Chemistry - Energy changes
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
Exothermic reactions
-reactions that release energy to the surroundings
-the products have less energy than the reactants
Why do exothermic reactions release energy?
Because more energy is released when new bonds form than is needed to break old bonds
Examples of exothermic reactions
combustion, respiration, neutralisation
Where are exothermic reactions used
-Hand warmers
-self heating cans
-burning fuels
Exothermic reaction change diagram
Endothermic reaction
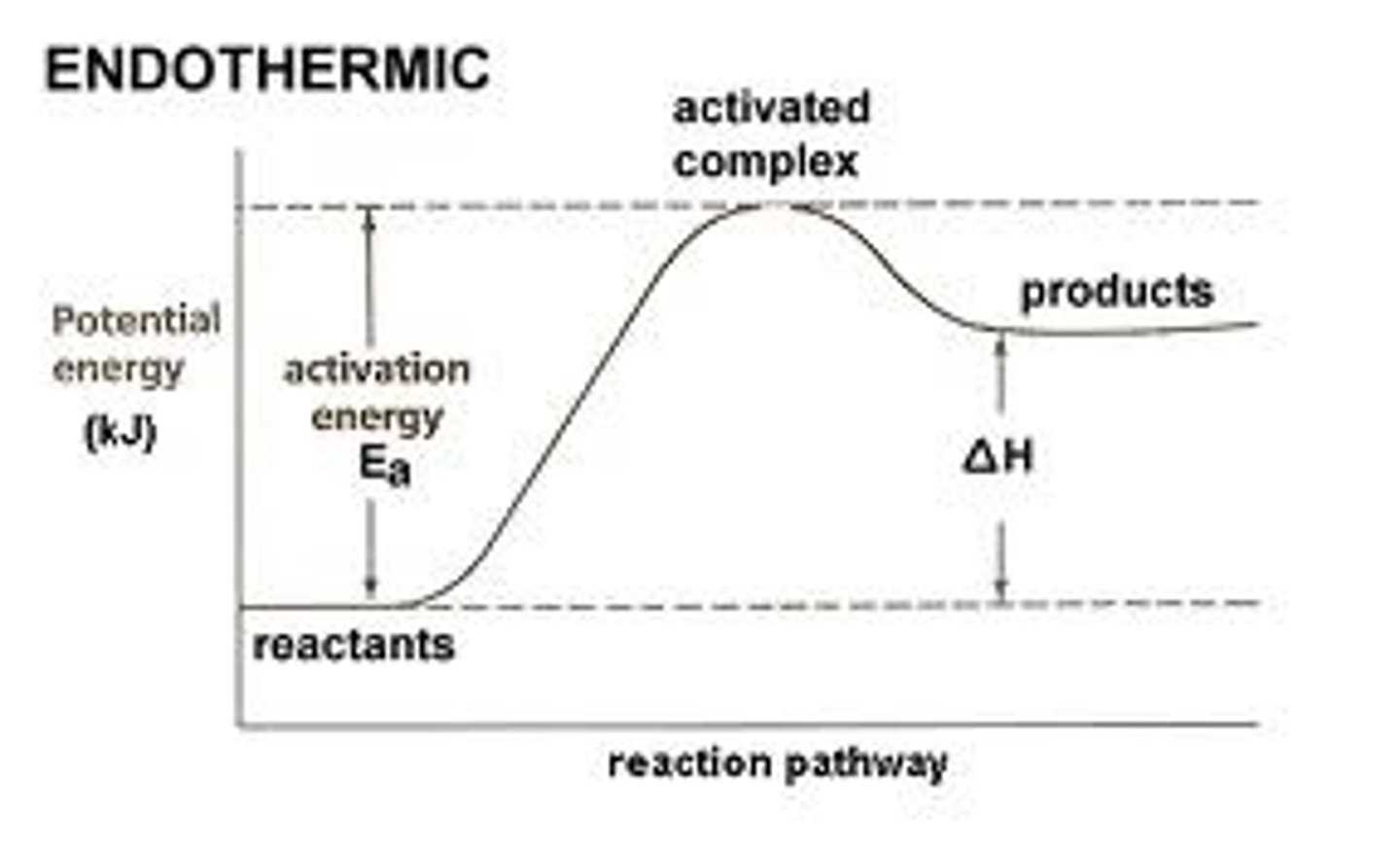
-A reaction in which energy is absorbed from the surroundings
-the products have more energy thaan the reactants
Why do endothermic reactions absorb energy?
More energy is needed to break the bonds than is released when new ones form
Examples of endothermic reactions
Thermal decomposition, photosynthesis
Where are endothermic reactions used
-ice packs
-evaporation
Endothermic reaction change diagram
activation energy
The minimum amount of energy required to start a chemical reaction.
breaking bonds
endothermic process absorbing energy
making bonds
Exothermic process releasing energy
energy change formula
Overall energy change = energy put in - energy taken out
negative overall energy change
exothermic reaction
positive overall energy change
endothermic reaction
chemical cells
a device that produces energy from a chemical reaction
How chemical cells work
-two metals act as electrodes
-the more reactive metal loses electrons and becomes the anode
-the less reactive one gains ean lectron and becomes the cathode
non-rechargeable batteries
Once the reactants are used up the battery is dead
rechargeable batteries
Chemical reactions can be reversed when an external circuit is supplied
Fuel cells advantages
higher efficiency, lower emissions, reduced noise pollution
Fuel cells disadvantages
high cost, limited fuel availability, efficiency losses, environmental impacts, durability, safety concerns
fuel cells
a type of electrochemical cell which provides electricity continuously, as long as it is supplied with a fuel (like hydrogen) and oxygen