Chemistry - Sem1 Final
0.0(0)
Card Sorting
1/111
Last updated 11:56 AM on 12/20/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
112 Terms
1
New cards
Democritus
440 BCE proposed that everything in the world was made out of particles: Atoms (indivisible)
2
New cards
John Dalton
\`1808: Atomic Theory (Accepted)
1. various compounds are combinations of atoms of differing elements
2. elements are of differeing size and mass
3. elements cannot be created nor destroyed
4. elements join together to create many different things
1. various compounds are combinations of atoms of differing elements
2. elements are of differeing size and mass
3. elements cannot be created nor destroyed
4. elements join together to create many different things
3
New cards
JJ Thomson
1897 discovers the electron; creates the “Plum Pudding Model”
4
New cards
Ernest Rutherford
“Father of the nuclear age”: though shooting alpha particles through gold foil, conclude that atomse had a lot of empty space with its mass at the center.
5
New cards
Niels Bohr
Electrons orbit the nuclues at fixes energies and distances. The can jumpdetwee the levels but cannot live in the space between levels. An atom is a “planetary model,” with the electrons acting as planets, with the abaily of a quantum leap
\
\
6
New cards
Quantum Leap
Niels Bhor, 1897
* “Quantum Leap”: As electron jump between “orbits” they lose a descrete amount of energy. This is known as a Quantum of energy. The more energy an electron has, the further away it is from the nucleus
* “Quantum Leap”: As electron jump between “orbits” they lose a descrete amount of energy. This is known as a Quantum of energy. The more energy an electron has, the further away it is from the nucleus
7
New cards
Werner Heisenberg
Impossible to determine the position ans speed of electrons
* Quantum model
* Quantum model
8
New cards
Atomic Number
Displays number of protons in an element
9
New cards
Isotopes
Atoms of a given element differing in number of neutrons
10
New cards
Mass Number
Number of protons + number of neutrons
11
New cards
Ions
Atoms that have gained or lost electrons
12
New cards
Cation
Positively charged ion
13
New cards
Anion
Negatively charged ion
14
New cards
Ionic Compound
Elements are held togeht by a difference of charge (one element gains electrons from the other element)
15
New cards
Covalent Compound
Bonding by sharing electrons
16
New cards
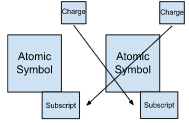
Atomic symbol
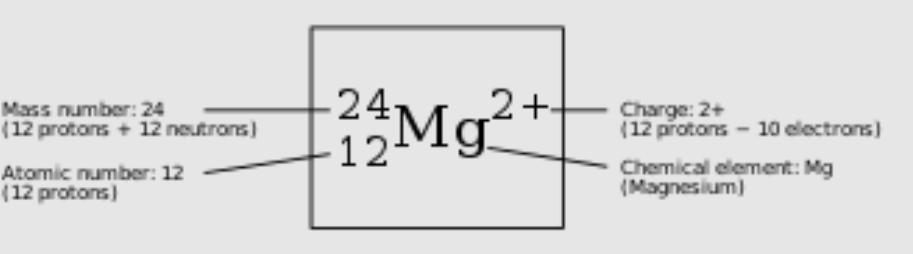
17
New cards
Matter
Anything that has mass and takes up space
18
New cards
Chemistry
The study of matter; almost everything outside of energy
19
New cards
Physical Proprty
Observed with the 5 senses
20
New cards
Chemical Property
Observation needing more than just the 5 senses
21
New cards
Physical Change
Change without changing the identity of a substance. Doesn’t change ts stucture/properties
* phase changes, breaking, tearing, dissolving
* phase changes, breaking, tearing, dissolving
22
New cards
Chemical Change
Matter changes its identity. Change in composition of a substance in a reactions.
* Release of energy (light/heat), color change, forms gas/precipitate, not easily reversed
* Release of energy (light/heat), color change, forms gas/precipitate, not easily reversed
23
New cards
Mixtures
Combination of matter with variable composition. Separated physically
* Distillation (sugar + water)
* Homogeneous: Uniform composition; equal throuhgout
* Heterogeneous: Depending on location within mixture
* Distillation (sugar + water)
* Homogeneous: Uniform composition; equal throuhgout
* Heterogeneous: Depending on location within mixture
24
New cards
Pure Substance
Not separated physically, if at all; same ratio of components but differing identities
* Elements: Cannot be separated chemically
* Compound: 1 or more elements combined; separated chemically
* Elements: Cannot be separated chemically
* Compound: 1 or more elements combined; separated chemically
25
New cards
Metric System
1 Meter:
* 1,000 milimeters
* 100 centimeters
* 10 decimeters
* .10 dekameters
* .001 hectometers
* .0001 kilometers
* Mili: 1/1000
* Centi: 1/100
* Deci: 1/10
* Base (meter, gram, liter): 1
* Deka: 10
* Hecto: 100
* Kilo: 1000
* 1,000 milimeters
* 100 centimeters
* 10 decimeters
* .10 dekameters
* .001 hectometers
* .0001 kilometers
* Mili: 1/1000
* Centi: 1/100
* Deci: 1/10
* Base (meter, gram, liter): 1
* Deka: 10
* Hecto: 100
* Kilo: 1000
26
New cards
Factor-Label System
Using units to solve measurement problems
27
New cards
Significant figures
Sig Figs; Numbers you can certify/guarantee
* Yes: non-zero numbers, Captive zeros, trailing zeros with a decimal
* No: Leading zeros, trailing zeros without a decimal
* Add/subtract: round to lost decimal place
* Multiply/divide: Round to lowest sig figs
* Yes: non-zero numbers, Captive zeros, trailing zeros with a decimal
* No: Leading zeros, trailing zeros without a decimal
* Add/subtract: round to lost decimal place
* Multiply/divide: Round to lowest sig figs
28
New cards
Accuracy
How close a measurement is to the accepted value
* Universal
* Universal
29
New cards
Precision
How close measurements of the smae item are to eachother
* Subjective
* Subjective
30
New cards
Density
Mass / Volume (M/V)
31
New cards
Naming Molecular Formulas
Nonmetals (4A - 7A)
1. Determine the names of the symbols
2. Determine the number of atoms of each element present
3. Write prefix corresponding with the quantity of each atom
4. End last element by changing the ending to -ide
1. Determine the names of the symbols
2. Determine the number of atoms of each element present
3. Write prefix corresponding with the quantity of each atom
