Biology Quiz 1 (ch. 1-4)
1/108
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
109 Terms
Biology is defined as
the scientific study of life
1 - biosphere
Life on earth, location found, atmosphere
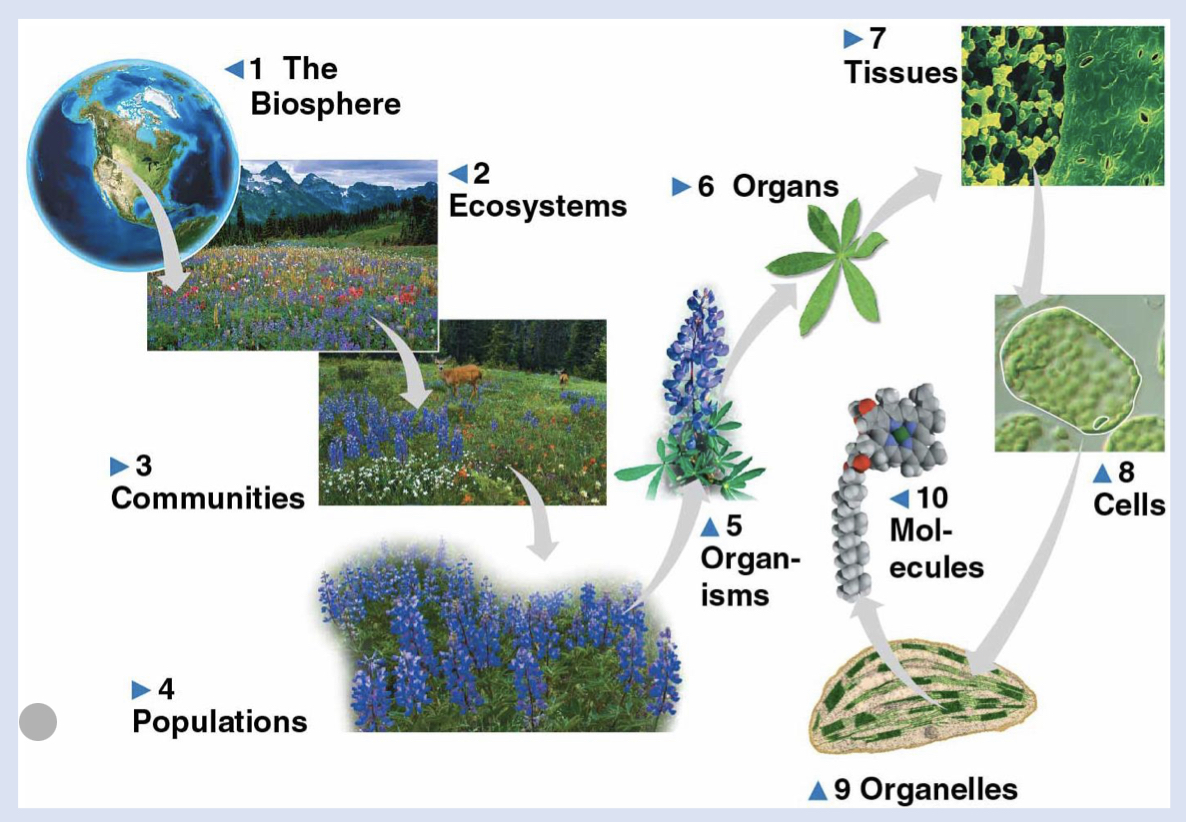
2 - ecosystems
all living things in a particular area: terrestrial…
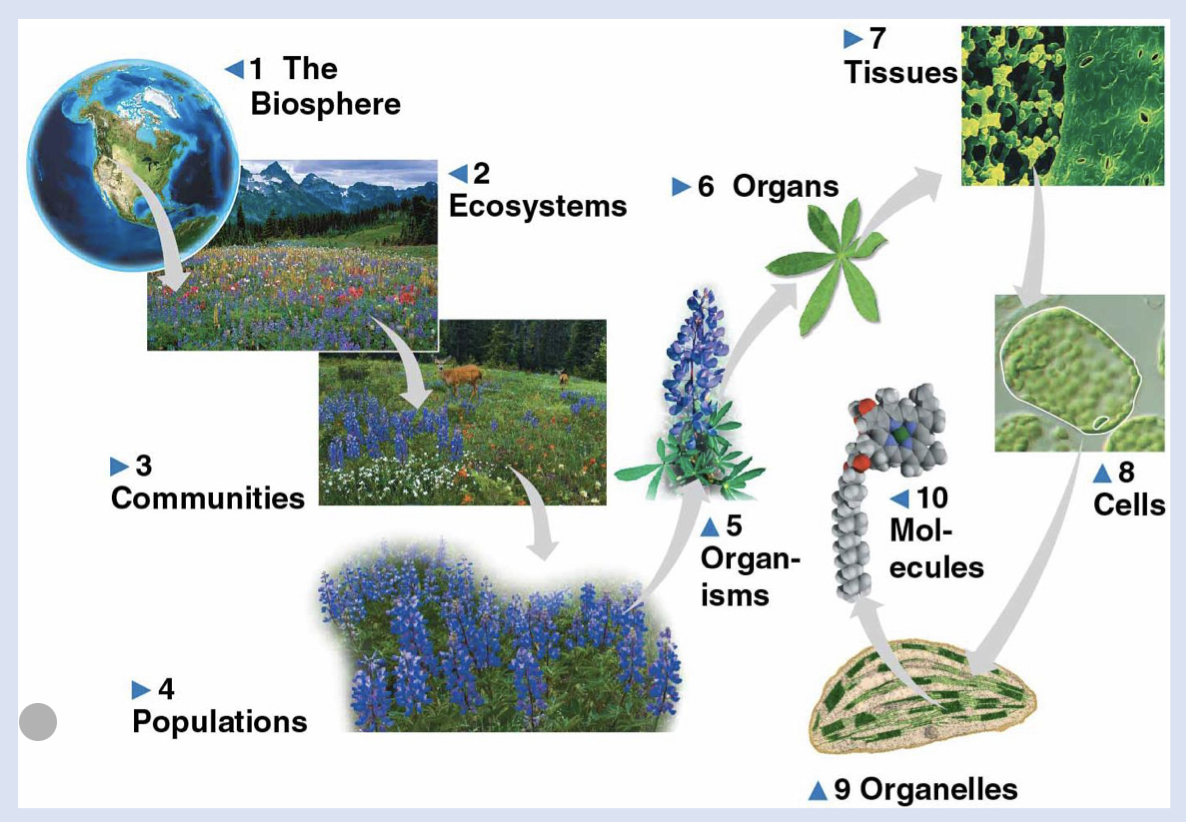
3 - communities
group of organisms in a specific ecosystem
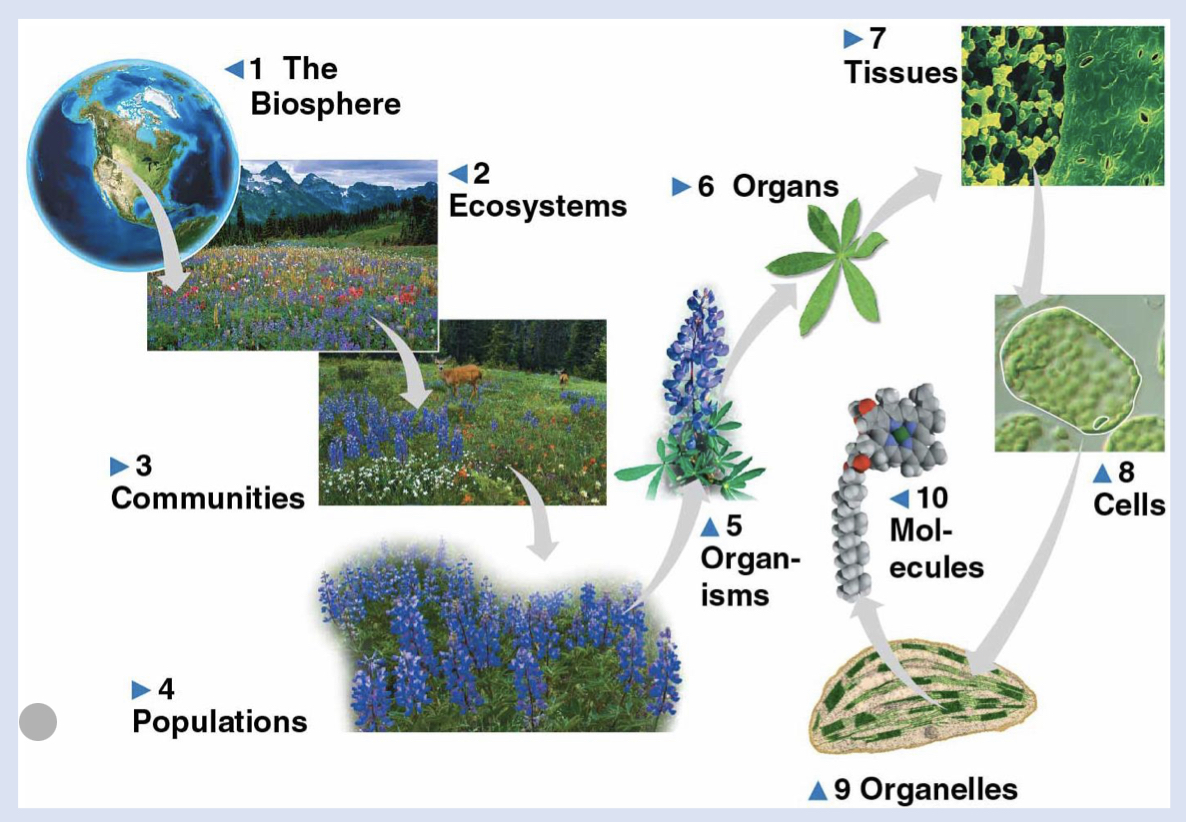
4 - population
individuals of a species living in a specific area
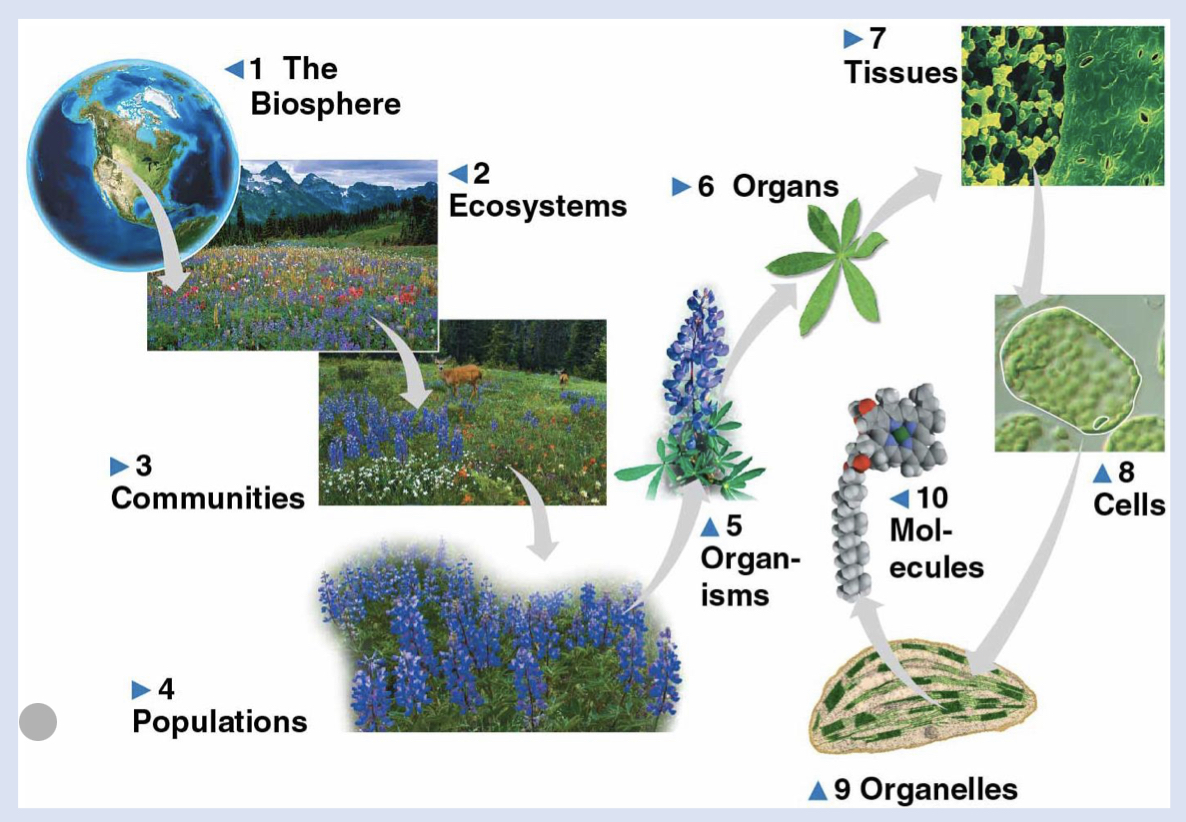
5 - organisms
individual living things
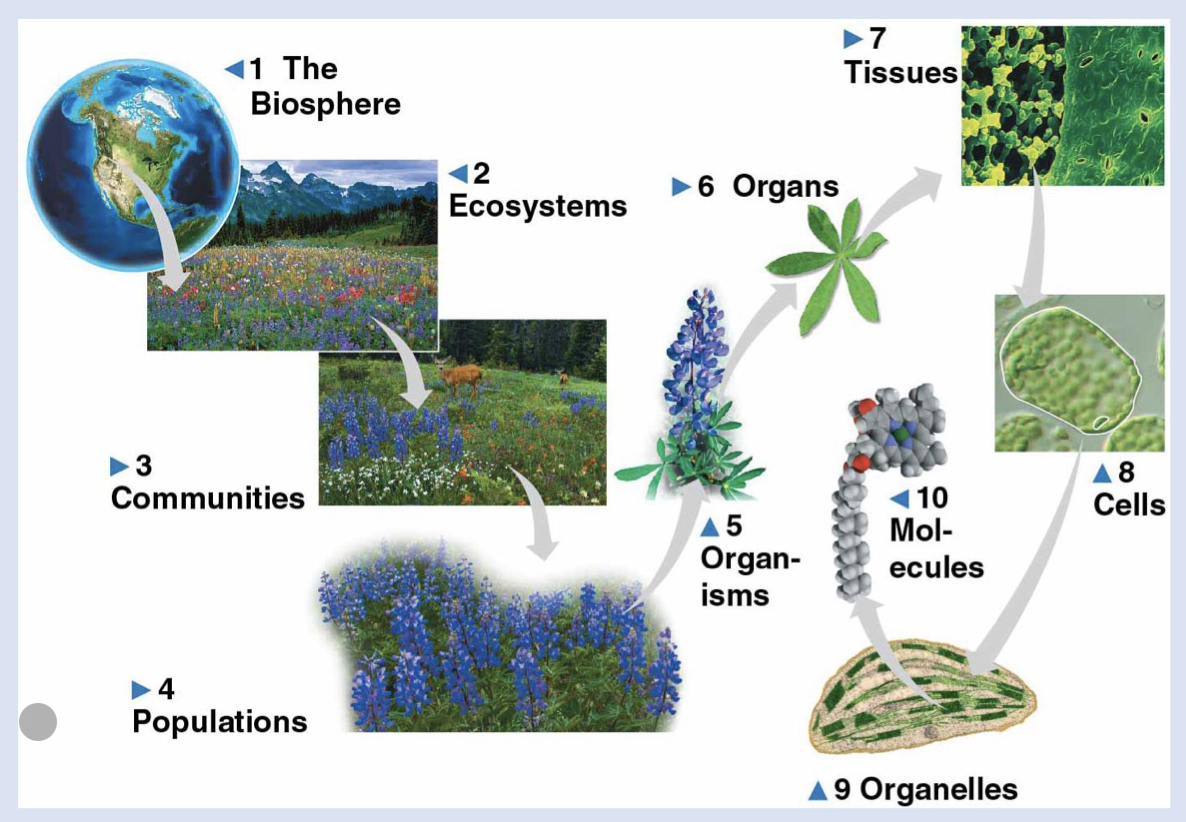
6 - organs
body part made of tissues with specific function
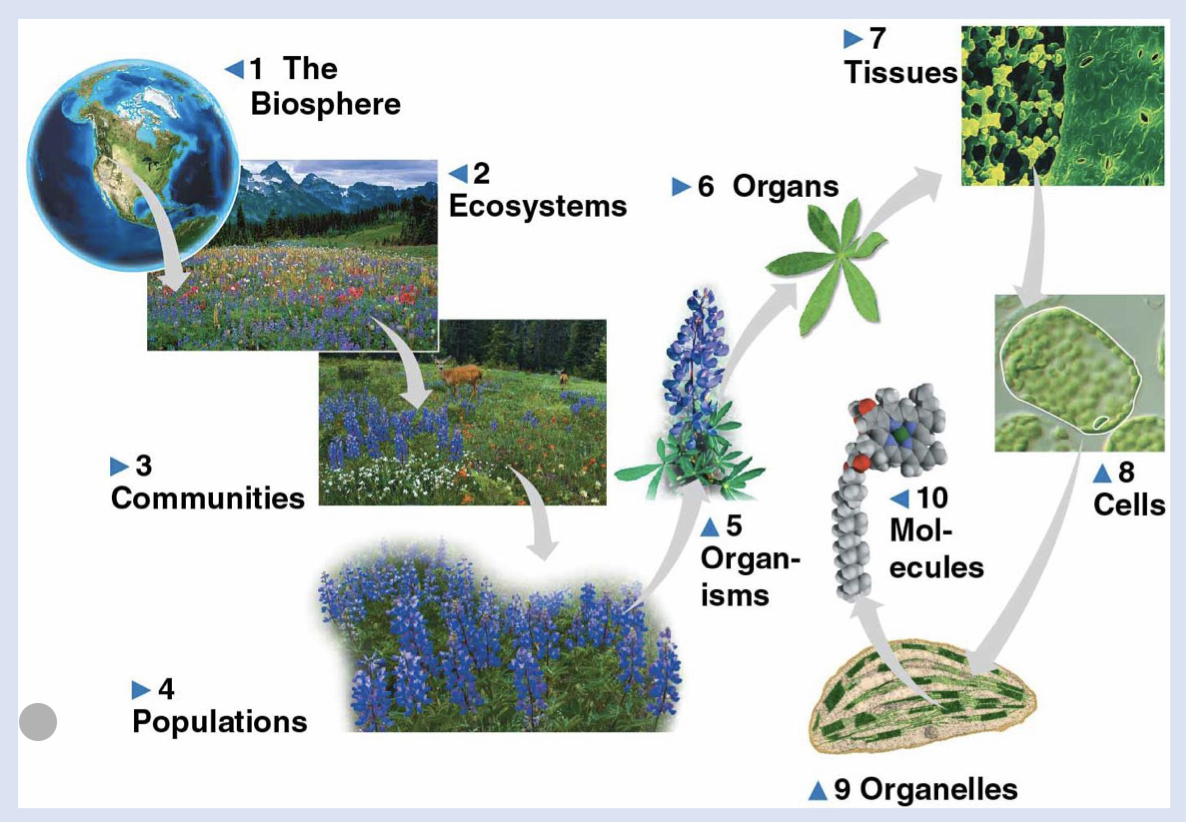
7 - tissues
group of cells that work together
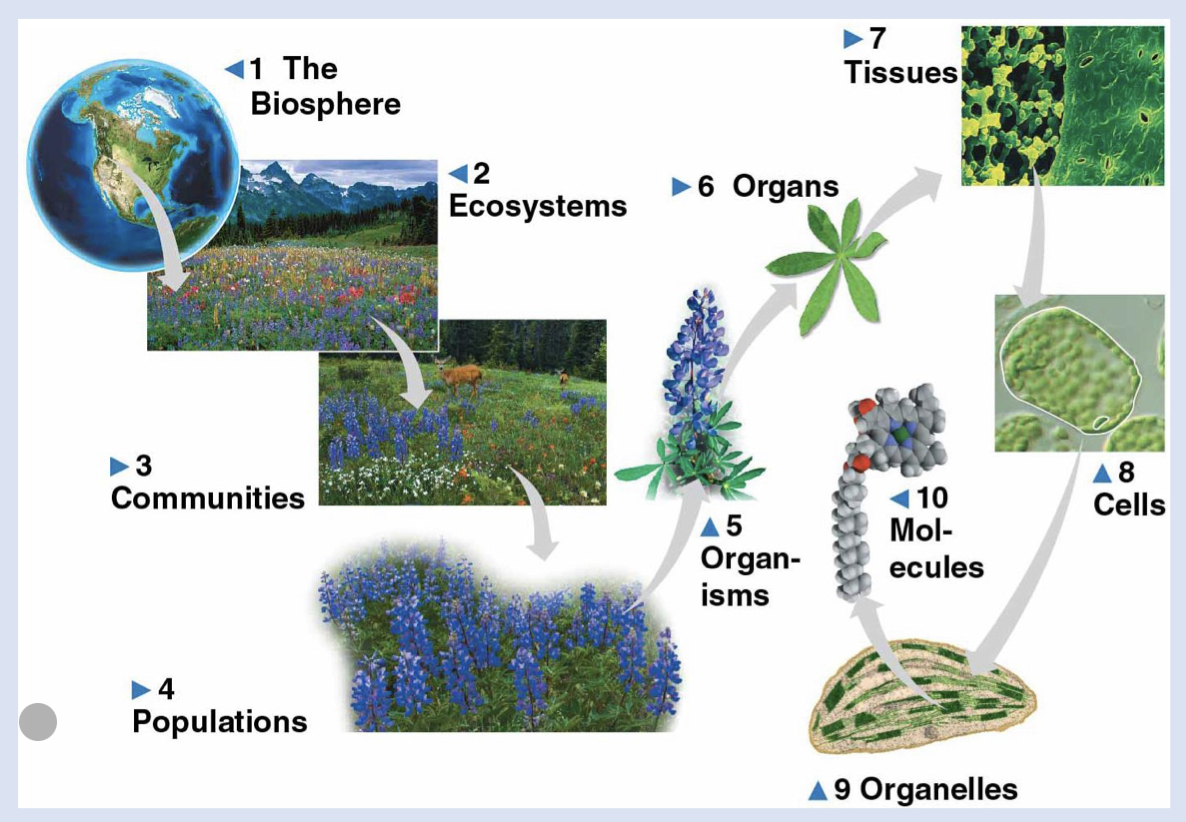
8 - cells
life’s fundamental unit of structure and function
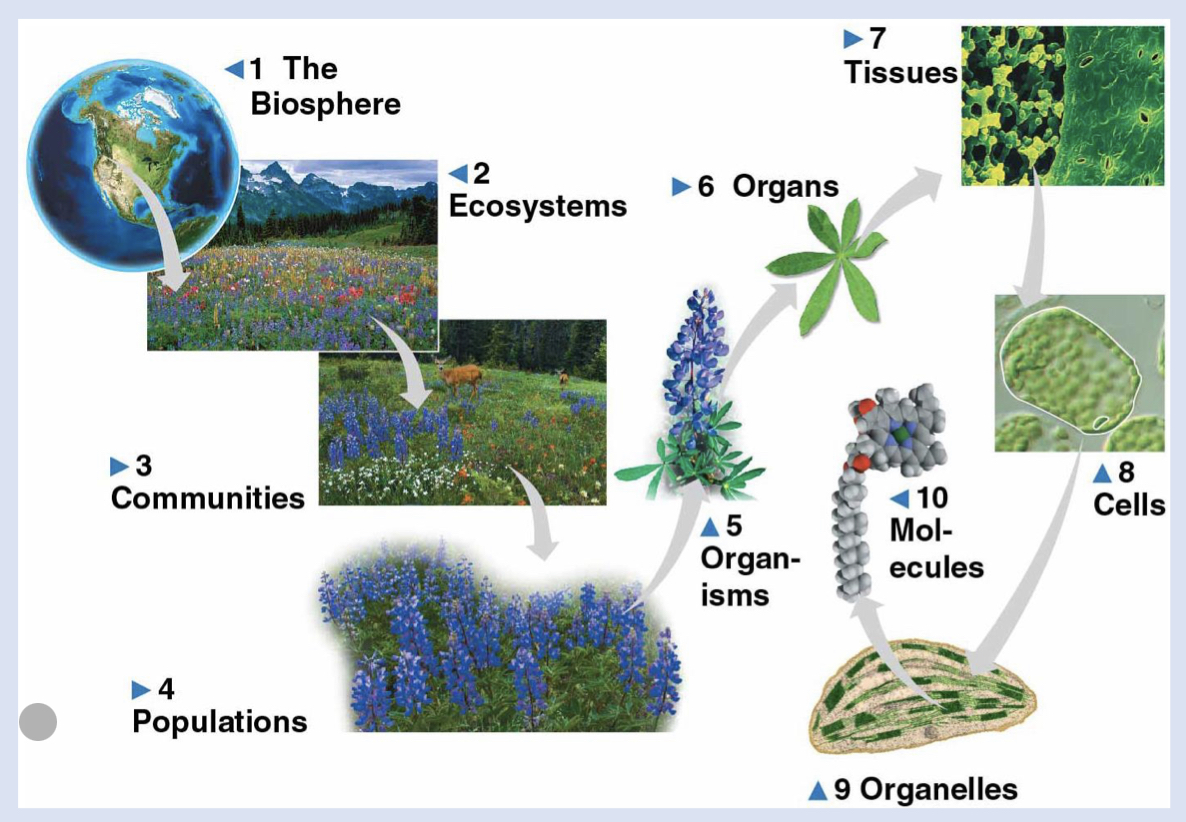
9 - organelles
functional components of a cell
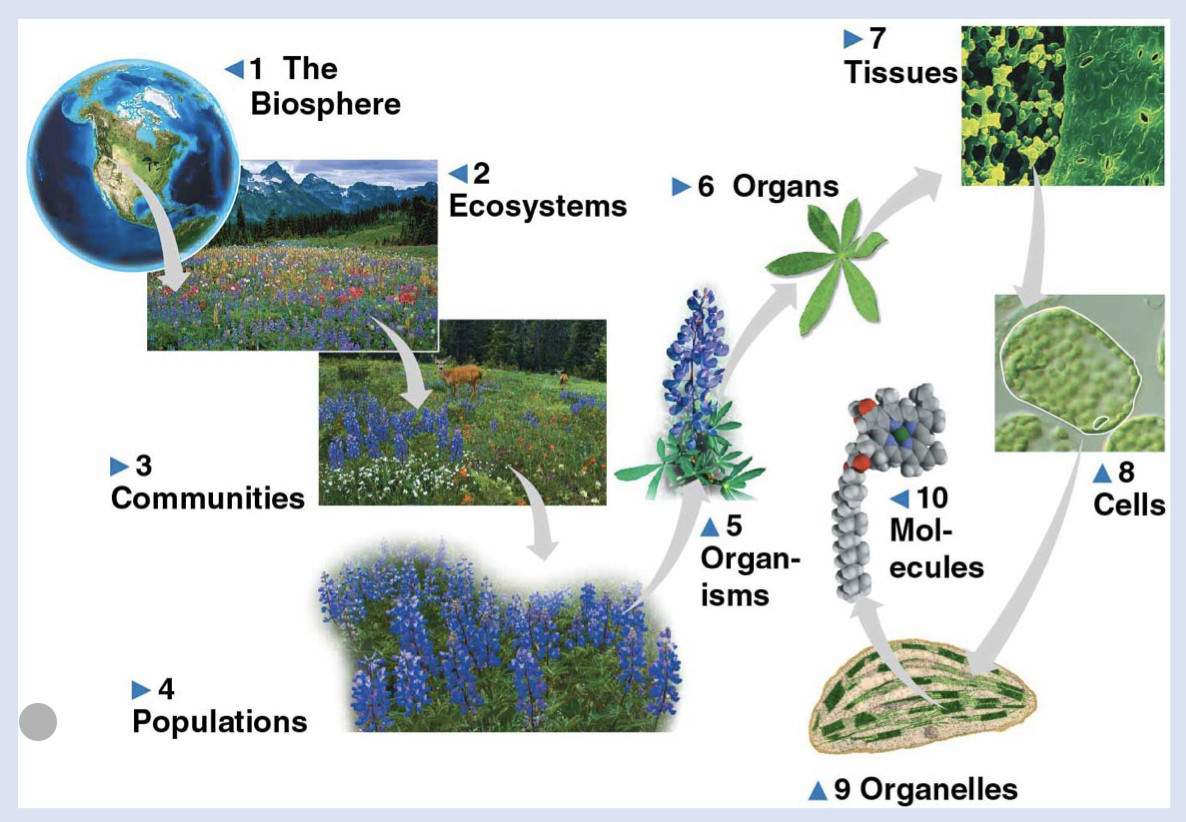
10 - molecules
chemical structure
correct order of the levels of biological organization from smallest to largest:
