ionic compounds
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
How is the structure of ionic compounds
Lattice (a closely packed regular arrangement).
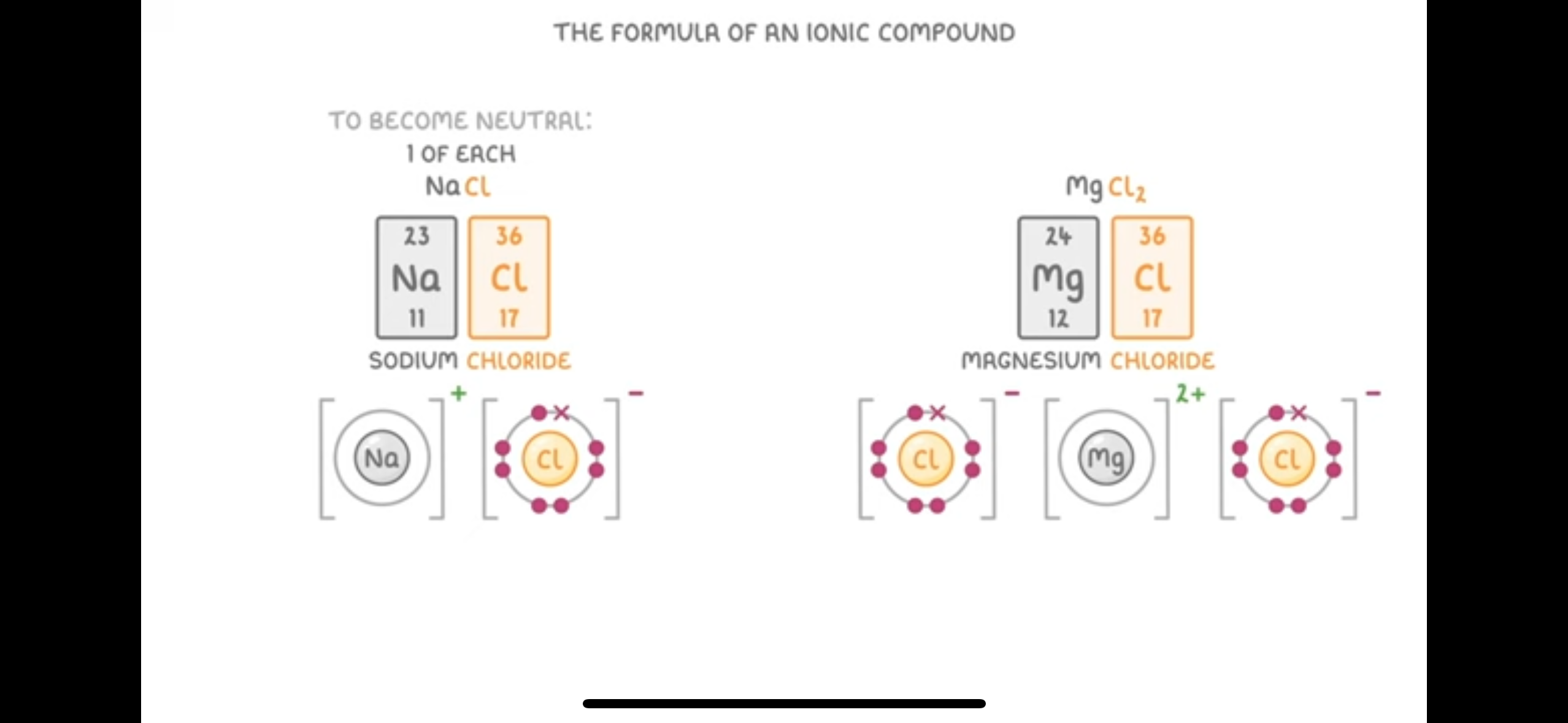
Ionic compounds are giant structures of ions held together in a lattice by electrostatic forces known as ionic bonds
How can ionic compounds be represented
In dot and cross diagrams
3 d models
Ball and stick models -which show different ions and put lines between to show different bonds
What are the properties of ionic compounds
And what is it determined by
Properties
Very high melting and boiling points
Can conduct electricity
Determined by
Melting and boiling points are determined by the strength of the bonds holding substance together. And Ionic compounds there are loads of ionic bonds all of which are very strong to break and all require loads of energy which will only be available at very high temperatures
-Conducting electricity depends on if there are charge particles that can move (can either be ions or elections)
Why can’t ionic compounds conduct electricity when solid
When I want it compounds are in solid form everything is fixed so they can’t conduct electricity. However when they’re melted or dissolved in water, the ions are free to move about and this movement of charge particles allows them to conduct electricity.
