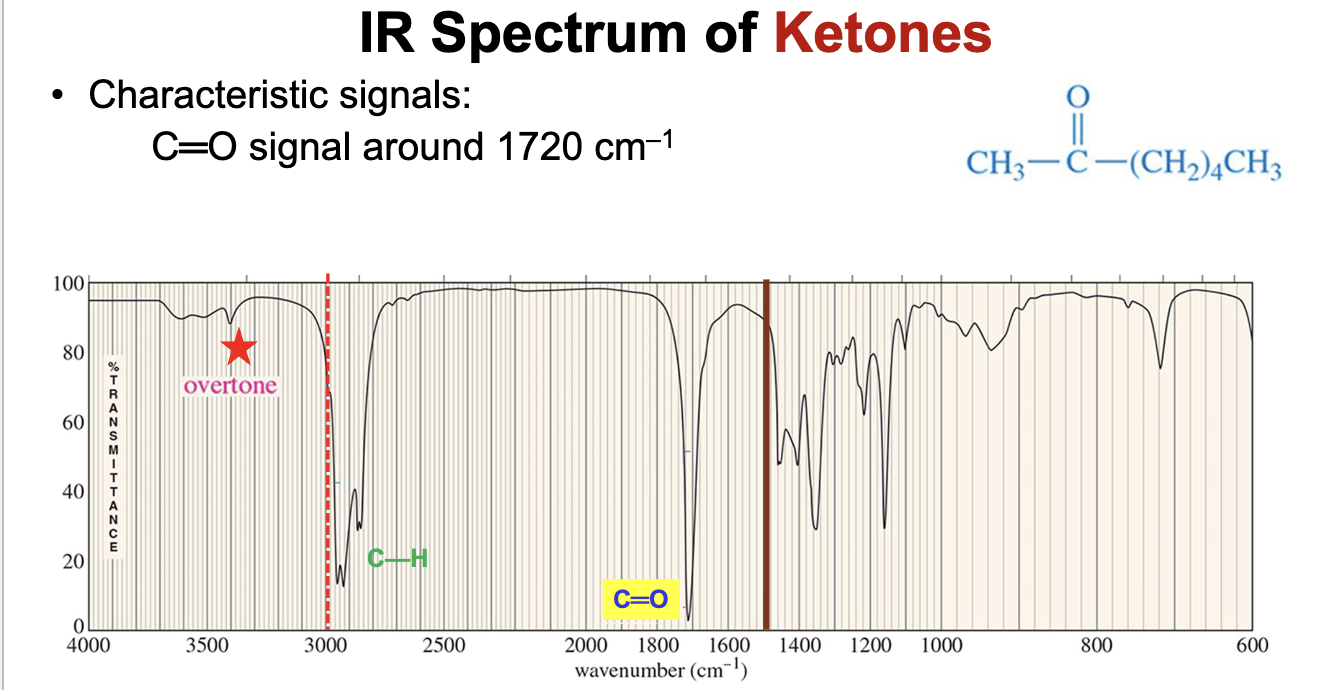
What is the characteristic IR signals (i.e. spectra) for ketones?
The carbonyl group will demonstrate a strong pinpoint signal around 1720 cm-1.
The IR spectra of ketones will also demonstrate signals just below 3000 cm-1 for the C-H bond stretchers.
Will also demonstrate the characteristic carbonyl overtone around 3400 cm-1.
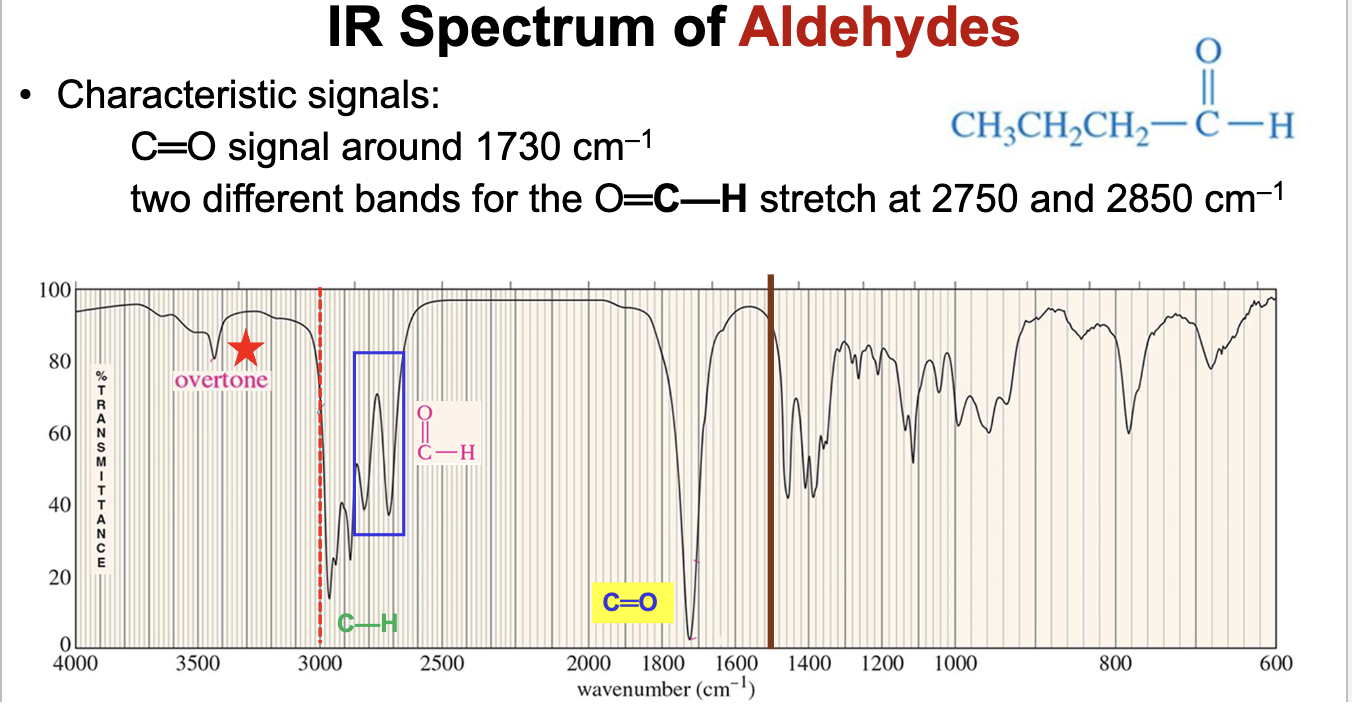
What is the characteristic IR spectra for aldehydes?