a. cells, organelles, organ system, community, ecosystems
b. molecules, organism, population, communities, biosphere
c. molecules, cells, tissues, ecosystems, communities
d. organelles, cells, population, biosphere, ecosystems
e. cells, organs, population, ecosystems, communities
e. cells, organs, population, ecosystems, communities
3 domains of life
archaea: consists of prokaryotic organisms (lacks nucleus) that live in many different environments
bacteria: consists of prokaryotes (lacks nucleus)
eukarya: consists of organisms that have cells with true nuclei (includes 4 subgroups: plants, fungi, animals, protists)
inquiry
inquiry is the search for information and explanations of natural phenomena
how we define hypothesis
a hypothesis is a tentative answer to a well- framed scientific question
2 important qualities of a hypothesis
testable and falsifiable
controlled experiment
compares an experimental group with a control group
types of data you can collect
qualitative data (descriptive data)
quantitative data (numbers)
deductive vs inductive reasoning
deductive reasoning uses general premises to make specific predictions (general to specific)
inductive reasoning states repeating specific observations can lead to important generalizations (specific to general)
experimental group vs control group
experimental group: group that receives the specific treatment or the variable being tested
control group: untreated group
dependent vs independent variables
independent: variable you are testing
dependent: data you get
Explain what is meant by a scientific theory by giving the three ways your text distinguishes a theory from a hypothesis or mere speculation
A scientific theory is an explanation that is broader in scope than a hypothesis, generates new hypotheses, and is supported by a large body of evidence
how are covalent bonds formed? give an example of a covalent bond
Covalent bonds are formed when two atoms share a pair of valence electrons. An example of a covalent bond is found in H2O, as oxygen shares two valence electrons with both hydrogens
what is the difference between Polar and Non Polar Covalent Bond with examples?