4. End last element by changing the ending to -ide
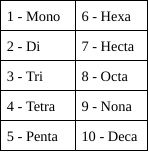
32
New cards
Writing Molecular Formulas
Nonmetals (4A - 7A)
1. Write sumbol for each element
2. Determin the subscribt from the prefix of each element
3. CANNOT REDUCE
1. Write sumbol for each element
2. Determin the subscribt from the prefix of each element
3. CANNOT REDUCE
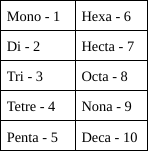
33
New cards
Naming Ionic I Formulas
Metals with only 1 charge (1A - 3A)
1. Name the metal and nonmenal
2. End nonmetal with -ide
3. NO PREFIXES OR NUMERALS
1. Name the metal and nonmenal
2. End nonmetal with -ide
3. NO PREFIXES OR NUMERALS
34
New cards
Writing Ionic I Formulas
Metals with only 1 charge (1A - 3A)
1. Write symbol for metal and nonmetal
2. Find both charges from the Periodic Table
3. Equalize with Criss-Cross
4. CAN REDUCE
1. Write symbol for metal and nonmetal
2. Find both charges from the Periodic Table
3. Equalize with Criss-Cross
4. CAN REDUCE
35
New cards
Naming Ionic Type II Formulas
Transition Metals w/ 1+ Charges
1. Name Metal and Nonmetal
2. Use __**Heeren’s Law**__ to find the charge
1. Multiply the charge of nonmetal by its subscript Add/Subtract it from rigth + left, divide right by subscript of metal
2. Subscript Metal x Charge Metal) + (Subscript Nonmetal x Charge Nonmetal) = 0
3. Use Roman Numerals to represent charge
4. End name of nonmetal in -ide
1. Name Metal and Nonmetal
2. Use __**Heeren’s Law**__ to find the charge
1. Multiply the charge of nonmetal by its subscript Add/Subtract it from rigth + left, divide right by subscript of metal
2. Subscript Metal x Charge Metal) + (Subscript Nonmetal x Charge Nonmetal) = 0
3. Use Roman Numerals to represent charge
4. End name of nonmetal in -ide

36
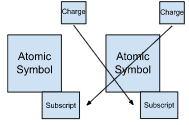
New cards
Writing Ionic Type II Formulas
Transition Metal w/ 1+ Charges
1. Write Symbol for metal and nonmetal
2. Determine charge from Periodic Table/Numeral
3. Criss-Cross charges
4. CAN REDUCE
1. Write Symbol for metal and nonmetal
2. Determine charge from Periodic Table/Numeral
3. Criss-Cross charges
4. CAN REDUCE
37
New cards
Combination Reaction
Combo; 2 Reactants to 1 Product
1. A + B = AB
1. Always check charges and balance
1. A + B = AB
1. Always check charges and balance
38
New cards
Decomposition
Decomp; 1 Reactant to 2 Products
1. AB = A + B
1. Check charges and balance
1. AB = A + B
1. Check charges and balance
39
New cards
Combustion
C.C.; Burning, needs fuel source
1. C_H_ + O2 = CO2 + H20
1. CO4 = Methane
1. C_H_ + O2 = CO2 + H20
1. CO4 = Methane
40
New cards
Single Replacement
SR; Element + Compound to New Element + New Compound
1. AB + C = AC + B
1. ALWAYS check activity series; only a higher-ranked element can replace a lower-ranked metal
1. AB + C = AC + B
1. ALWAYS check activity series; only a higher-ranked element can replace a lower-ranked metal
41
New cards
Double Replacement
DR; 2 Ionic Compounds to 2 New Compounds
1. AB + CD = AD + CB
1. Usually forms a precipitat, gas (bubbling), or water
1. AB + CD = AD + CB
1. Usually forms a precipitat, gas (bubbling), or water
42
New cards
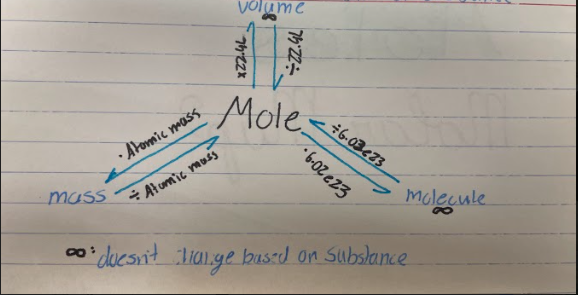
Mole
SI (Metric) Standard unit to measure the amount of a substance
\
1 mole is equivalent to:
1. Atomic mass of an element
2. 6.02e23 atoms (∞) - __*Avogadro’s Number*__
3. 22.4L (∞)
\
1 mole is equivalent to:
1. Atomic mass of an element
2. 6.02e23 atoms (∞) - __*Avogadro’s Number*__
3. 22.4L (∞)
43
New cards
Dimentional Analysis
Relationship between quantities based on fundamental quanlity
1. State Given
2. Multiply by conversion factor (atomic mass, atoms, liter)
3. Arrange Step 2 so desire units are on top
4. Solve and round to sig figs
1. State Given
2. Multiply by conversion factor (atomic mass, atoms, liter)
3. Arrange Step 2 so desire units are on top
4. Solve and round to sig figs
44
New cards
Molar Mass
The molar mass of a total compound
1. Find number of atoms of each element
2. Find mass of each individual atom
1. From Periodic Table
3. Find total mass of the element available
4. Find total mass of compound
1. Add up step three of each element
1. Find number of atoms of each element
2. Find mass of each individual atom
1. From Periodic Table
3. Find total mass of the element available
4. Find total mass of compound
1. Add up step three of each element
45
New cards
Percent Composition
Comparison of all elements in a compound : compound mass
1. (Mass of element) / (Mass of compound) x 100
1. (Mass of element) / (Mass of compound) x 100
46
New cards
Hydrates: Finding anhydrate
Hydrate: Substance w/ water as an additional component
1. Find molar mass of each reactant
2. Add up molar masses to find hydrate mass
3. Divide molar mass of individual reactant by hydrate mass
4. Divide by 100, multiply by quantity of anhydrate
1. Find molar mass of each reactant
2. Add up molar masses to find hydrate mass
3. Divide molar mass of individual reactant by hydrate mass
4. Divide by 100, multiply by quantity of anhydrate
47
New cards
Hydrates: Coefficient of Hydration
Mole Ration
1. Find mass of both reactants in substance
2. Convert masses of both into moles
3. Divide both moles quantities by the smallest mole quatitity
4. Quotients are the equtation coeffients
1. Quotient that is >1 should be your coefficient of hydration
1. Find mass of both reactants in substance
2. Convert masses of both into moles
3. Divide both moles quantities by the smallest mole quatitity
4. Quotients are the equtation coeffients
1. Quotient that is >1 should be your coefficient of hydration
48
New cards
Empirical Formula
A formula giving the __proportions__ of the elements present in a compound but not the actual numbers or arrangement of __atoms__.