The carbonyl group will demonstrate a strong pinpoint signal around 1730 cm-1, and two distinct moderate/weak intensity signals will appear for the O=C-H (carbonyl carbon to hydrogen stretch) at 2750 and 2850 cm-1.
Also has the same carbonyl overtones around 3400 cm-1.
Will also display C-H bond stretch signals below 3000 cm-1.
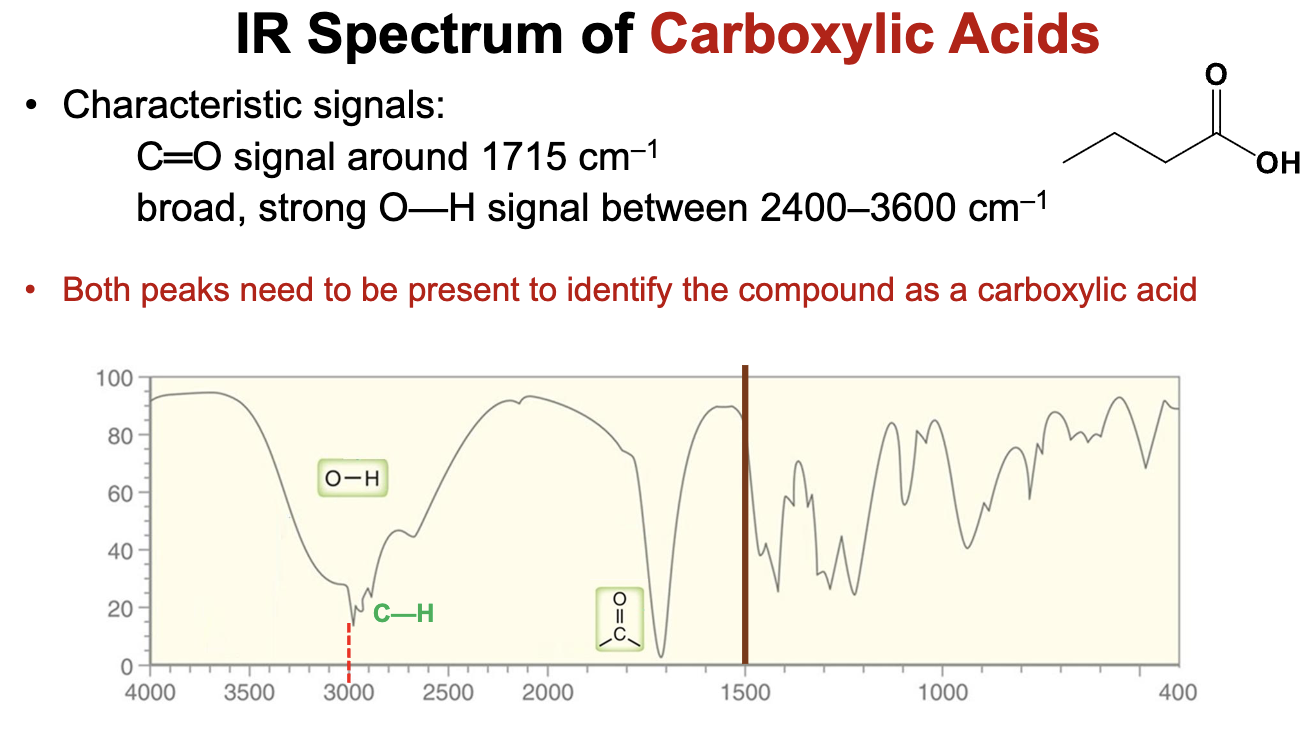
What is the characteristic IR spectra for carboxylic acids?
The carbonyl group will demonstrate a strong pinpoint signal around 1715 cm-1, while the O-H stretch of the alcohol group will show a broad signal with a tip peak ranging from 2400 to 3600 cm-1.
Both peaks need to be present to identify the compound as a carboxylic acid.
Will display C-H bond stretch signals below 3000 cm-1 as well.
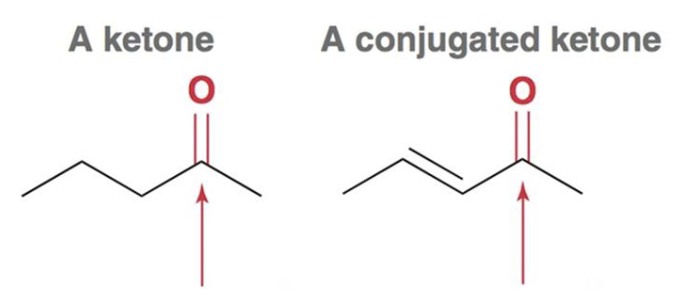
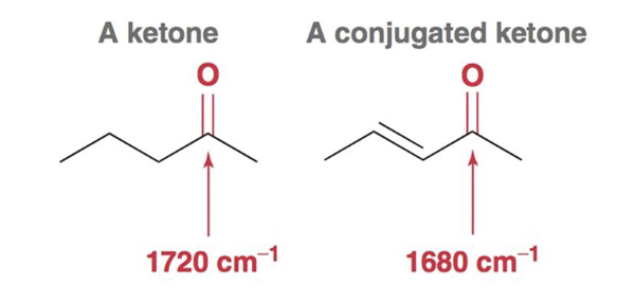
What is the characteristic IR spectra for a conjugated carbonyl-containing compound?
The carbonyl signal will lower in frequency to around 1680 cm-1 due to conjugation and resonance effects.