A non-polar covalent bond is when there is equal sharing of electrons
A polar covalent bond is when there is unequal sharing of electrons
An example of a non-polar covalent bond would be the single bond of H2 or the double bond of O2
An example of a polar covalent bond would be the bonds between the hydrogen and oxygen in H2O
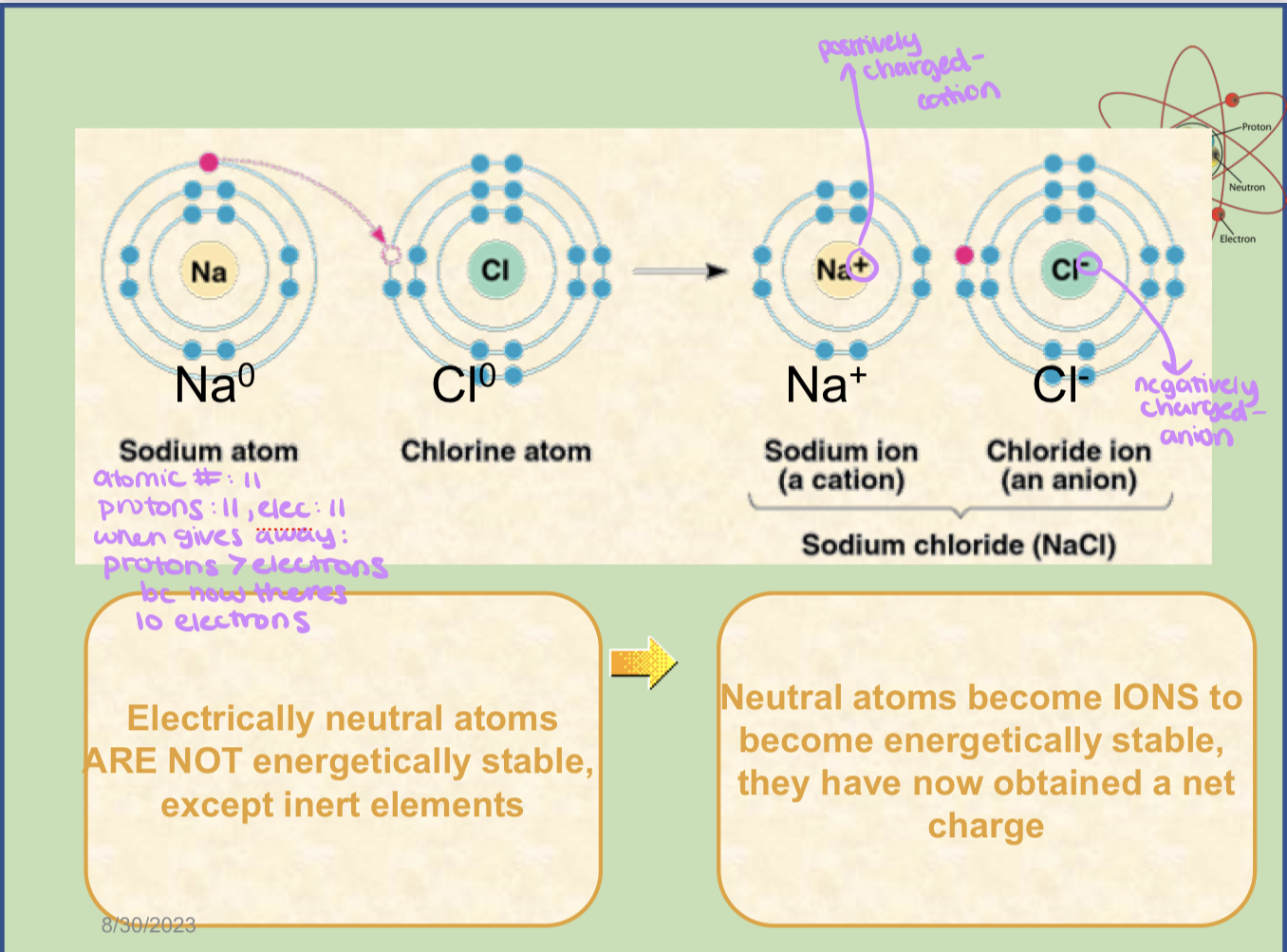
what is an Ionic bond? + example
an ionic bond is a bond that consists of transferring electrons that results in the atoms gaining charges
an example of an ionic bond would be a sodium atom giving an electron to a chlorine atom. After the exchange, the sodium ion would become a cation (positively charged) and the chloride ion would become an anion (negatively charged)
cation vs anion
A cation is a positively charged ion, while an anion is a negatively charged ion
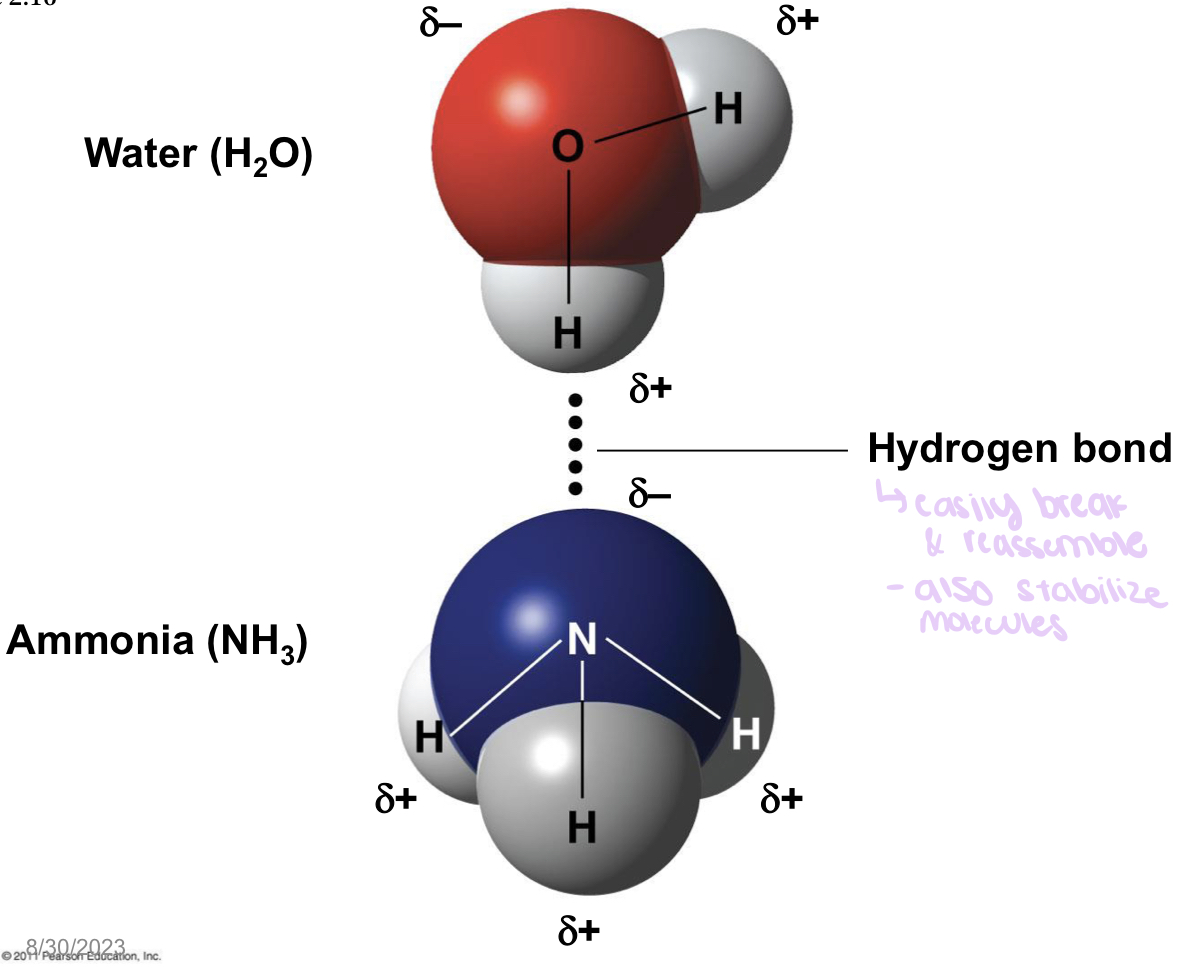
what is a Hydrogen Bond?