1. If given percentages, assume that you have a 100g sample
2. Convert percentages into grams
3. Convert mass into moles
4. Divide quantities by the smallest mole quantity
5. Results are the equation subscripts
1. Is the basic, lowest ratio that you can have for a substance
1. If given percentages, assume that you have a 100g sample
2. Convert percentages into grams
3. Convert mass into moles
4. Divide quantities by the smallest mole quantity
5. Results are the equation subscripts
1. Is the basic, lowest ratio that you can have for a substance
49
New cards
Molecular Formula
The real compound that still shares the same ratio as its empirical formula
1. A multiple of the empirical formula
1. A multiple of the empirical formula
50
New cards
Stoichiometry
Relating the Amount of one substance to another
1. Balance Chemical equation
2. Convert given into moles
3. Convery step 2 into moles of desired substance
1. Use mol/mol ration using moles from equation
4. Convert step 3 into desired unit
1. Balance Chemical equation
2. Convert given into moles
3. Convery step 2 into moles of desired substance
1. Use mol/mol ration using moles from equation
4. Convert step 3 into desired unit
51
New cards
Percent Composition
The percentage your answer is from the theoretical result
1. (Actual yield) / (Theoretical yield) x 100
1. Normally
1. (Actual yield) / (Theoretical yield) x 100
1. Normally
52
New cards
Limiting Reactant
You are limitd by whatever runs out first
1. Balance given equation
2. Use Stoichiometry to determine how much of your porduct each substance can make
3. Whichever theoretical yield is lower is your limiting reactant
1. Balance given equation
2. Use Stoichiometry to determine how much of your porduct each substance can make
3. Whichever theoretical yield is lower is your limiting reactant
53
New cards
Properties of gases
1. Compressible: Able to be compressed/moveable
2. Expanding: Will take up full volume of structure
3. Shape: Takes shape of its container
4. Low Density: Many float (Helium)
5. Homogeneous: Equal distribution within a mixture
6. Similarity: Properties of all gases are similar
54
New cards
Kinetic Molecular Theory
Takes idea of what we can see in order to explain what we cannot see
1. Gas particles are so small that their volume is considered to be 0L
2. Gas particles are in constant motion.
1. Collisions with wall creates pressure
3. Gas particles do not attrct or repel eachother