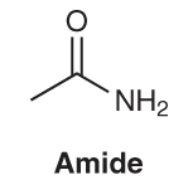
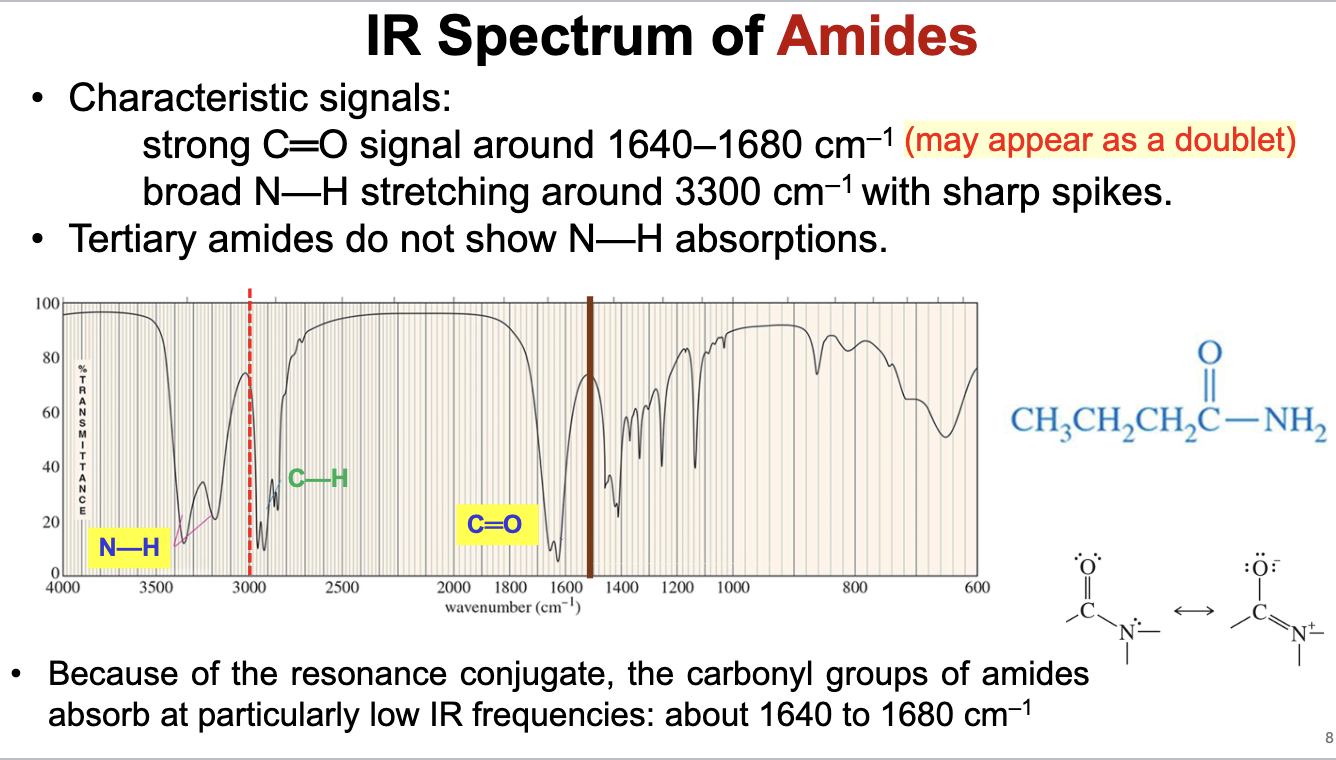
What is the characteristic IR spectra for amides?
The carbonyl in an amide will show a strong somewhat broad signal that may appear as a doublet around 1640 to 1680 cm-1, and will also display strong N-H stretch signals around 3300 cm-1 with secondary or primary amides
The carbonyl frequency is lowered to conjugation/resonance between the O=C—N conjugated system.
Tertiary amides do not show an N-H stretch signal because they are not bound to any hydrogen atoms.
Will also have C-H bond stretch signals just below 3000 cm-1.
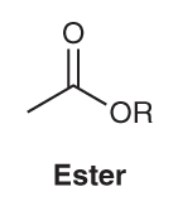
What is the characteristic IR spectra for an ester compound ?
Ester compounds will show a strong pinpoint carbonyl signal around 1735 cm-1, and also demonstrate C-H bond stretch signals just below 3000 cm-1.
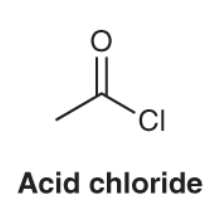
What is the characteristic IR spectra for an acid chloride compound?
The carbonyl group will show a strong pinpoint signal around 1790 to 1800 cm-1, further away from the carbonyl group-based compounds.
Will also show C-H bond stretches just below 3000 cm-1.
Can also demonstrate carbonyl overtone as well around 3400 cm-1.
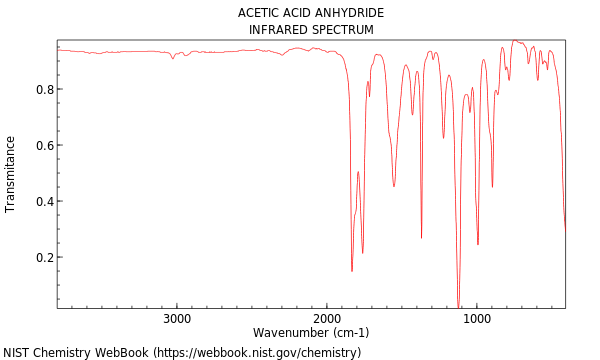
What is the characteristic IR spectra for an anhydride compound?
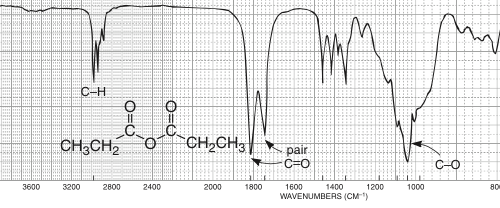
The two carbonyl groups of an anhydride result in two carbonyl signals, one at 1820 cm-1 and one at 1760 cm-1.
Will also demonstrate C-H bond stretch signals just below 3000 cm-1 (not shown in image).