A hydrogen bond forms when a hydrogen ion covalently bonded to an electronegative atom has a slight positive charge and in result is attracted to another nearby electronegative atom
What is an electronegative element? Find examples, how does this help in forming Hydrogen Bond
An electronegative element is an element that attracts electrons in a covalent bond. Elements with high electro negativity include oxygen, nitrogen, and neon. This helps in forming hydrogen bonds because hydrogen ions that are already attached to an electronegative atom are attracted to other nearby electronegative atoms, which is what forms hydrogen bonds in the first place.
matter is made up of __
elements
compounds
different elements coming together
molecule
elements of the same kind in a group
96% of living matter is made up of these four elements: ___; the remaining 4% are these others: ___
Hydrogen (H), Carbon (C), Nitrogen (N), Oxygen (O); Potassium (K), Calcium (Ca), Phosphorous (P), Sulfur (S)
trace elements
elements required in small amounts
subatomic particles of an element
neutrons (neutral)
protons (positive)
electrons (negative)
an elements atomic number is equal to
the number of protons
the number of protons is equal to
the number of electrons
the mass number is the sum of
protons and neutrons
atomic mass can be approximated by
the mass number
how to find number of neutrons
by subtracting the atomic number from the atomic mass
isotopes
all atoms of an element have same number of protons but may differ in number of neutrons (so atomic mass varies)
isotopes are two atoms of an element that differ in number of neutrons
radioactive isotopes decay spontaneously, giving off particles and energy, and become diff element altogether
the more shells of electrons an atom has, the more ___ they have
energy
covalent bonds
strong
the sharing of a pair of valence electrons bony two atoms
can form between atoms of same or different element(s)
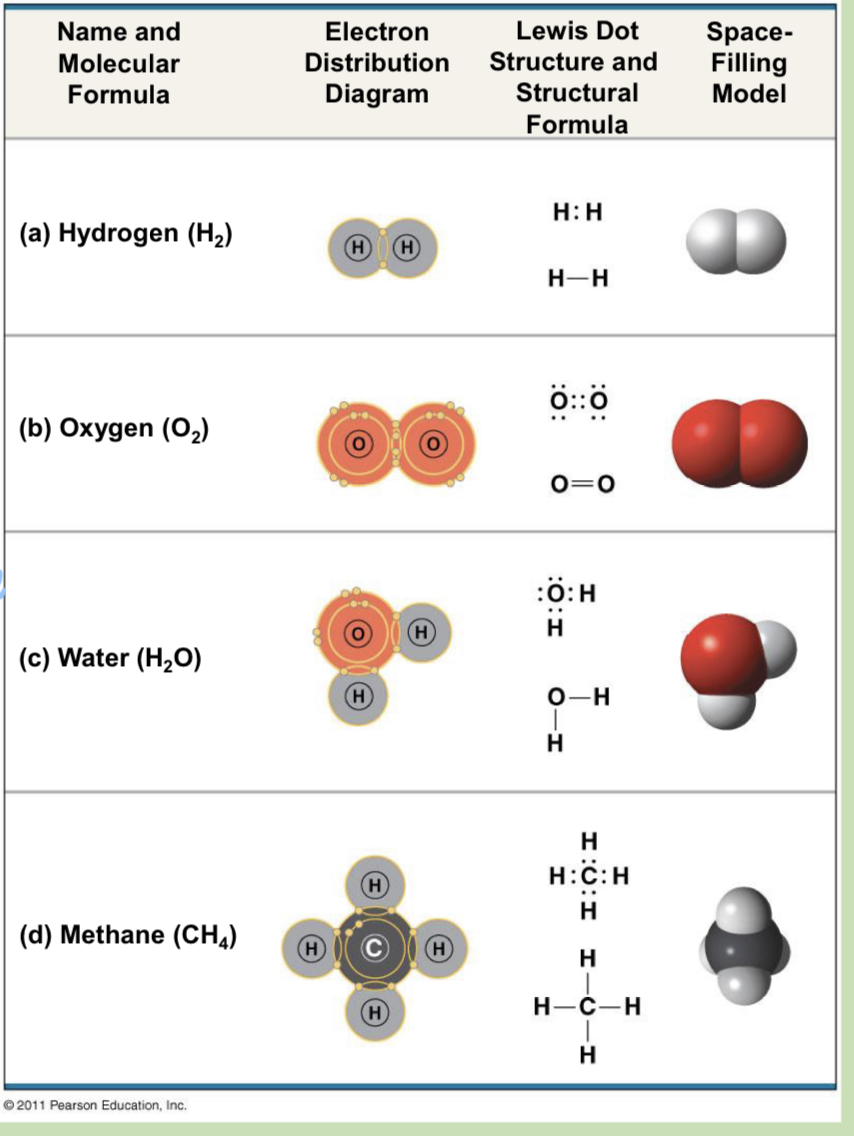
bonding capacity in covalent bonds:
atom’s valence
notation used to represent atoms and bonding is called a ___ formula, or can be further abbreviated with a _____ formula
structural formula (ex: H—H); molecular formula
nonpolar covalent bond vs polar covalent bond
nonpolar: equal sharing of electrons; polar: unequal sharing of electrons (when one atom is more electronegative and the atoms do not share the electrons equally)
unequal sharing of electrons causes a partial positive or negative charge for each atom or molecule
water is called a ___ molecule. why?
polar; because it has polar covalent bonds (O is electronegative and so H has + charge and O has - charge)
partial charge
partial charged occur when an H atom is covalently bonded to a very electronegative atom
H bonds occur between two molecules with ___ charges
partial
electronegativity
an atoms attraction fore the electrons in a covalent bond; the more electronegative an atom, the more strongly it pulls shared electrons toward itself
ionic bonds
can be weak or strong - depends on environment (typically more weak)
transfers electrons - does not share
ex: transfer of an electron from sodium to chlorine
after the transfer of an electron both atoms have charges - ions
hydrogen bonds
form when a hydrogen atom covalently bonded to one electronegative atom is also attracted to another electronegative atom
van der waals interactions
if electrons are distributed asymmetrically in mol/atoms, “hot spots” form of positive and negative charge (congregation in one area increases attraction)
attractions between mols that are close together as a result of these charges
interactions can be strong
seen in proteins
ex: gecko walking on wall

why is water considered a polar molecule?
because electrons are shared unequally between the oxygen and hydrogen atoms
explain how hydrogen bonding contributes to water’s high specific heat
hydrogen bonding contributes to waters high specific heat because water molecules can move freely when hydrogen bonds beak due to heat being absorbed
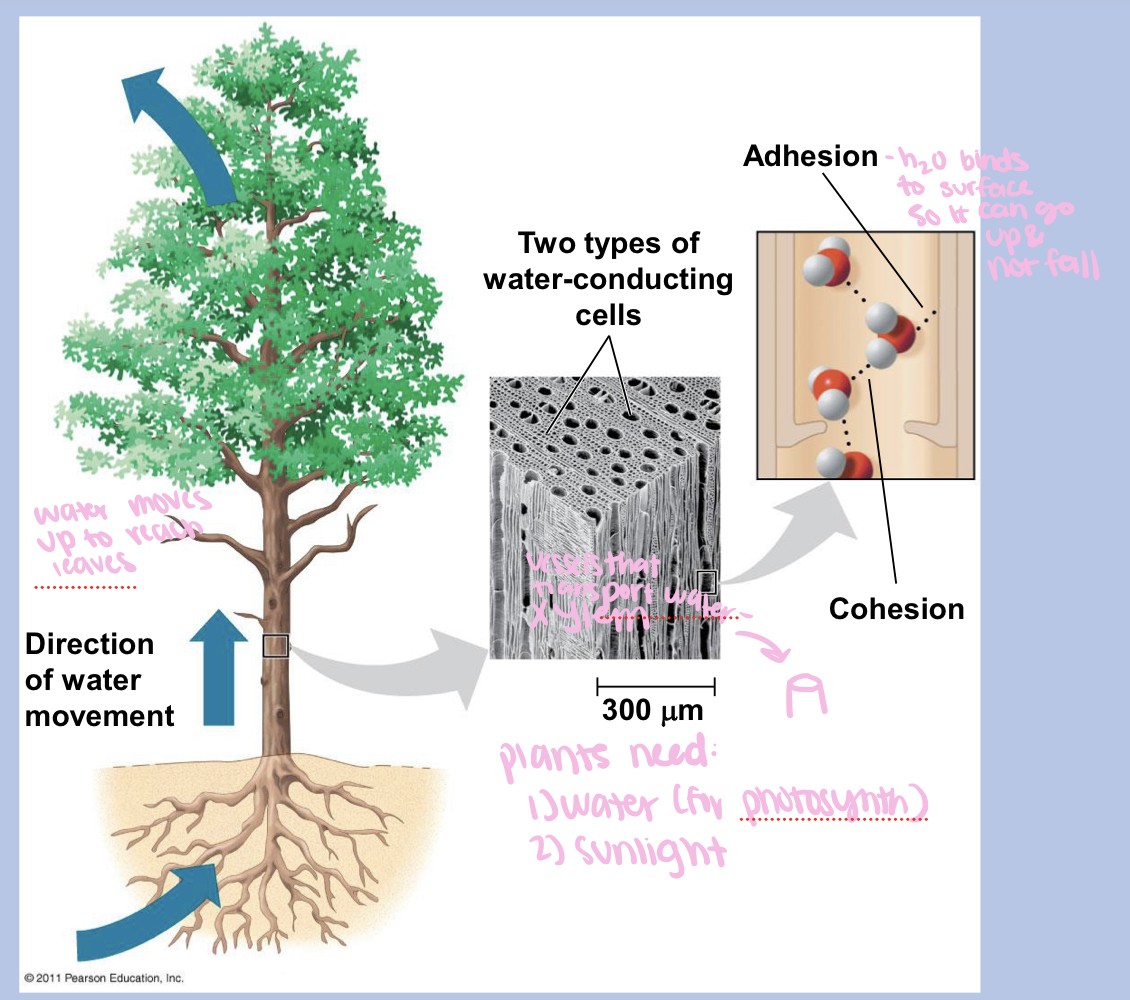
cohesion vs adhesion
cohesion is a phenomenon in which hydrogen bonds hold the molecules within water closely together
adhesion is the attraction that exists between two diff substances
solvent
dissolves something within a solution (ex: water)
solution
homogenous mixture of substances (liquid)
solute
is what dissolves in a solution
aqueous solution
solution that has water serving as the solvent
hydrophobic vs hydrophilic substances + examples
A hydrophobic substance does not have an affinity for water (does not dissolve in water), while a hydrophilic substance has an affinity for water (will dissolve). An example of a hydrophobic substance would be oils bc they have relatively nonpolar bonds. An example of a hydrophilic substance would be sugar.