4. Average kinetic energy is proportional to temperature
1. Cold Temperature: Less energy
2. Warm Temperature: More Energy
\
1. Gas particles are so small that their volume is considered to be 0L
2. Gas particles are in constant motion.
1. Collisions with wall creates pressure
3. Gas particles do not attrct or repel eachother
4. Average kinetic energy is proportional to temperature
1. Cold Temperature: Less energy
2. Warm Temperature: More Energy
\
55
New cards
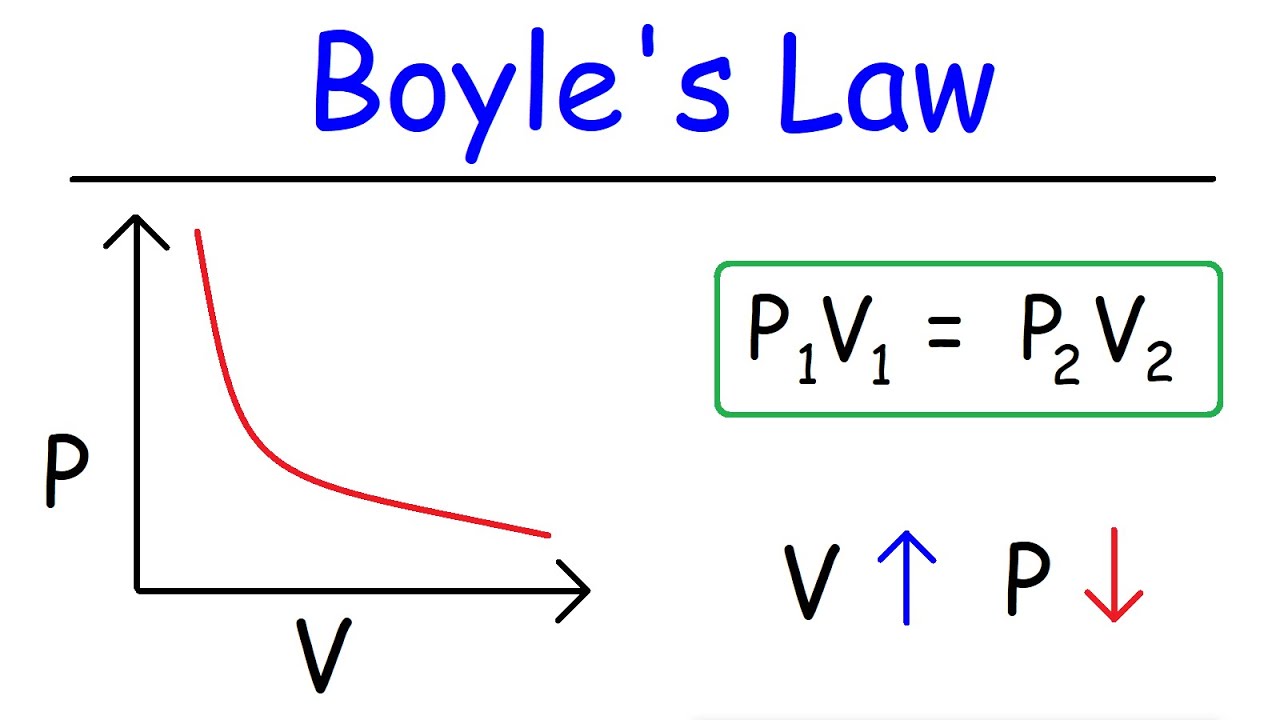
Boyle’s Law
Pressure and Volume are inversely related when temperature is constant
1. P1V1 = P2V2
1. P1V1 = P2V2
56
New cards
Charle’s Law
If temperature of a gas increases, its volume increases IF pressure reamins the same
V1 / T1 = V2 / T2
V1 / T1 = V2 / T2
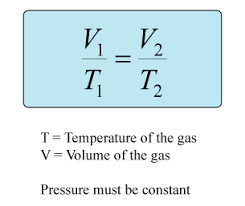
57
New cards
Temperature
Measure of Average Kinetic Energy of Particles
1. Kelvin: Starts at absolute zero
1. Absolute zero: All particles stop moving
2. K = °C + 273
3. Used in ALL gas-related problems
1. Kelvin: Starts at absolute zero
1. Absolute zero: All particles stop moving
2. K = °C + 273
3. Used in ALL gas-related problems
58
New cards
Combined Gas Law
Combination of Boyle’s Law and Charle’s Law; When everything changes
1. Anything constant will cancel
1. Anything constant will cancel
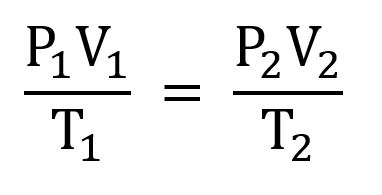
59
New cards
STP
Standard Temperature and Pressure
1. P = 1 atmosphere or its equivalents
2. T = 273 K (0 °C)
1. P = 1 atmosphere or its equivalents
2. T = 273 K (0 °C)
60
New cards
Dalton’s Law of Partical Pressures
The pressure of each gas is proportional to the moles available
1. “x” : Mole fraction - Mole gas 1 / total moles
2. P(gas1) = (X - gas 1)(P - total)
3. P -Total = Sum of pressures of each gas
1. “x” : Mole fraction - Mole gas 1 / total moles
2. P(gas1) = (X - gas 1)(P - total)
3. P -Total = Sum of pressures of each gas

61
New cards
Avogadros Law
Volume is directly proportional to the number of gas molecules if Pressure and Temperature are constant
1. From Gay-Lussac: Gases comine in volumes that have smmple whole number ratios; pressure and temperature are directly proportional
1. From Gay-Lussac: Gases comine in volumes that have smmple whole number ratios; pressure and temperature are directly proportional
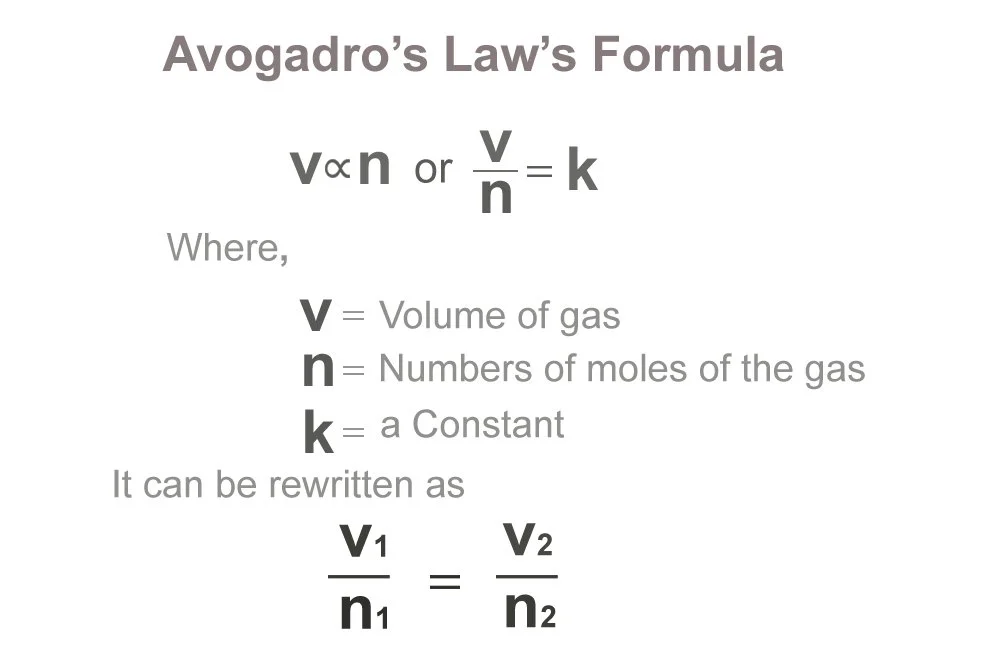
62
New cards
Ideal Gas Law
Ideal Gas: Occupy ngligible space and have no interactions; obeys all gas laws.