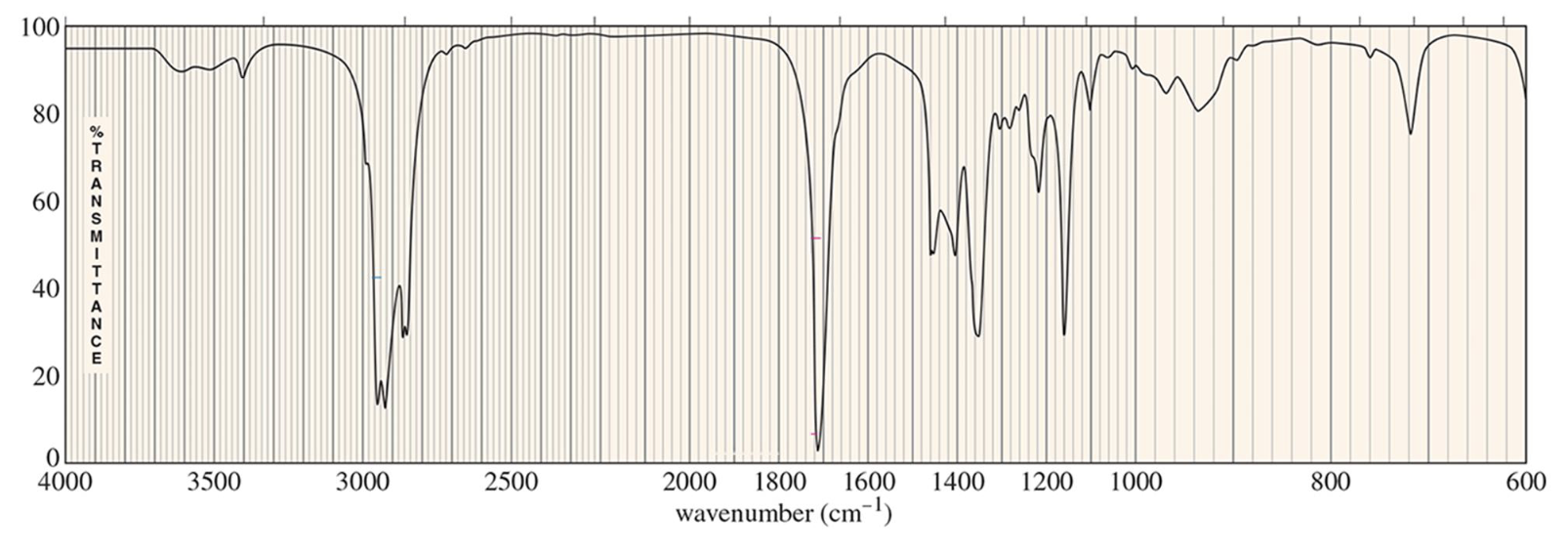
For which compound is this IR spectrum likely for?
A ketone, due to the sharp strong carbonyl signal at 1720 cm-1, with C-H stretch signals just below 3000 cm-1, and the distinct carbonyl overtones around 3400 cm-1.
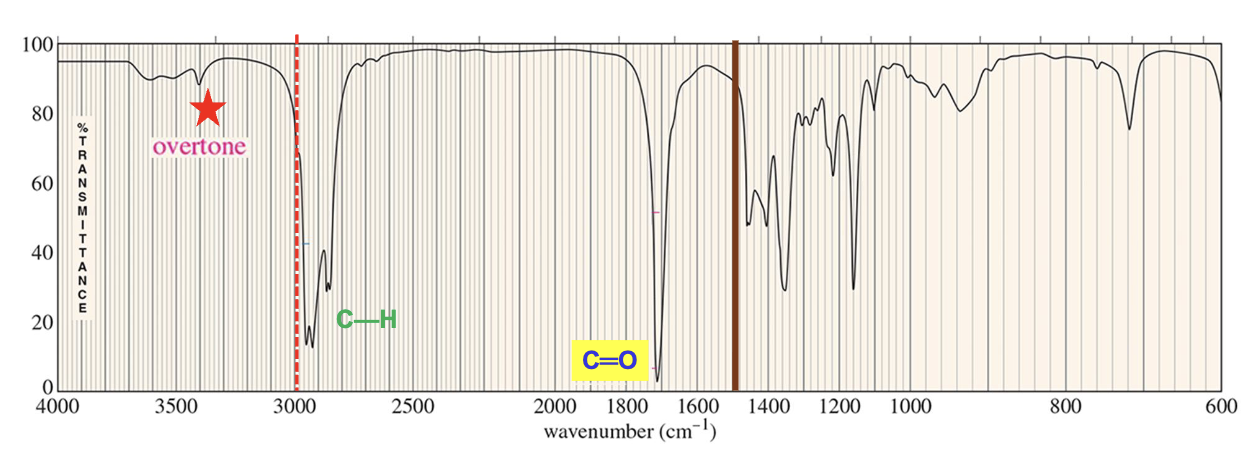
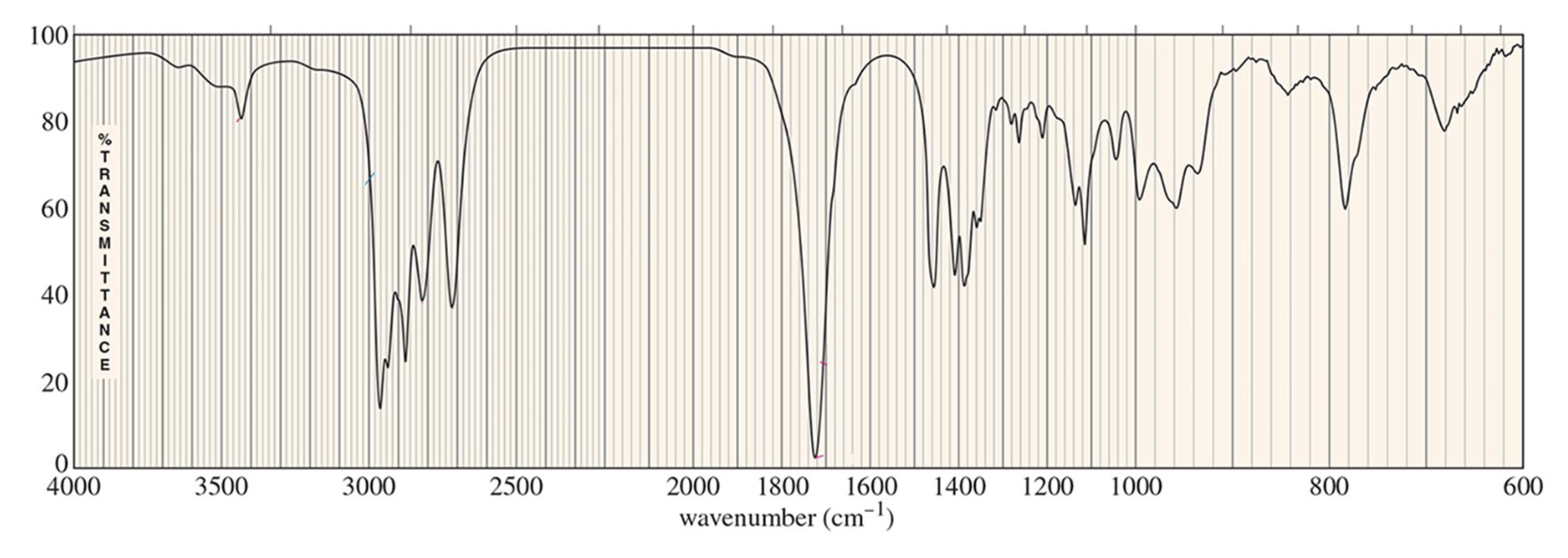
For which compound is this IR spectrum likely for?