4 properties of water that are important for life + how they result from hydrogen bonding
cohesive behavior (sticks together) results from hydrogen bonding bc hydrogen bonds hold water molecules together - cohesion helps transport of water against gravity in plans
surface tension is related to cohesion, and is a measure of how hard it is to break the surface of a liquid. water has an unusually high surface tension due to hydrogen bonding between mol at air-water interface and to the water below
moderation of temperature results from hydrogen bonding bc water absorbs heat from warmer air and releases stored heat to cooler air, can absorb or release a large amount of heat with only a slight change in own temp, heat transfer: ice it water or water to ice
waters expansion upon cooling results because hydrogen bonds spread out when water freezes; hydrogen bonds form a pattern called a lattice, which prevents the mols from packing close together - evaporative cooling
waters versatility as a solvent is because of its polarity, which allows it to form hydrogen bonds easily and helps dissolve many compounds
what is the buffering system that minimizes blood pH changes?
bicarbonates - natural buffers that neutralize acidic substances in blood. When any acidic substance enters the bloodstream, the bicarbonate ions neutralize the hydronium ions forming carbonic acid and water. Carbonic acid is already a component of the buffering system of blood. Thus hydronium ions are removed, preventing the pH of blood from becoming acidic.
how do buffers moderate pH change?
most of them work by minimizing the changes in concentrations of H+ and OH-. most of them have an acid-base pair that reversible combines with H+
the factors that allow water to travel up plants to reach leaves
cohesion and adhesion
specific heat definition
the amount of heat that must be absorbed or lost for 1 g of that substance to change its temp by 1 degree celsius
the specific heat of water is 1 cal/g/degrees C
an acid is any substance that increases the __ concentration of a solution
H+
a base is any substance that reduces the __ concentration of a solution
H+
intermolecular and intramolecular bonds
The covalent bonds between the hydrogen and oxygen atoms in a water molecule are called intramolecular bonds
The hydrogen bonds between hydrogen and other oxygen atoms (due to their electronegativity) are called intermolecular bonds
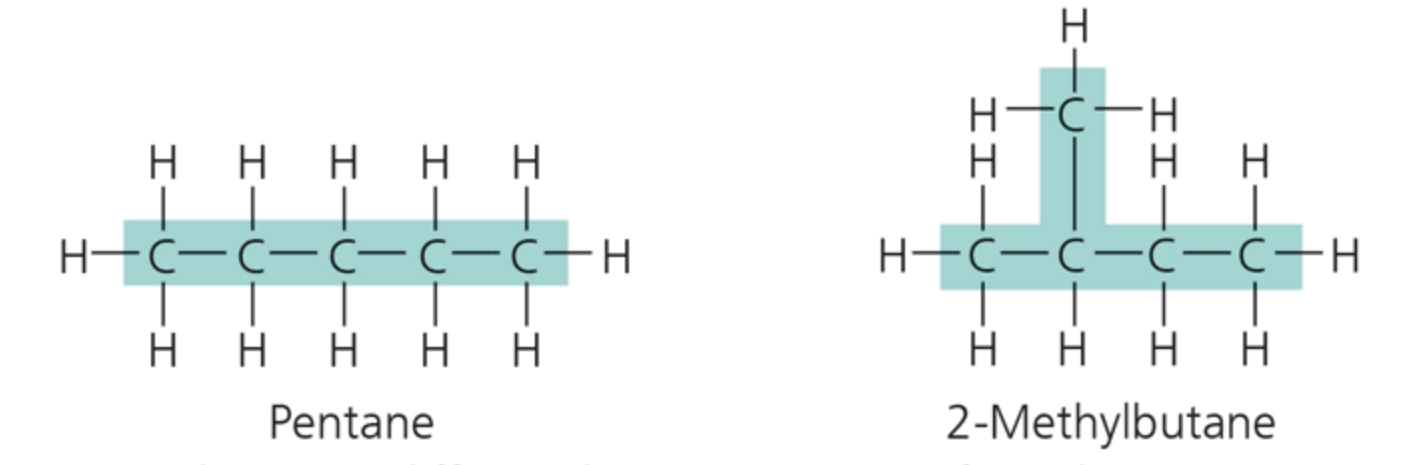
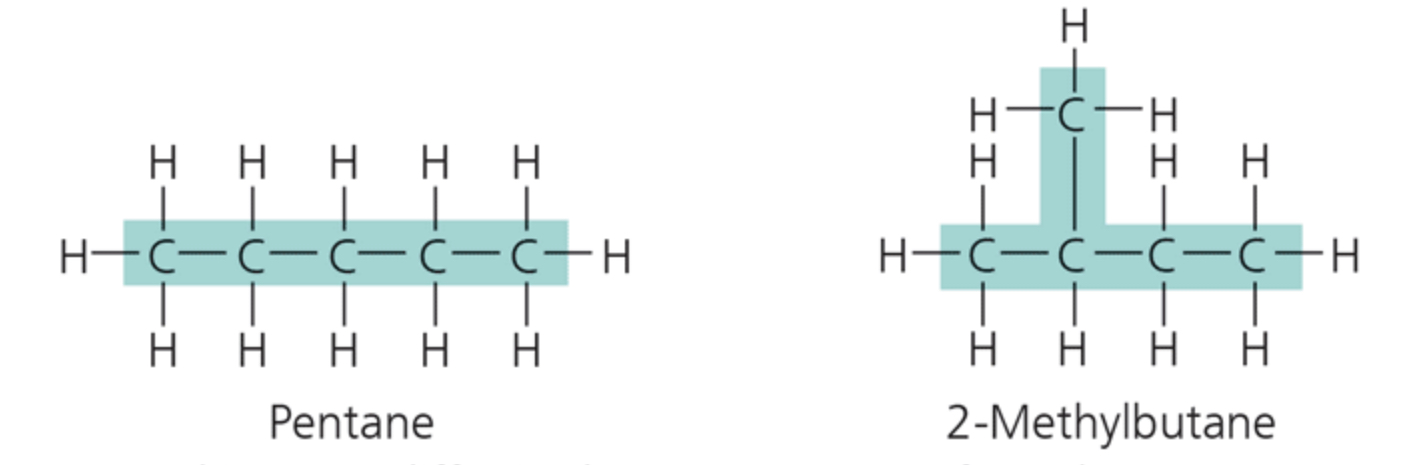
label the isomer
structural isomers
Structural isomers differ in the arrangement of covalent bonding partners, as shown in these two isomers of C§H12.

label the isomer
cis-trans isomers
cis isomer: The two Xs are on the same side
trans isomer: The two Xs are on opposite sides. Cis-trans isomers differ in arrangement about a double bond.
In these diagrams, X represents an atom or group of atoms attached to a double-bonded carbon.

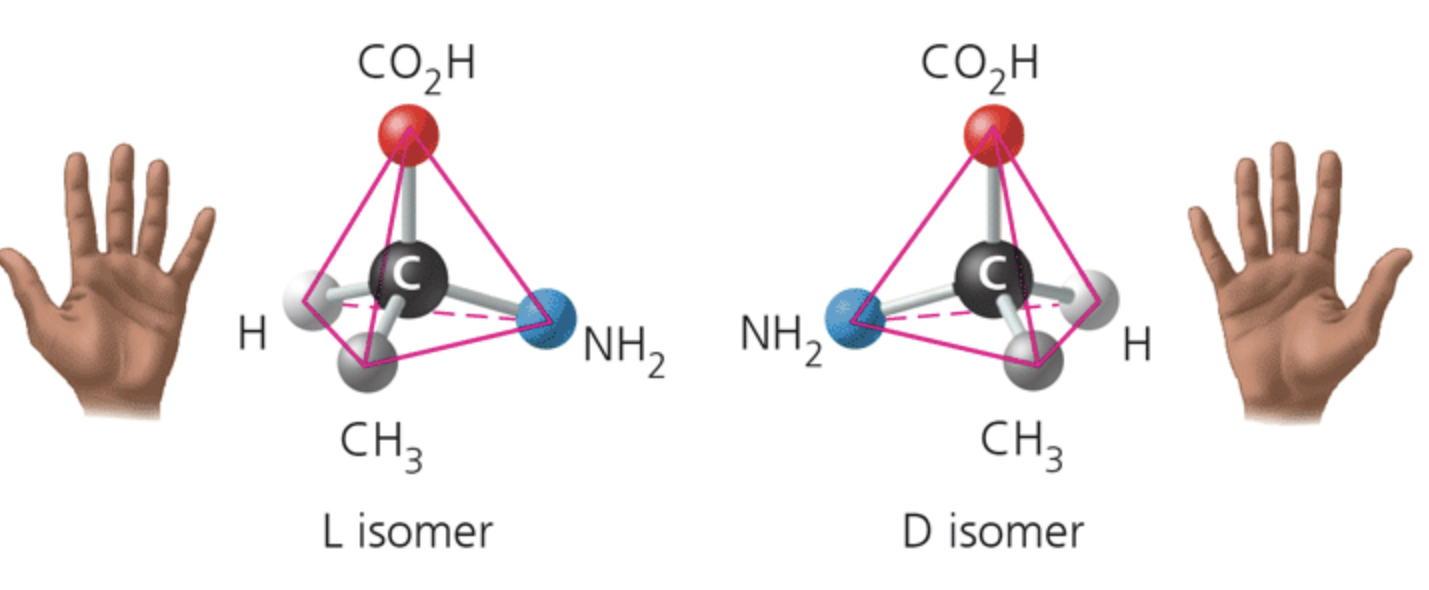
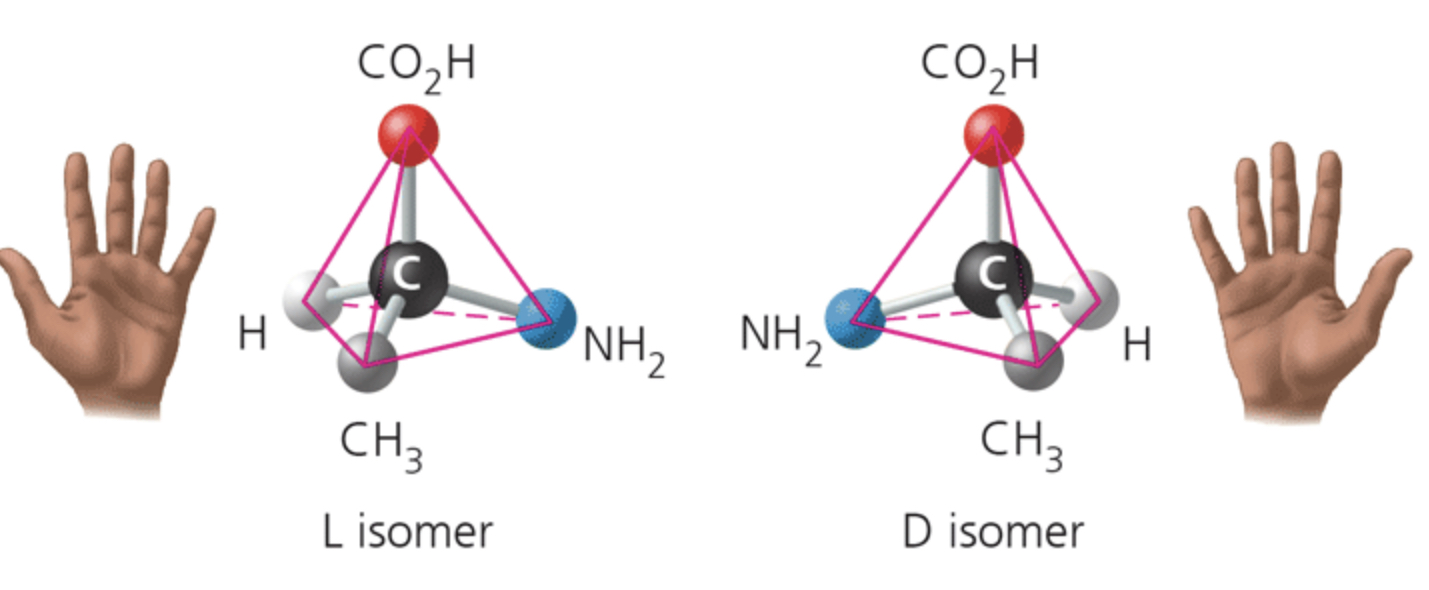
label the isomer
enantiomers
Enantiomers differ in spatial arrangement around an asymmetric carbon, resulting in molecules that are mirror images, like left and right hands. The two isomers here are designated the L and D isomers from the Latin for "left" and "right" (levo and dextro)
Enantiomers cannot be superimposed on each other.