1. 1 situation; NOT CHANGING
2. PV = nRT
1. P = Pressure
2. V = Volume
3. n = Moles of gass
4. R = 0.08206 (when P = atm); 8.314 (when P = kPa); 62.36 (when P = Torr)
5. T = Temperature
1. 1 situation; NOT CHANGING
2. PV = nRT
1. P = Pressure
2. V = Volume
3. n = Moles of gass
4. R = 0.08206 (when P = atm); 8.314 (when P = kPa); 62.36 (when P = Torr)
5. T = Temperature
63
New cards
Ideal Gas Law: Molar Mass
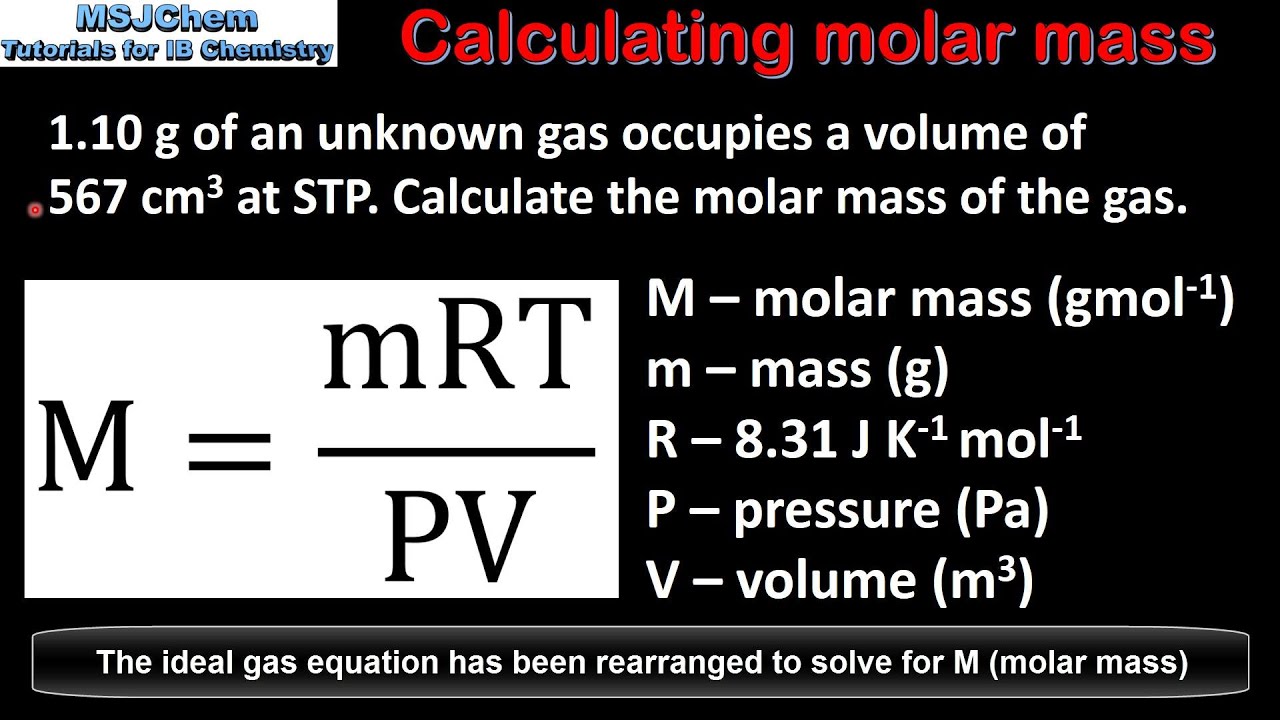
64
New cards
Ideal Gas Law: Density
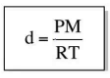
65
New cards
calorie
cal: Heat required to raise 1g water by 1 °C
* 1 cal = 4.184 J
* 1 cal = 0.0001 kcal
* 1 cal = 4.184 J
* 1 cal = 0.0001 kcal
66
New cards
Calorie/Kilocalorie
Cal/kcal; Heat required to raise 1,000g water by 1 °C
* 1 Cal = 4,184 J
* 1 Cal = 1,000 cal
* 1 Cal = 4,184 J
* 1 Cal = 1,000 cal
67
New cards
Joule
J; Standard SI unit for heat/energy
* 4.184 J = 1 cal
* 4,184 J = 1 Cal/ 1 kcal
* 4.184 J = 1 cal
* 4,184 J = 1 Cal/ 1 kcal
68
New cards
Specific Heart
* Specific Heat Capacity = C
* Quantity of heat (J, cal, Cal/kcal) absorbed per unit mass (g, kg) when temperature increases / decreases by 1 °C
* Reason why some materials hold heat longer / change heat faster
* Higher C = More energy to increase temperature
* Quantity of heat (J, cal, Cal/kcal) absorbed per unit mass (g, kg) when temperature increases / decreases by 1 °C
* Reason why some materials hold heat longer / change heat faster
* Higher C = More energy to increase temperature
69
New cards
Change in Heat - ΔH
Equation to find change in heat
* m = Mass
* C = Specific Heat
* ΔT = Change in temperature (final temp - first temp)
* m = Mass
* C = Specific Heat
* ΔT = Change in temperature (final temp - first temp)
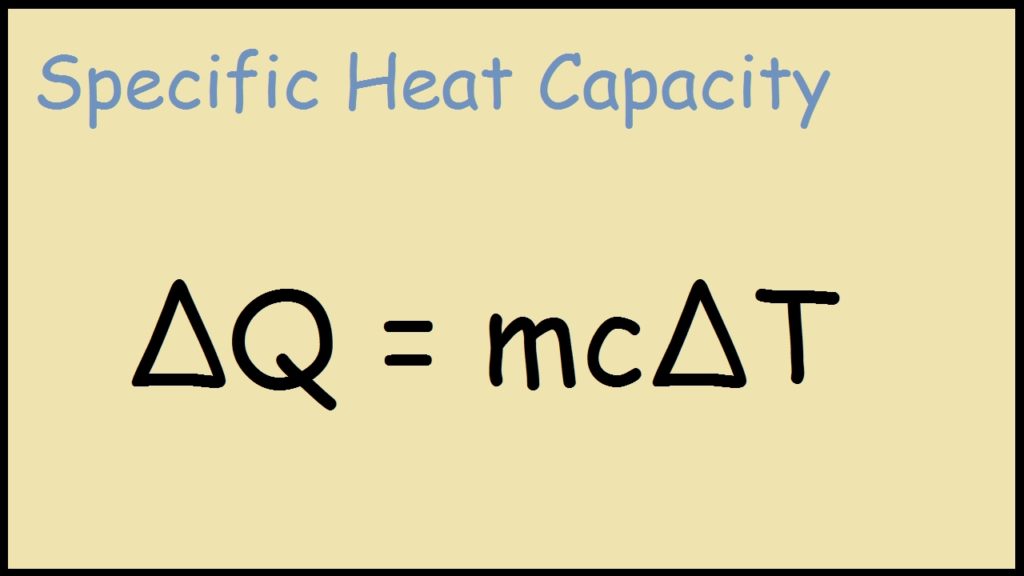
70
New cards
Equilibrium
mCΔT = -mCΔt
Occurs when liquid is added to a calorimeter
Occurs when liquid is added to a calorimeter
71
New cards
Calorimetry
* Calorimeter: Device to measure heat of a given object/reaction
* Heat lost = -Heat gained
* Heat lost by an object is gained by its surroundings
* Detects calories in food
* Heat lost = -Heat gained
* Heat lost by an object is gained by its surroundings
* Detects calories in food
72
New cards
0th Law
2 thermodynamic systems are in equilibrium with another
* |A|B|C|
* (A + C) / 2 = B
* Underlying principle in temperature found in other laws
* found after Laws 1-3
* |A|B|C|
* (A + C) / 2 = B
* Underlying principle in temperature found in other laws
* found after Laws 1-3
73
New cards
1st Law
As heat increases, so does the amount of potential work
* W = P(ΔV); P = Pa; V = m3
* W = P(ΔV); P = Pa; V = m3
74
New cards