An aldehyde, due the sharp strong carbonyl signal at 1730 cm-1, the C-H stretch signal just below 3000 cm-1, and distinct carbonyl overtone around 3400 cm-1.
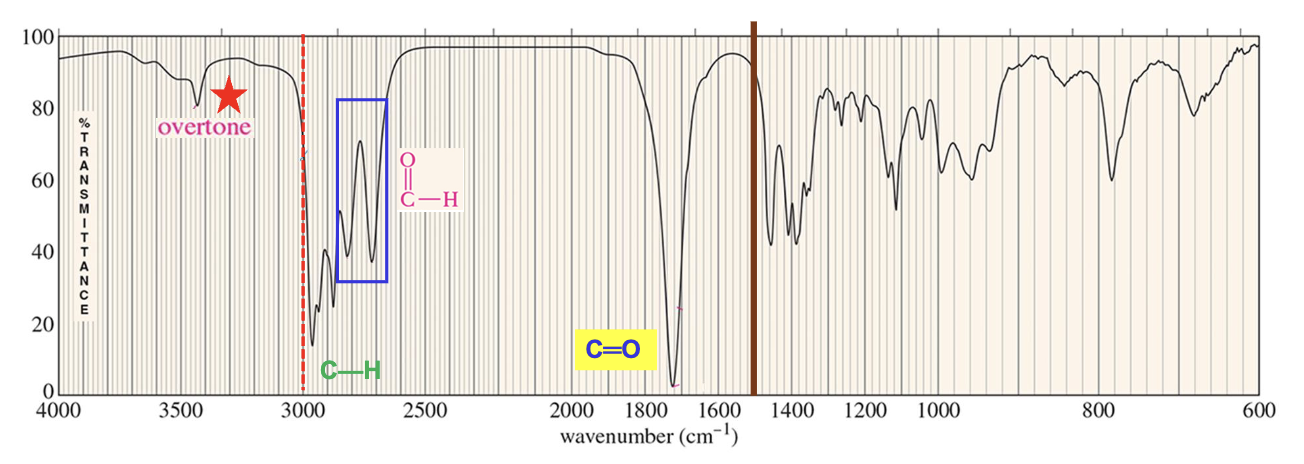
For which compound is this IR spectrum likely for?
A carboxylic acid, due to the sharp strong carbonyl signal around 1750 cm-1, the broad and strong signal at 2400-3600 cm-1 for the OH stretch, and the C-H stretch signals just below 3000 cm-1.