what is a hydrocarbon? name two. are they hydrophobic or hydrophilic?
a hydrocarbon is an organic mol that only consists of carbon and hydrogen
they are hydrophobic
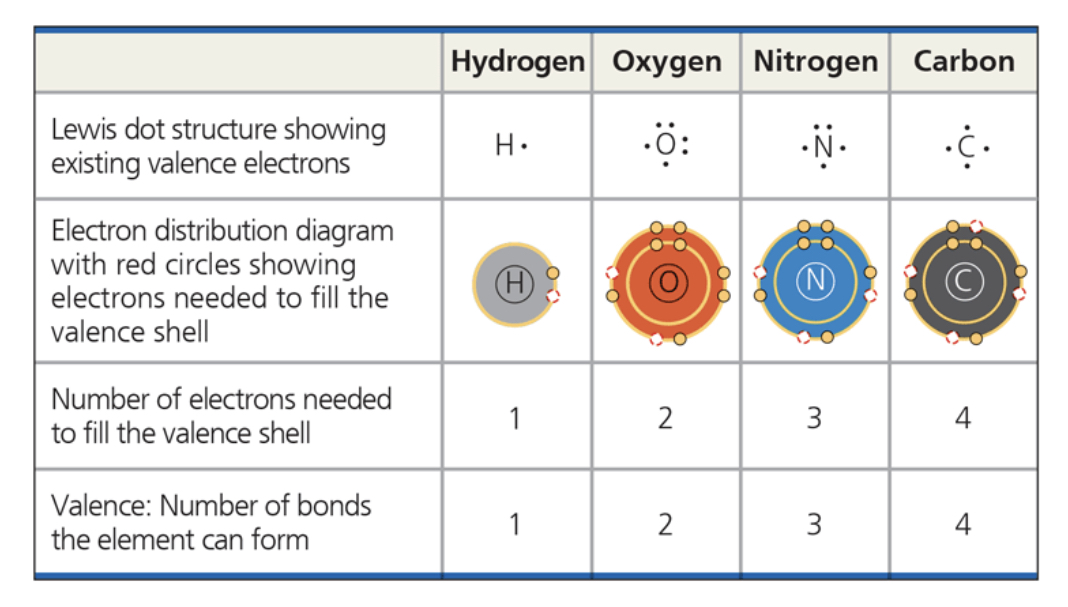
how many valence electrons does carbon have? how many bonds can carbon form? what type of bonds does it form with other elements?
4, 4, covalent bonds
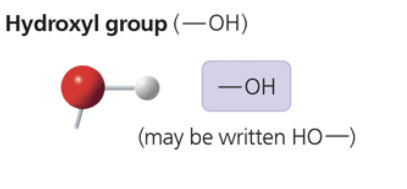
hydroxyl group
—OH (may be written as HO—)
is polar due to electronegative oxygen
forms hydrogen bonds with water, helping dissolve compounds such as sugars
compound name: alcohol (specific name usually ends in -ol)
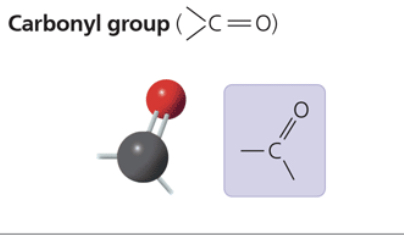
carbonyl group
sugars with ketone groups are called ketones; those with aldehydes are called aldoses
compound name: ketone (carbonyl group is within carbon skeleton) or aldehyde (carbonyl group is at the end of a carbon skeleton)
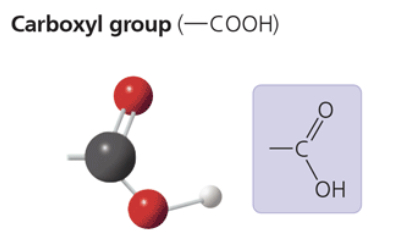
carboxyl group
—COOH
acts as an acid (can donate H+) bc the covalent bond between oxygen and hydrogen is so polar
compound name: carboxylic acid, or organic acid
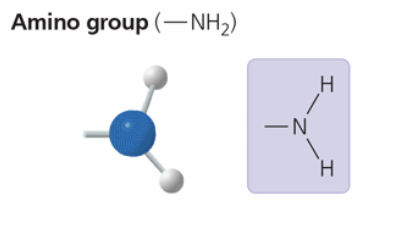
amino group
—NH2
acts as a base; can pick up an H+ from the surrounding solution (water, in living organisms)
compound name: amine
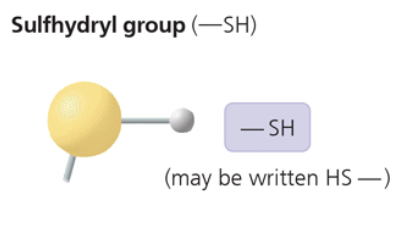
sulfhydryl group
—SH (may be written as HS—)
two —SH groups can react, forming a “cross-link” that helps stabilize protein structure
hair protein cross-links maintain straightness/curling’s of hair
compound name: thiol
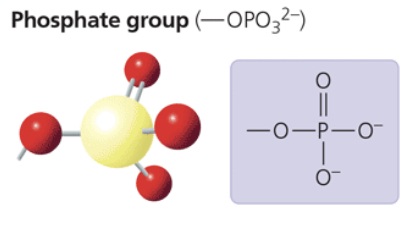
phosphate group
—OPO32-
contributes negative charge (1 - when positioned inside a chain of phosphates; 2 - when at the end)
when attacked, confers on a mol the ability to react with water, releasing energy
compound name: organic phosphate
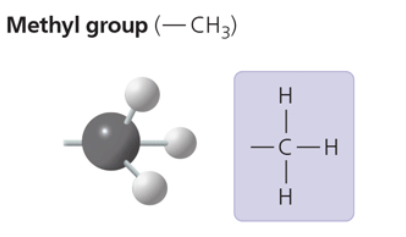
methyl group
—CH3
affects the expression of genes when bonded to DNA or to proteins that bind to DNA
affects shape and function of male and female sex hormones