Carnot Efficiency
Efficiency of a system is based off of the difference between hot and cold temperatures
* E = \[(T-hot ) - (T-cold)\]/T-hot x 100
* E = \[(T-hot ) - (T-cold)\]/T-hot x 100
![Efficiency of a system is based off of the difference between hot and cold temperatures
* E = \[(T-hot ) - (T-cold)\]/T-hot x 100](https://knowt-user-attachments.s3.amazonaws.com/baa9223bfe3b405d814c4d7791a85cc0.jpeg)
75
New cards
2nd Law
Heat flows from Hot to cold
* Can flow in opposite direct but needs a lot of help
* Can flow in opposite direct but needs a lot of help
76
New cards
Entropy
Amount of energy given off with every change in temperature
* Measure of the disorder within a system (gs has more entropy than liquid)
* Measure of the disorder within a system (gs has more entropy than liquid)
77
New cards
3rd Law
As temperature approaches absolute zero (0k) entropy is minimized and all functions stop
* Cannot do Charle’s Law at 0 K
* Cannot do Charle’s Law at 0 K
78
New cards
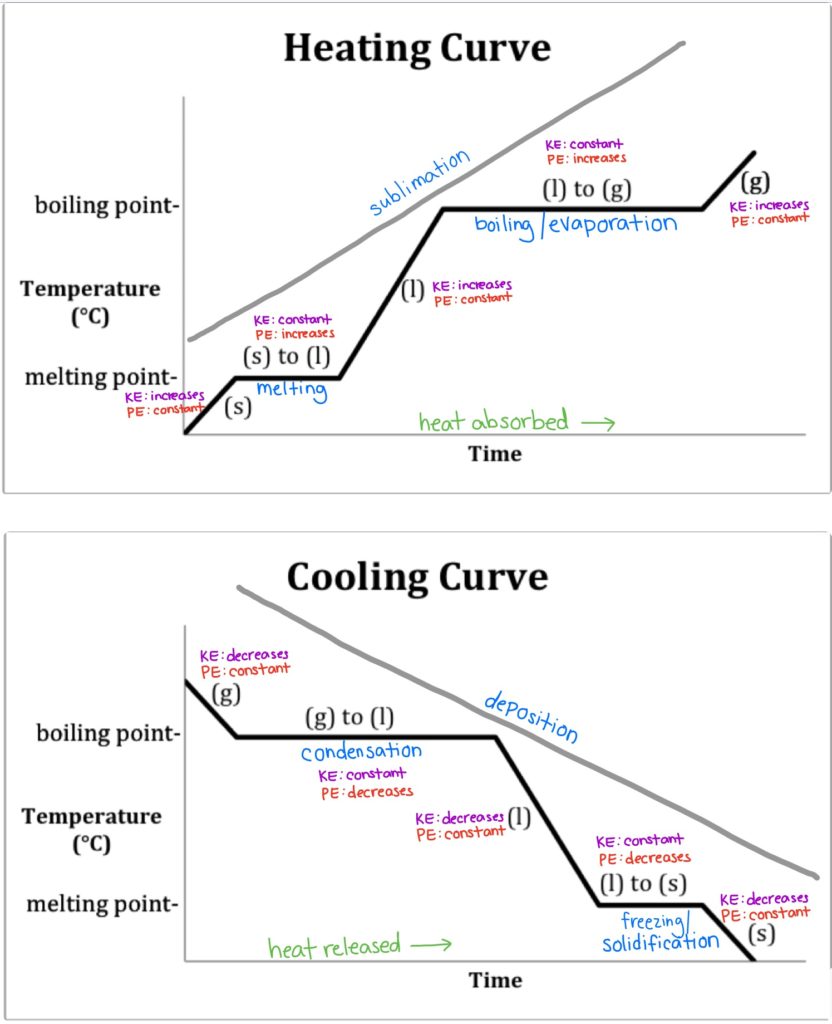
State Change
* Freezing: Process of taking a liquid to a solid (0 °C)
* Melting: Process of taking a solid to a liquid (0 °C)
* Evaporation: Process of taking a liquid to a gas (100 °C)
* Condensation: Process of taking a gas to a liquid (100 °C)
* Sublimation: Process of taking a solid to a gas
* Melting: Process of taking a solid to a liquid (0 °C)
* Evaporation: Process of taking a liquid to a gas (100 °C)
* Condensation: Process of taking a gas to a liquid (100 °C)
* Sublimation: Process of taking a solid to a gas
79
New cards
Heating / Cooling Curve
Heating: Graph of phase changes thorugh Increasing Temperature
\
Cooling: Graph of phase changes through Decreasing Temperatures
\
Cooling: Graph of phase changes through Decreasing Temperatures
80
New cards
Heat of Vaporation and Fusion
Vaporation: Amount of energy to boil a liquid
* Hvap
* Water = 2,260 J/g
* 100 °C
\
Fusion: Amount of energy to freeze a liquid
* Hfusion (Hf)
* Water: 333 J/g
* 0 °C
* Hvap
* Water = 2,260 J/g
* 100 °C
\
Fusion: Amount of energy to freeze a liquid
* Hfusion (Hf)
* Water: 333 J/g
* 0 °C
81
New cards
Heat Change with Phase Change
During a phase change, you have to account for any phase changes that occur
* ΔH = (mCΔT) + H(v or f)
* Can have multipl mCΔT’s and H(v or f)’s depending on how many phase changes occur
* ΔH = (mCΔT) + H(v or f)
* Can have multipl mCΔT’s and H(v or f)’s depending on how many phase changes occur
82
New cards
Enthalpy
The sum of internal energy and product of pressure and volume
* 1st Law
* Directioni of heat transfer in a reaction; ΔH Products - ΔH Reactants
* Endothermic: Heat absorbed (+)