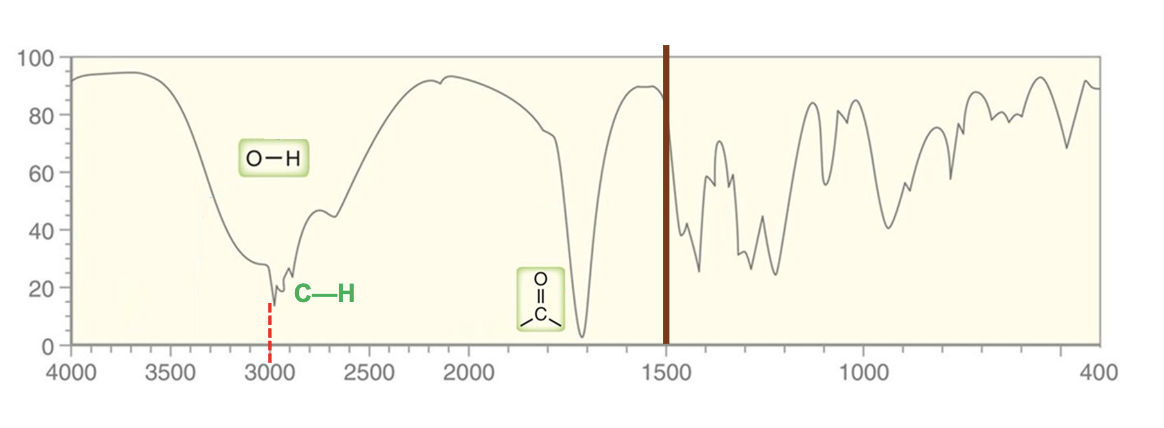
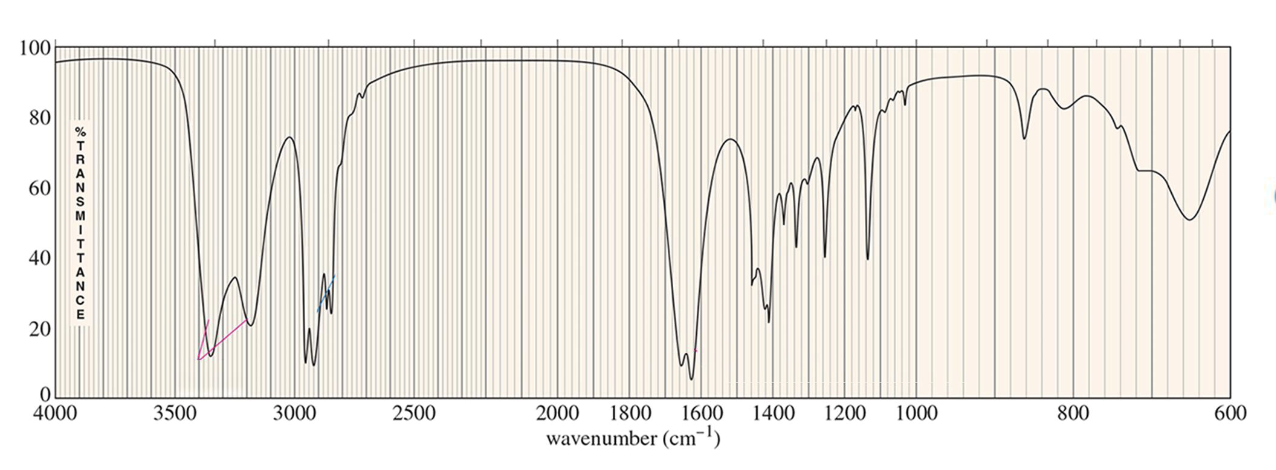
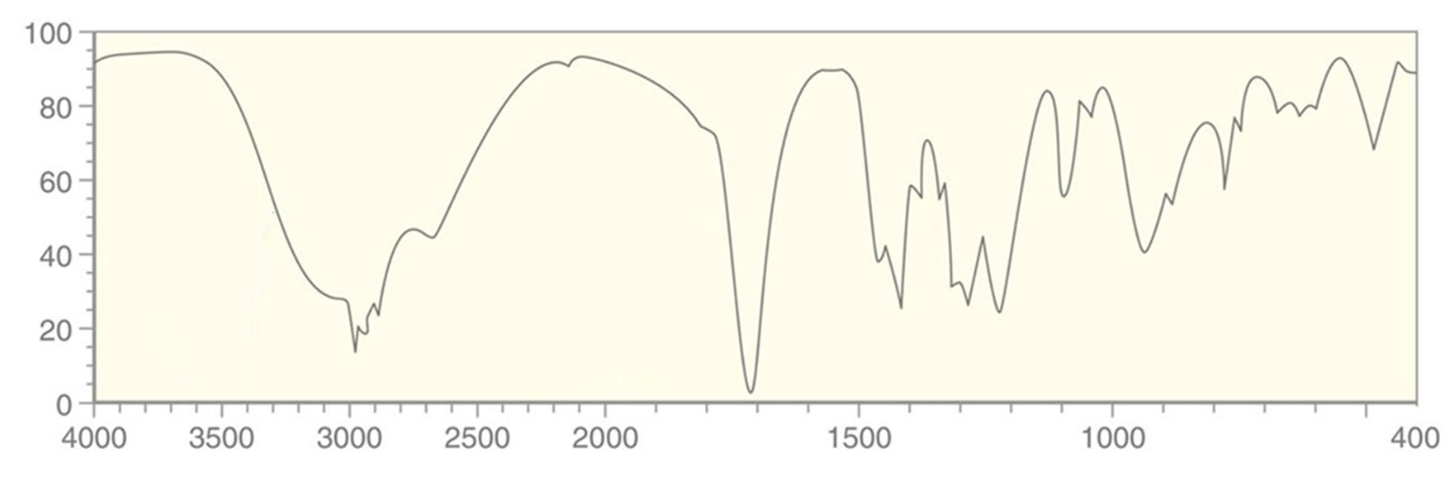
For which compound is this IR spectrum likely for?
An amide, a primary amide in particular, due to the sharp strong carbonyl signal around 1640 to 1680 cm-1, C-H stretch signals just below 3000 cm-1, and strong, somewhat broad N-H stretch signals with peaks around 3300 cm-1.