compound name: methylated compound
—NH2
amino group
can form covalent cross-links that stabilize protein structure
sulfhydryl group
key component of ATP
phosphate group
can affect gene expression
methyl group
—CH3
methyl group
chem group that is always polar
hydroxyl group
determines two groups of sugars
carbonyl group
has acidic properties
carboxyl group
COOH
carboxyl group
acts as base
amino group
electron distribution diagram of carbon
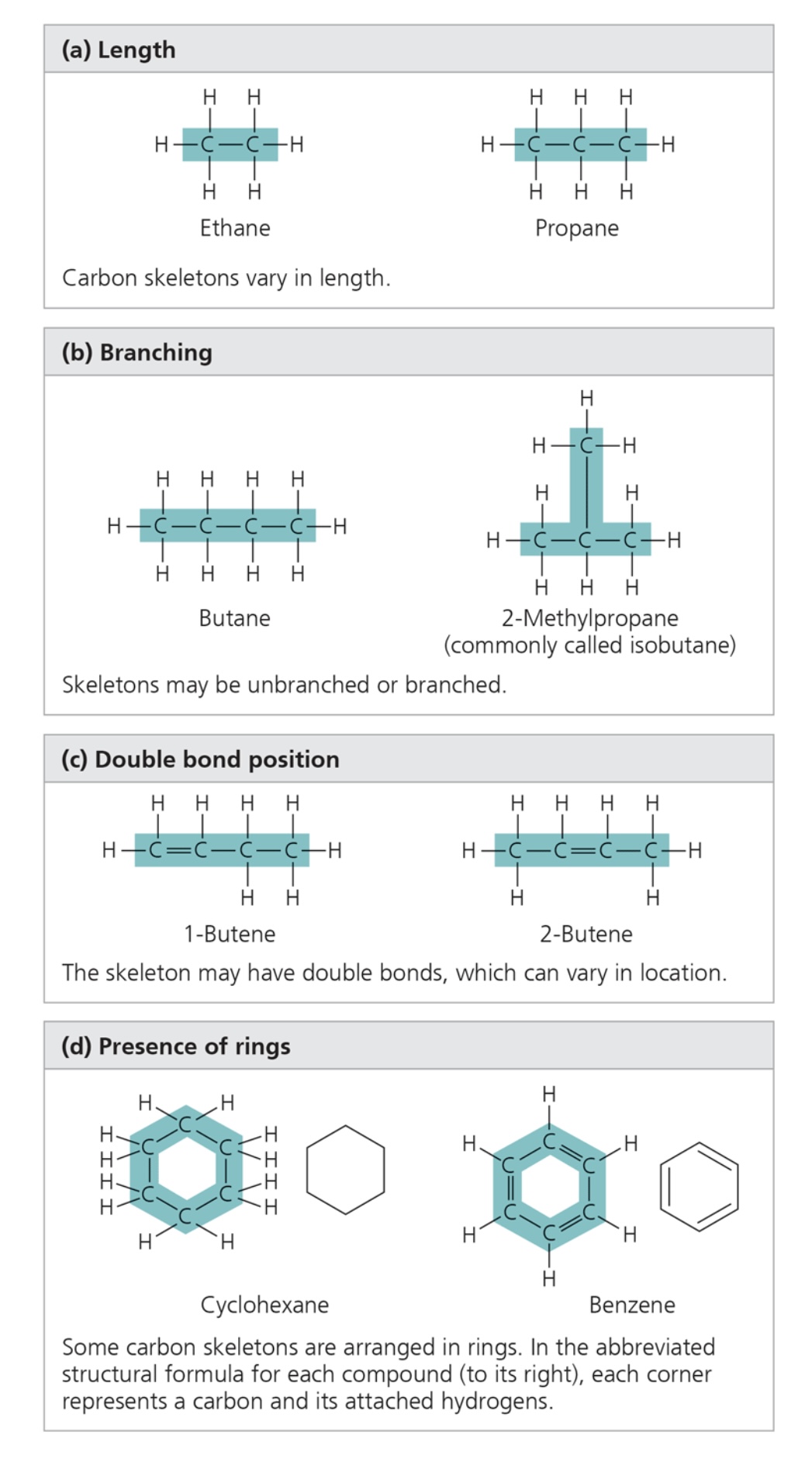
4 ways carbon skeletons can vary
length, branching, double bond position, presence of rings
some carbon chains have double bonds, which can vary in number and location
organic compounds
compounds containing carbon
organic chemistry
the study of organic compounds (compounds containing carbon)
what makes carbon special?
it has the ability to form 4 bonds, meaning it can be used to build an inexhaustible variety of organic mol
different species and different organisms are distinguished by variations in types of org mol they make
the key to an atoms chemical characteristics is ____ ____
electron configuration
determines kinds/#s of bonds an atom forms w/ others
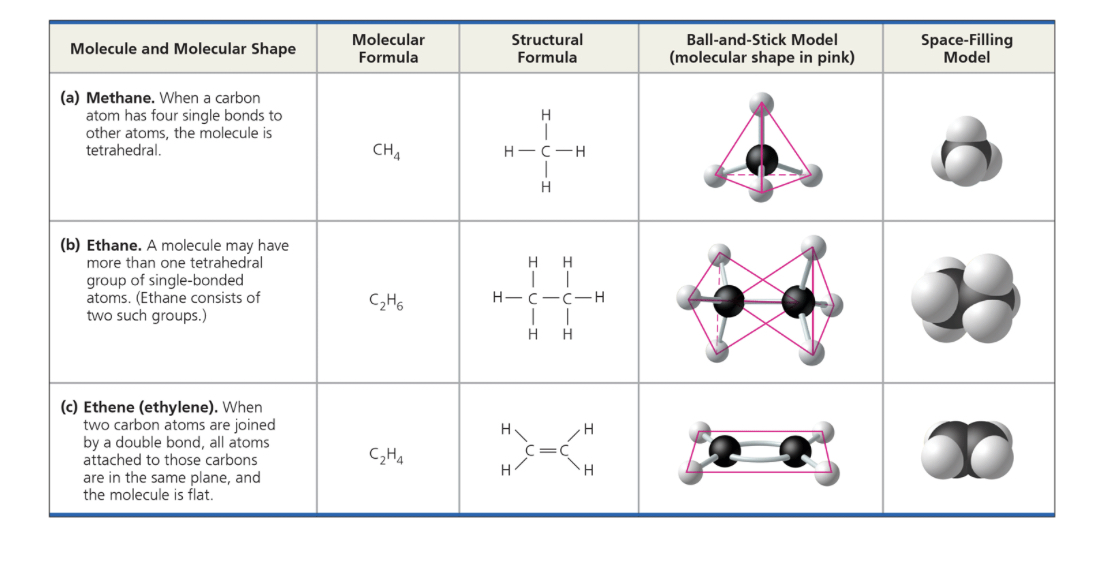
when carbon forms 4 single cov bonds, the bonds angle toward the contents of an imaginary ____; when 2 carbon atoms are joined by a double bond, the bonds from both carbons are in the ___ plane, so atoms joined to those carbons are as well
tetrahedron, same
most frequent bonding partners of carbon
hydrogen, oxygen, nitrogen - 4 main atoms in org mols
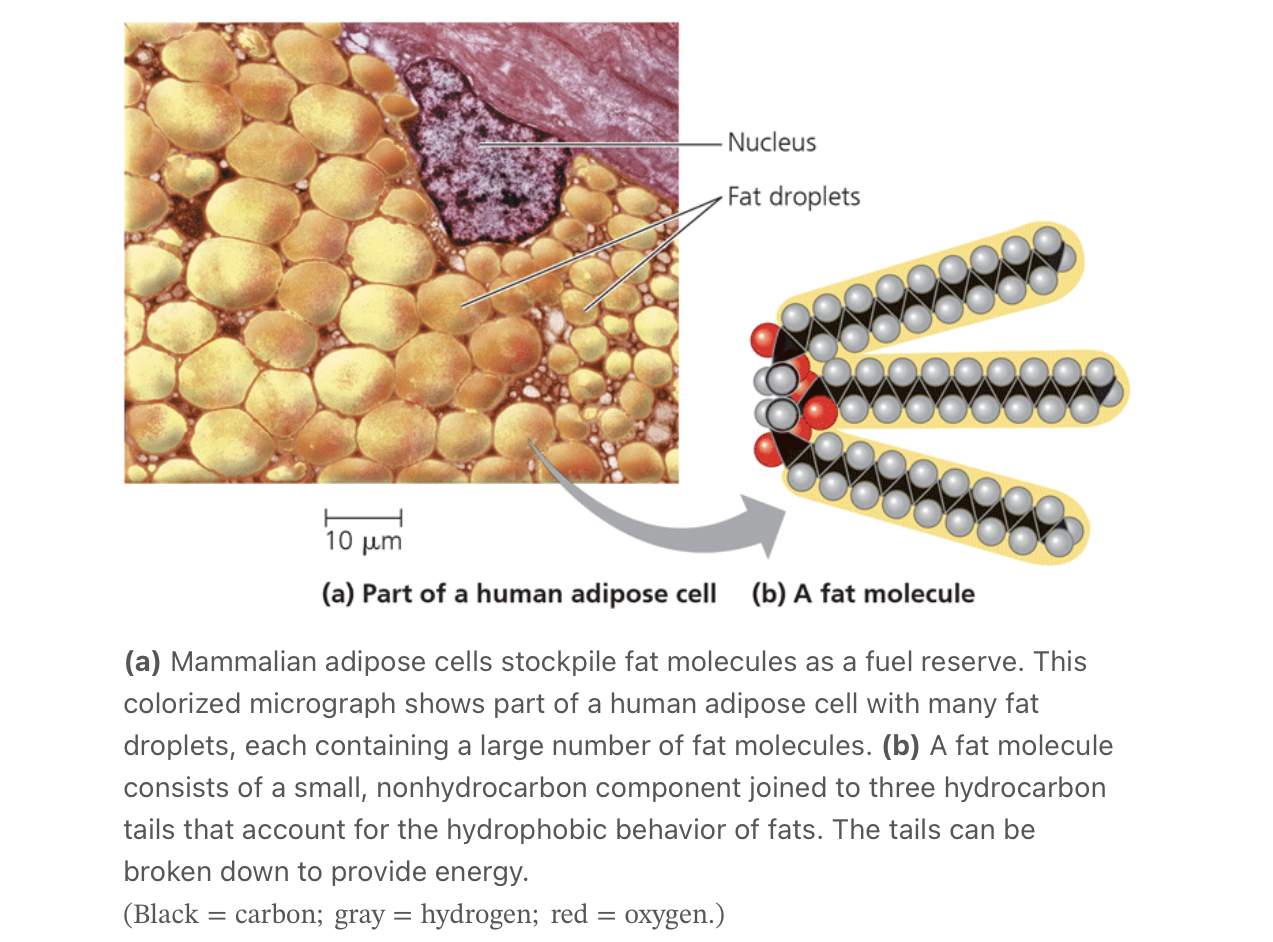
function of hydrocarbons
can undergo reactions that release a relatively large amount of energy
the gas that fuels a car consists of hydrocarbons, and the hydrocarbon tails of fats serve as stored fuel for plant embryos (seeds) and animal as
isomers are ___
compounds that have the same numbers of atoms of the same elements but different structures and hence different properties