* Exothermic: Heat release (-)
* Use enthalpy to fin the ΔH of variables
* 1st Law
* Directioni of heat transfer in a reaction; ΔH Products - ΔH Reactants
* Endothermic: Heat absorbed (+)
* Exothermic: Heat release (-)
* Use enthalpy to fin the ΔH of variables
83
New cards
Hess’s Law
Enthalpu is the function of the state of matter
\
If desired reaction is multiple steps, show the enthalpy for each step
\
If desired reaction is multiple steps, show the enthalpy for each step
84
New cards
Solving Hess’s Law equations
* Undesired variables: Put on opposite sides
* Flip: Products to reactants and vice versa; change sign of given ethaply
* Desired Variables: On desired side with desired quantity
* Multiply the whole equation by factor to get desired quantity; given enthaply is multiplied by same factor
* Add up your ending enthalpies to find final enthalpu
* Flip: Products to reactants and vice versa; change sign of given ethaply
* Desired Variables: On desired side with desired quantity
* Multiply the whole equation by factor to get desired quantity; given enthaply is multiplied by same factor
* Add up your ending enthalpies to find final enthalpu
85
New cards
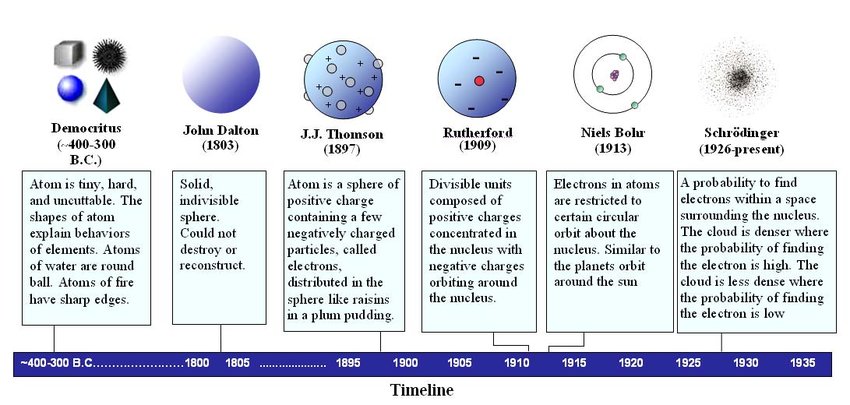
The History of Quantum Mechanics
* Democritus: 400 BC - Proposed the “atom”
* Dalton: 1803 - Atomic Theory
* Small Particles
* Indivisible
* Unique
* Comine and Separate
* Golstein: 1886 - “Anoderays” lead to the discovery of the Proton
* Thomson: 1897 - “Cathoderays” lead to the discovery of the electrom
* Plum Pudding Model
* Rutherford: 1911 - Discovers te nucleus during the Gold Foil Experiement
* Dalton: 1803 - Atomic Theory
* Small Particles
* Indivisible
* Unique
* Comine and Separate
* Golstein: 1886 - “Anoderays” lead to the discovery of the Proton
* Thomson: 1897 - “Cathoderays” lead to the discovery of the electrom
* Plum Pudding Model
* Rutherford: 1911 - Discovers te nucleus during the Gold Foil Experiement
86
New cards
Particles and Waves
Particles: Electons are assumed to be particles
* Particles have mass and occupy space
* Very small mass (9.11e-31 kg)
\
Waves have no mass but carry energy
* If electons are “massless” can they carry energy?
\
Much knowledge of the electron comes from the study of light
* Particles have mass and occupy space
* Very small mass (9.11e-31 kg)
\
Waves have no mass but carry energy
* If electons are “massless” can they carry energy?
\
Much knowledge of the electron comes from the study of light
87
New cards
Light
* Colors: Absorbed and Reflected
* Fast, no mass, constant motion
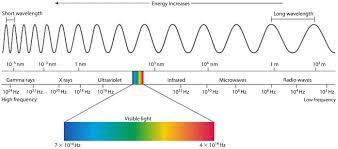
* Electromagnetic Spectrum: R. O. Y. G. B. I. V.
