A level Chem 1.4 Energetics
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
State the meaning of the term standard enthalpy of combustion. (2)
Enthalpy change when one mole of a substance burns completely in oxygen (1)
With all substances in their standard states (1)
A student does an experiment to determine the enthalpy of combustion of propan-1-ol (CH3CH2CH2OH, Mr = 60.0).
Combustion of 0.497 g of propan-1-ol increases the temperature of 150 g of water from 21.2 °C to 35.1 °C
Calculate a value, in kJ mol–1 , for the enthalpy of combustion of propan-1-ol in this experiment. The specific heat capacity of water is 4.18 J K–1 g (3)
q = m c ∆T = 150 × 4.18 × 13.9 = 8715.3 J (1)
n(propan-1-ol)=0.497/60.0=0.00828 mol (1)
∆H = − 8.7153/0.00828 = −1050 kJ mol-1 (1)
The enthalpy of combustion determined experimentally is less exothermic than that calculated using enthalpies of formation.
Give one possible reason for this, other than heat loss (1)
Incomplete combustion (1)
Define the term enthalpy change (1)
Heat (energy) change at constant pressure (1)
Propane undergoes complete combustion.
C3H8(g) + 5 O2(g) ⟶ 3 CO2(g) + 4 H2O(l)
∆H = –2046 kJ mol–1
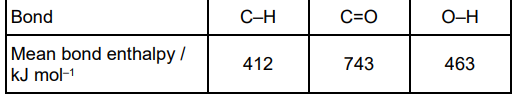
The table below shows some bond enthalpy data.
The bond enthalpy for O=O is 496 kJ mol–1
For H2O(l) ⟶ H2O(g) ∆H = +41 kJ mol–1 Use these data to calculate a value for the C–C bond enthalpy in propane. (4)
Bonds broken
2(C–C) + 8(C–H) + 5(O=O)
(5776 + 2(C-C)) (1)
Bonds made
including 4(41) for vaporisation of water
2(C-C) = 6(C=O) + 8(O-H) + 4(41) - 2046 - 8(C-H) - 5(O=O) = 6(743) + 8(463) + 4(41) - 2046 - 8(412) - 5(496) = 504 (1)
(C-C) = 504/2= 252 (kJ mol-1 )(1)
Propane undergoes complete combustion.
C3H8(g) + 5 O2(g) ⟶ 3 CO2(g) + 4 H2O(l)
∆H = –2046 kJ mol–1
The table below shows some bond enthalpy data.
The bond enthalpy for O=O is 496 kJ mol–1
Explain why the value given for the O=O bond enthalpy in part (b) is not a mean value. (1)
Oxygen / O2 is the only substance that has O=O bond (1)
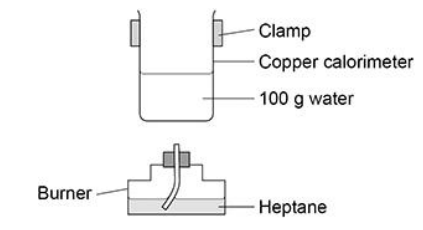
A student does an experiment to determine a value for the enthalpy of combustion of heptane. The figure below shows some of the apparatus used.
The student considered using a glass beaker on a tripod and gauze instead
of the clamped copper calorimeter.
Suggest two disadvantages of using a glass beaker on a tripod and gauze.(2)
Glass is a poorer conductor than copper (1)
Tripod and gauze would reduce heat transfer (1)
A student determines the enthalpy change for the reaction between
calcium carbonate and hydrochloric acid.
CaCO3(s) + 2 HCl(aq) → CaCl2(aq) + CO2(g) + H2O(l)
The student follows this method:
• measure out 50 cm3 of 1.00 mol dm–3 aqueous hydrochloric acid
using a measuring cylinder and pour the acid into a 100 cm3 glass
beaker
• weigh out 2.50 g of solid calcium carbonate on a watch glass and tip
the solid into the acid
• stir the mixture with a thermometer
• record the maximum temperature reached.