Primary amides will have two peaks for the N-H stretches as the nitrogen atom has two hydrogen atoms bound to it.
Secondary amides will have one peak for the N-H stretch due to only one hydrogen atom.
Tertiary amides show no N-H signal.
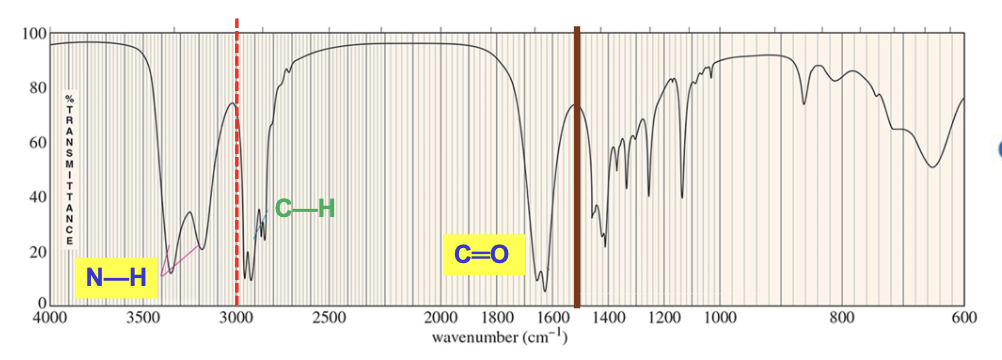
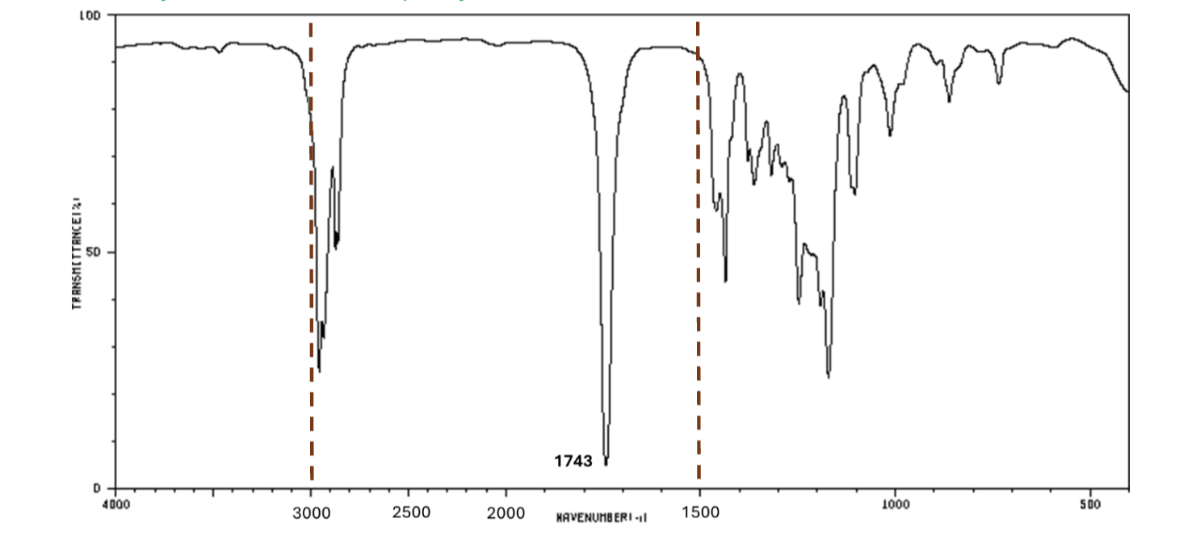
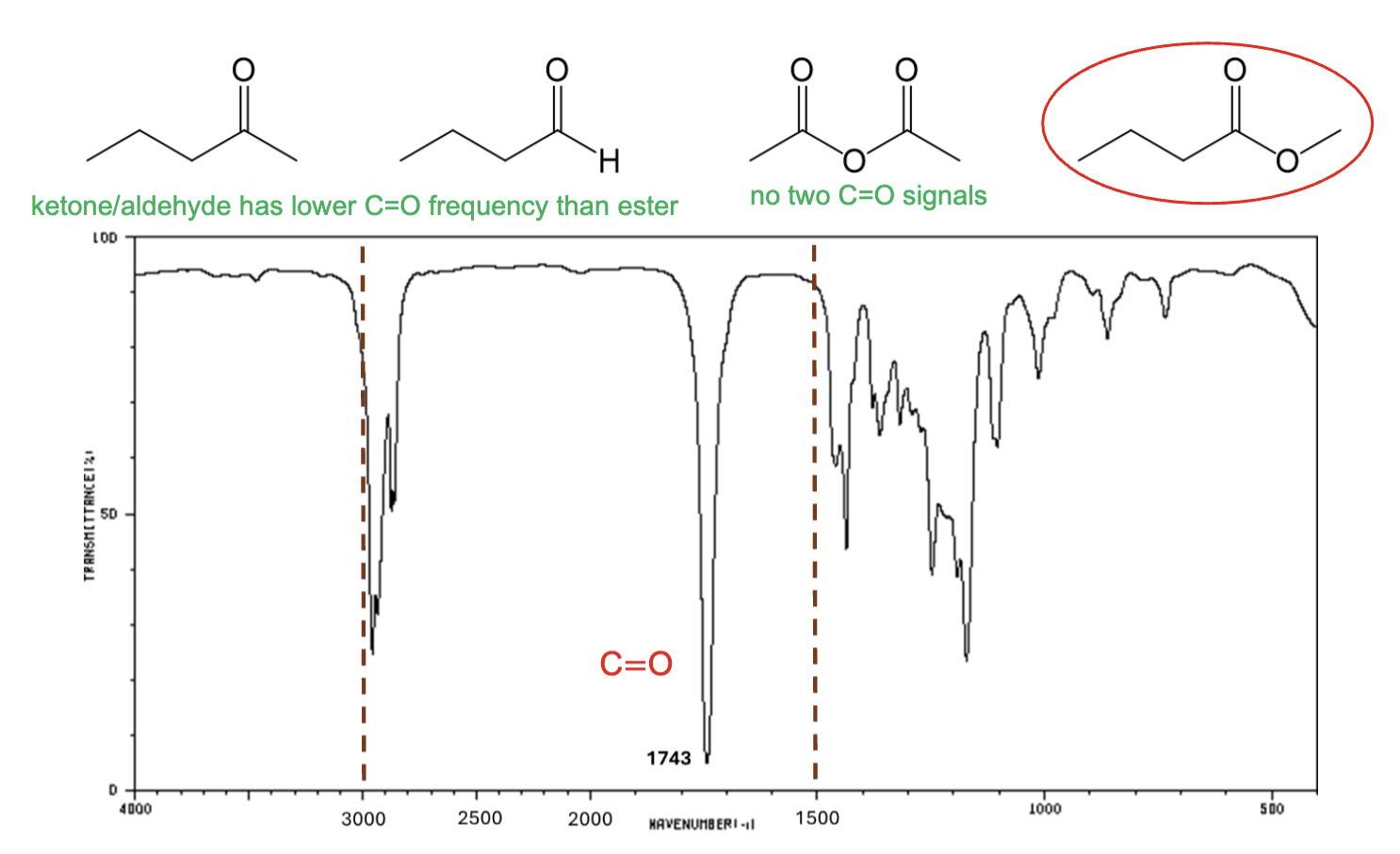
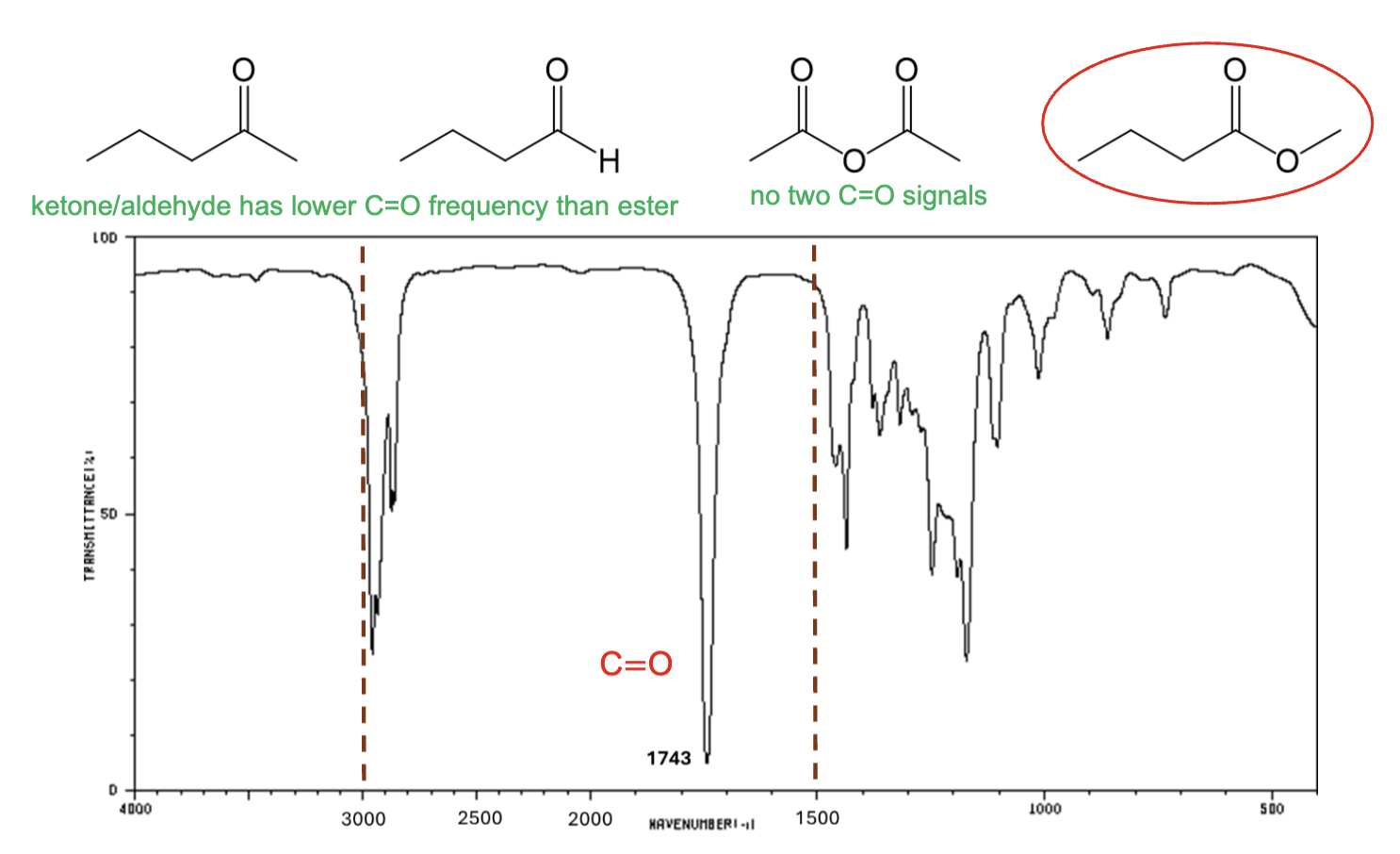
For which compound is this IR spectrum likely for?