* Fast, no mass, constant motion
* Electromagnetic Spectrum: R. O. Y. G. B. I. V.
88
New cards
Photon
Quantum of light carrying energy proportional to radiation frequency; NO MASS
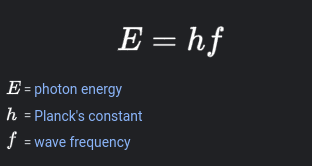
89
New cards
Frequency
* 𝜈: (nu); represents frequency
* Number of waves travelling every second
* Hertz: Measurement of Frequency
* 1 Hz = 1 wave per second
* kHx (kilohertz) and MHz (megahertz)
\
INVERSE OF WAVELENGTH
* Number of waves travelling every second
* Hertz: Measurement of Frequency
* 1 Hz = 1 wave per second
* kHx (kilohertz) and MHz (megahertz)
\
INVERSE OF WAVELENGTH
90
New cards
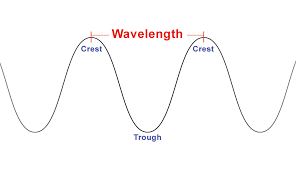
Wavelength
λ: (lambda); symbol of wavelength
* Distance between 2 consecutive crest/troughs of a wave
\
Monometers: Measurement of a wavelength
\
INVERSE OF FREQUENCY
* Distance between 2 consecutive crest/troughs of a wave
\
Monometers: Measurement of a wavelength
\
INVERSE OF FREQUENCY
91
New cards
Speed of light
* c: Speed of Light
* All light travels at same speed
* 3.00e8 m/s
* Sp = λ • 𝜈
* All light travels at same speed
* 3.00e8 m/s
* Sp = λ • 𝜈
92
New cards
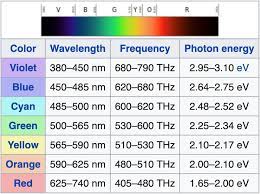
Wave - Particle of Light
Different color = different wavelength = different frequency = different energy
* Energ of light travels in “packets” or “quanta” which cna be measure by function of frequency
\
E = h𝜈
* E = Energy
* H = Planck’s Constant: 6.626e035 j/sc
* Energ of light travels in “packets” or “quanta” which cna be measure by function of frequency
\
E = h𝜈
* E = Energy
* H = Planck’s Constant: 6.626e035 j/sc
93
New cards
Color: Photon Energy
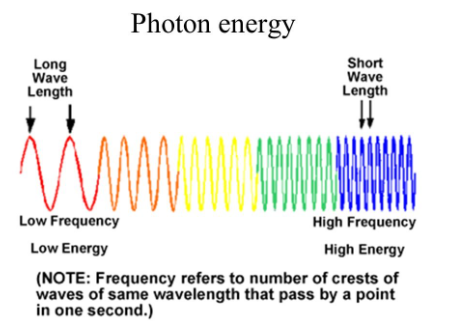
94
New cards
Emission Spectra
* A spectroscope separates the light into bands (lines) of specific wavelengths
* light given off = emission spectrum
* light energy gained = absorption spectrum
* Each element has a unique spectrum which can be used to identify it
* light given off = emission spectrum
* light energy gained = absorption spectrum
* Each element has a unique spectrum which can be used to identify it
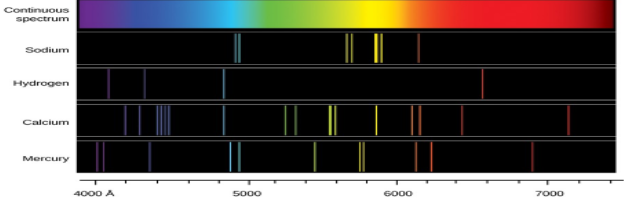
95
New cards
Excitement
* When atom gains energy, electron “jumps” to a higher energy level
* Also called quantum level
* As the electron “drops” to a lower energy level, the energy is released in the form of light
* Also called quantum level
* As the electron “drops” to a lower energy level, the energy is released in the form of light
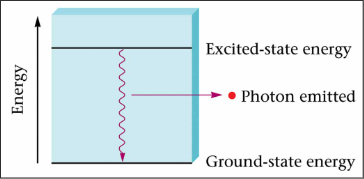
96
New cards
Atomic Spectra
* Atoms may gain extra energy - become excited - and they release that energy in the form of light
* EX: Luminol, Glow Sticks
* EX: Luminol, Glow Sticks
97
New cards
Quantum Theory
* Atoms emit or absorb only radiation with certain specific frequencies.
* Energy is directly proportional to frequency
* So only certain energy states/levels of the electrons are possible
* “Quantized”
* Energy is directly proportional to frequency
* So only certain energy states/levels of the electrons are possible
* “Quantized”
98
New cards
Electromagnetic Radiation and Color
* Compounds absorb light when the difference in energy levels corresponds to a wavelength that is visible to the eye.
* When some ionic compounds are heated, the heat of the flame provides enough energy to excite an electron to a higher level.
* Then the light is emitted when the electron falls back.
* When some ionic compounds are heated, the heat of the flame provides enough energy to excite an electron to a higher level.
* Then the light is emitted when the electron falls back.
99
New cards
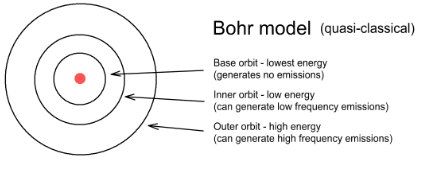
Bohr Model
Electron “orbi” the nucleus like planets orbit the sun
* Held together by force of attraction between opposite forces
\
Energy related to the distance from the nucleus (close = higher attraction)
* Held together by force of attraction between opposite forces
\
Energy related to the distance from the nucleus (close = higher attraction)
100
New cards
Bohr Model Diagram
1. 2
2. 8
3. 8
4. 18
5. 18
6. 32
7. 32