The student uses the data to determine a value for the enthalpy change. Explain how the experimental method and use of apparatus can be improved to provide more accurate data. Describe how this data from the improved method can be used to determine an accurate value for the temperature change. (6)
Use a burette/pipette (instead of a measuring cylinder) (1)
Use a polystyrene cup (instead of a beaker) / insulate beaker (1)
Measure and record the initial temperature of the solution for a few minutes before addition (1)
Measure and record the temperature after the addition at regular intervals (eg each minute) for 8+ minutes/until a trend is observed (1)
Plot a graph of temperature against time (1)
Determine ΔT at the point of addition (1)
In a different experiment 50.0 cm3 of 0.500 mol dm–3 aqueous hydrochloric
acid are reacted with 50.0 cm3 of 0.500 mol dm–3 aqueous sodium
hydroxide.
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l) ΔH = –57.1 kJ mol–1
The initial temperature of each solution is 18.5 °C
Calculate the maximum final temperature of the reaction mixture.
Assume that the specific heat capacity of the reaction mixture, c = 4.18 J
K–1 g–1
Assume that the density of the reaction mixture = 1.00 g cm–3(5)
n(HCl) or n(NaOH) = 50 x 0.500 / 1000 = 0.025 moles (1)
q = –ΔH x n = 57.1 x 0.025 = 1.4275 kJ (1)
ΔT = q/mc (1)
ΔT = (1.4275 x 1000) / (100 x 4.18) = 3.4(2) °C 1)
Final Temperature = 18.5 + 3.4 = 21.9 °C (1)
In a different experiment 50.0 cm3 of 0.500 mol dm–3 aqueous hydrochloric
acid are reacted with 50.0 cm3 of 0.500 mol dm–3 aqueous sodium
hydroxide.
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l) ΔH = –57.1 kJ mol–1
The initial temperature of each solution is 18.5 °C
Calculate the maximum final temperature of the reaction mixture.
Assume that the specific heat capacity of the reaction mixture, c = 4.18 J
K–1 g–1
Suggest how, without changing the apparatus, the experiment in part (c) could be improved to reduce the percentage uncertainty in the temperature change. (1)
Increase the concentration of the solutions(1)
The temperature changed from 21.8 °C to 19.2 °C during a calorimetry
experiment.
The uncertainty of each reading of the thermometer is ±0.1 °C
What is the percentage uncertainty in the temperature change?
A 0.5%
B 1.0%
C 3.8%
D 7.7% (1)
D (1)
Each reading: ±0.1 °C
Two readings → total uncertainty in ΔT = ±0.2 °C
What is Hess’s law(1)
his law states that the total enthalpy change (ΔH) for a chemical reaction is independent of the pathway (1)
An experiment is done to determine the enthalpy of combustion of a fuel using a calorimeter containing water.
b = mass of fuel burned / g
w = mass of water heated / g
ΔT = temperature rise of water / K
Mr = relative molecular mass of fuel
c = specific heat capacity of water / J K–1 g–1
Which expression gives the enthalpy of combustion (in J mol–1 ), assuming there is no heat loss?
A (-cwΔTMr )/b
B (-cbΔTMr )/w
C (-cbwMr )ΔT
D (-cbwΔT)Mr
A (1)
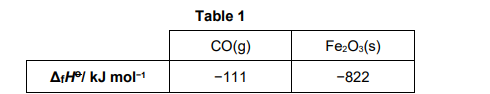
Table 1 contains some standard enthalpy of formation data.
Fe2O3(s) + 3CO(g) ⟶ 2Fe(s) + 3CO2(g) ΔH = −19 kJ mol−1
Use these data and the equation for the reaction of iron(III) oxide with carbon monoxide to calculate a value for the standard enthalpy of formation for carbon dioxide.
Show your working.
Correct cycle or equation (1)
3 × ΔHfCO2) = −19 + (−822) + 3(−111) − 0 (3 ×ΔHfCO2) = −1174 (1)
ΔHfCO2 = −391 kJ mol−1 (1)
Rmb Fe is not included it is 0!!!
Give one reason why the bond enthalpy that you calculated in part (c) is different from the mean bond enthalpy quoted in a data book (388 kJ mol−1 ). (1)
Data book value derived from (a number of) different compounds (not just different NH3 molecules) (1)
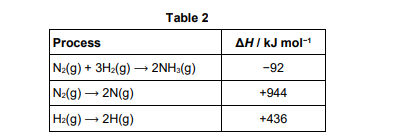
Some enthalpy data are given in Table 2.
Use the data from Table 2 to calculate the bond enthalpy for N−H in ammonia (3)
N2+3H2→2NH3 /Correct Hess’s law cycle or equation (1)
(6(N−H)) = 944 + 3(+436) + 92
(6(N−H))=2344 (1)
N−H =(+)391kJmol-1 (1)
A student planned and carried out an experiment to determine the enthalpy of
reaction when magnesium metal displaces zinc from aqueous zinc sulfate.