An ester, due to the sharp strong carbonyl signal around 1735 cm-1, and the C-H stretch signals just below 3000 cm-1.
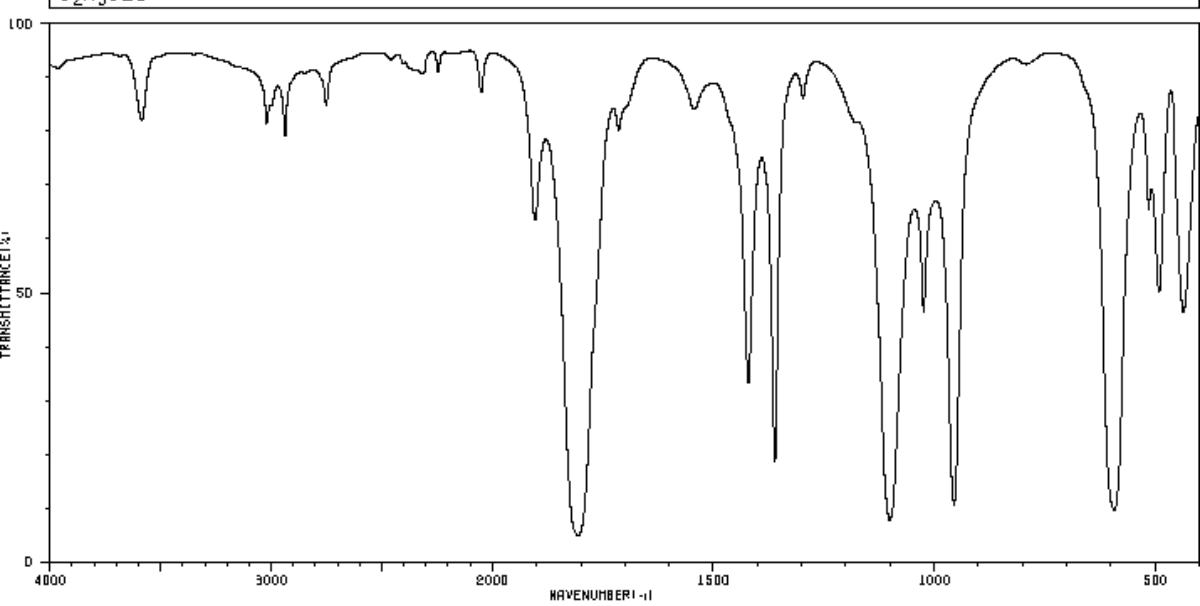
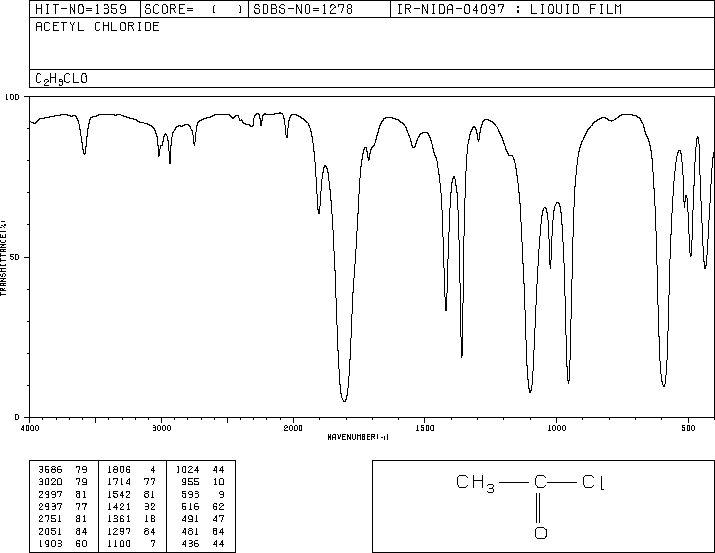
For which compound is this IR spectrum likely for?
An acid chloride, due to the sharp strong carbonyl signal around 1790 to 1800 cm-1, the C-H stretch signals just below 3000 cm-1 (small in this IR spectra image), and the distinct carbonyl overtone around 3400 cm-1.
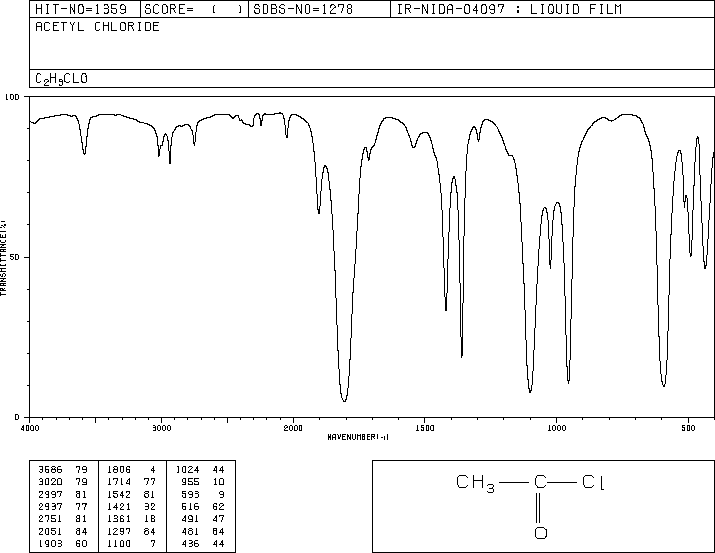
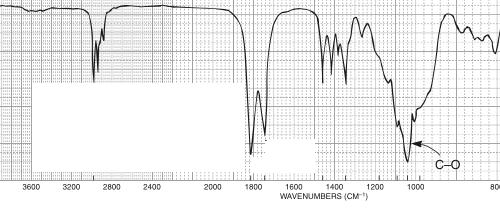
For which compound is this IR spectrum likely for?
An anhydride due to the dual strong sharp signals at 1760 and 1820 cm-1, and C-H stretch signals just below 3000 cm-1.