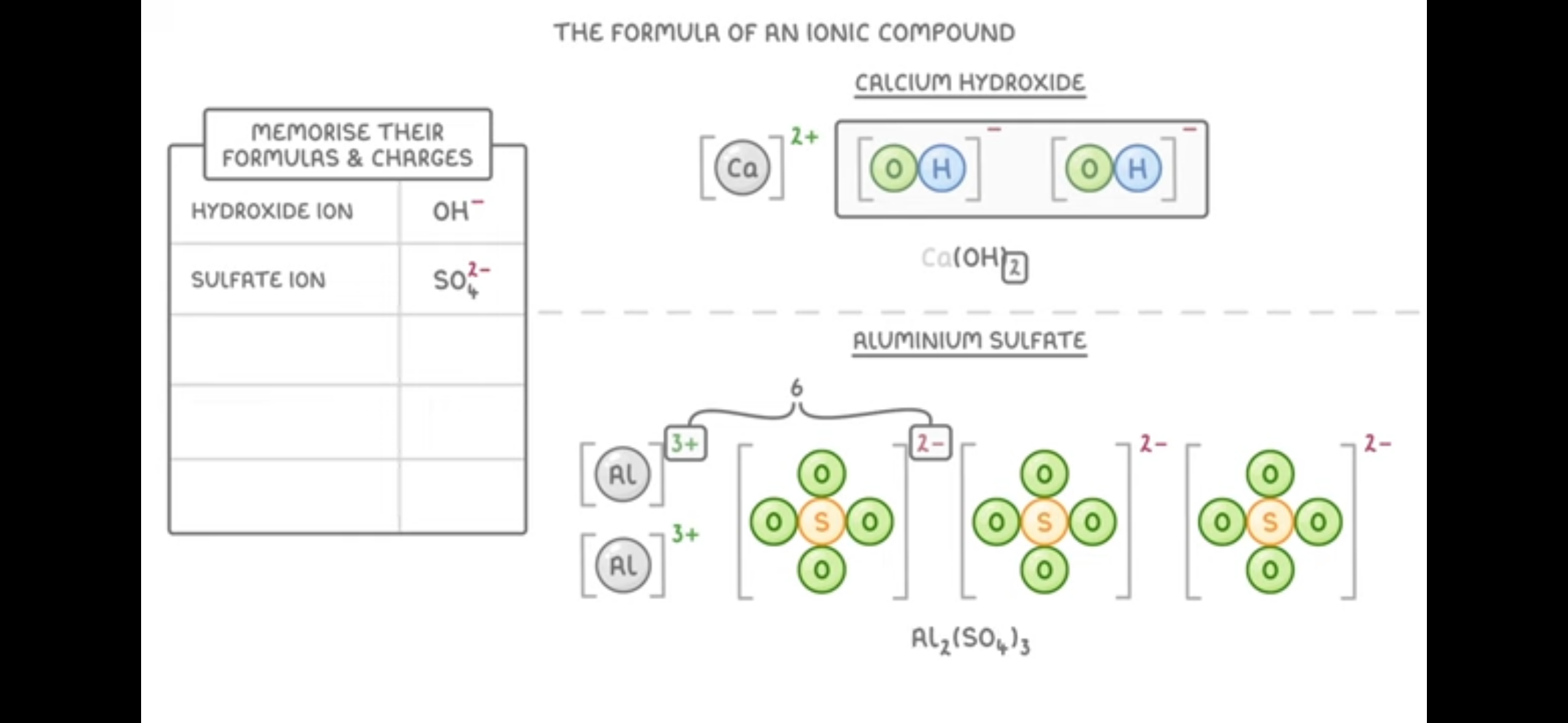
Write Symbol for hydroxide ions, sulfate ion, nitrate ions, carbonate ions , ammonia ions
Hydroxide ion- OH-
sulphate ions- SO4 2-
Nitrate ions- NO3-
Carbonate ions- CO3 2-
Ammonia- NH4+
Creating formulas