Mg(s) + Zn2+(aq) ⟶ Mg2+(aq) + Zn(s)
The student used this method:
• A measuring cylinder was used to transfer 50 cm3 of a 1.00 mol dm−3
aqueous solution of zinc sulfate into a glass beaker.
• A thermometer was placed in the beaker.
• 2.08 g of magnesium metal powder were added to the beaker.
• The mixture was stirred and the maximum temperature recorded.
The student recorded a starting temperature of 23.9 °C and a maximum
temperature of 61.2 °C.
Suggest how the students’ method, and the analysis of the results, could be improved in order to determine a more accurate value for the enthalpy of reaction.
Justify your suggestions. Do not refer to the precision of the measuring equipment. Do not change the amounts or the concentration of the chemicals. (6)
You must include all stages in order to achieve full marks
Stage 1 Improved insulation
1a Insulate the beaker or use a polystyrene cup or a lid
1b To reduce heat loss
Stage 2 Improved temperature recording
2a Record the temperature for a suitable time before adding the metal
2b To establish an accurate initial temperature
OR
2c Record temperature values at regular time intervals
2d To plot the temperature results against time on a graph
Stage 3 Improved analysis of results
3a Extrapolate the cooling back to the point of addition
3b To establish a (theoretical) maximum temperature OR temperature
change (e.g. at the 4th minute) OR adjust for the cooling /apply a cooling
correction
3a and 3b could be seen on an extrapolated sketch graph (6)
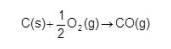
State the name given to the enthalpy change represented by the following chemical equation.
Explain why this enthalpy change would be difficult to determine directly (2)
(Standard) enthalpy of formation (1)
Difficult to prevent C reacting with O2 to form some CO2(1)
Suggest a reason why the value calculated in part (d) differs from the mean Xe–F bond enthalpy quoted in a data source. (1)
Mean bond enthalpy found by taking an average for Xe–F in a range of compounds (1)
What is the temperature rise, in K, when 504 J of heat energy are absorbed by
0.110 kg of solid iron?
Specific heat capacity of iron = 0.448 J K−1 g−1
A 9.78 × 10−2
B 1.02 × 101
C 2.83 × 102
D 1.02 × 104(1)
B (1)
a temperature change (ΔT) is the same number in kelvin and in degrees Celsius.

Calculate the enthalpy change, in kJ, for this dissociation of mole of propan-1-ol. C3H7OH(g) ⟶ 3C(g) + 8H(g) + O(g) (1)
Sum of reactants = 4403
enthalpy change = 4403 (1)
A 50.0 g sample of water was used in this experiment.
Explain how you could measure out this mass of water without using a balance (2)
Water has a known density (of 1.0 g cm–3) (1)
Therefore, a volume of 50.0 cm3 could be measured out(1)
Suggest why the enthalpy change for the thermal decomposition of solid silver nitrate(III) is difficult to determine experimentally (1)
The silver nitrate has to be heated so you cannot measure the temperature change caused by the reaction. (1)
( it only decomposes at very high temperatures!)
Silver nitrate(V) solution reacts with solid sodium chloride.
AgNO3(aq) + NaCl(s) ⟶ AgCl(s) + NaNO3(aq)
A student does an experiment to determine the enthalpy change for this reaction.
The student follows this method:
1. Measure out 50 cm3 of 0.10 mol dm–3 aqueous silver nitrate(V) using
a clean, dry measuring cylinder.
2. Pour the silver nitrate(V) solution into a glass beaker.
3. Weigh out 2.00 g of solid sodium chloride (an excess) using a
weighing boat and tip the solid into the silver nitrate(V) solution.
Reweigh the weighing boat to determine the mass of sodium chloride
added.
4. Add a lid to the beaker that has two small holes for a stirring rod and
for a thermometer.
5. Stir the mixture with a plastic stirring rod whilst recording the
temperature with a thermometer.
6. Record the maximum temperature reached
Identify three aspects of this method which could cause inaccurate results. Describe how the student could improve these three aspects of the method to obtain more accurate results. (6)
Inaccuracy 1 - Use of a glass beaker. (1)
Improvement 1 - Use a polystyrene cup or use insulation (to reduce heat
loss.) (1)
Inaccuracy 2 - Did not record the initial temperature. (1)
Improvement 2 - Record an initial starting temperature before adding the
solid sodium chloride/Recording the starting temperature of the silver
nitrate solution.(1)
Inaccuracy 3 - Does not take into account heat loss/method to determine maximum temperature not effective (1)
Improvement 3 - Measure the temperature at suitable time intervals after the addition, plot a graph and extrapolate to determine the maximum change. (1)
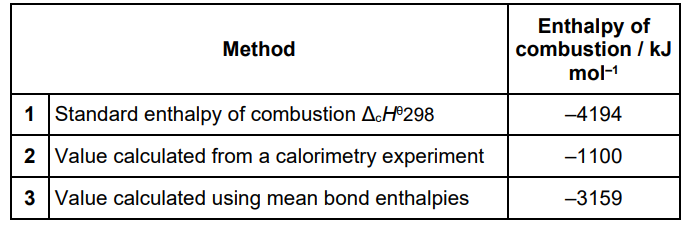
The table below shows values, obtained by different methods, for the enthalpy of combustion of a different liquid hydrocarbon.
Suggest reasons for the differences between the values obtained by each of Methods 2 and 3, and the value obtained by Method 1 in the table above (5)
Value from calorimetry less exothermic/lower/smaller (than method 1 value) (1)
(Calorimetry = ) because of heat/energy loss (1)
(Calorimetry = ) incomplete combustion (1)
Mean bond enthalpies values use enthalpies taken across a range of compounds (1)
Value from bond enthalpy data ignores energy changes in vaporisation of the fuel or condensing the water (1)

Table 1 shows the enthalpies of combustion of some alcohols.
Explain how your answer to part (a) suggests that the alcohol is butan-1-ol. (If you have been unable to obtain an answer for part (a), assume that the answer is –2120 kJ mol–1) (2)
idea that value measured is not accurate due to due to heat loss / incomplete combustion (1)
idea that the result from the experiment must be less exothermic than the true value and so cannot be propan-1-ol (1)
The equation for the complete combustion of gaseous pentan-1-ol is shown.
CH3(CH2)3CH2OH(g) + 7.5O2(g) → 5 CO2(g) + 6 H2O(g) ΔH = –3388 kJ mol–1
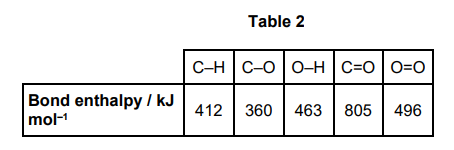
Table 2 shows some bond enthalpy data.
Use data from Table 2 to calculate a value for the mean C–C bond enthalpy in pentan-1-ol. (3)
ΔH (or −3388) = ΣBonds broken - ΣBonds formed (1)
−3388 = 4 B(C−C) + 11(412) + 360 + 463 + 7½(496) − 10(805) − 12(463) (1)
B(C-C) =M2/4 =(+) 286 kJ mol-1
The energy stored in fuels can be compared using energy density values measured in kJ dm–3
Calculate the energy density of butan-1-ol.
enthalpy of combustion of butan-1-ol = –2676 kJ mol–1
density of butan-1-ol = 0.810 kg dm–3
relative molecular mass (Mr) of butan-1-ol = 74.0 (2)
amount of butan-1-ol in 1 dm3 =810/74 (=10.95 mol) (1)
energy from 1 dm3 = M1 × 2676 = 29300 (kJ dm-3) (1)
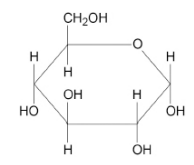
Ethanol is a biofuel that can be produced by the fermentation of glucose
C6H12O6 → 2 C2H5OH + 2 CO2
Glucose has the structural formula shown
Table 1 shows some mean bond enthalpy values.
C-H : 412
C-C : 348
C-O : 360
C=O : 805
O-H : 463
Use the equation and the data in Table 1 to calculate an approximate value of ΔH for the fermentation of glucose. For this calculation you should assume that all the substances are in the gaseous state. (3)
bonds broken = 9459 kJ mol-1
(5C-C + 7C-O + 7C-H + 5O-H) (1)
Bonds formed = 9682 kJ mol-1
(2C-C + 10C-H + 2C-O + 2O-H + 4C=O) (1)
∆H = M1 - M2 = -223 kJ mol-1(1)