Chapter 21 - Alpha Carbon Chemistry
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
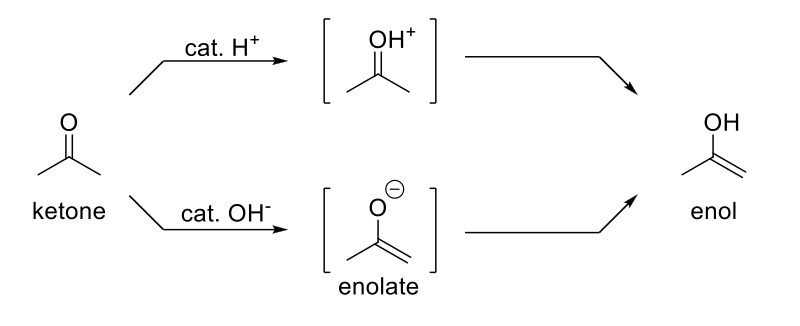
Acidic Keto-Enol Tautomerization
Mechanism:
1) C=O is protonated.
2) Resonance structure.
3) Elimination.
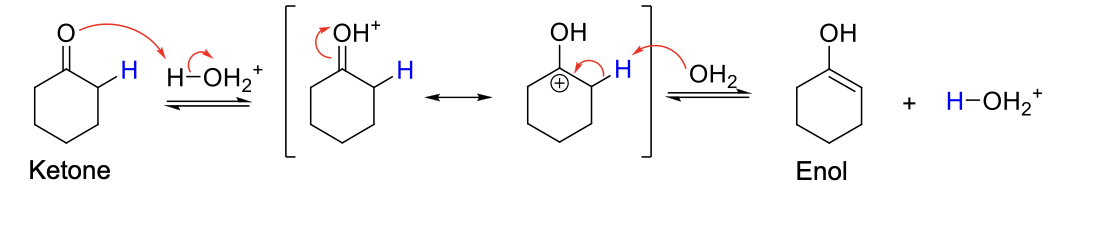
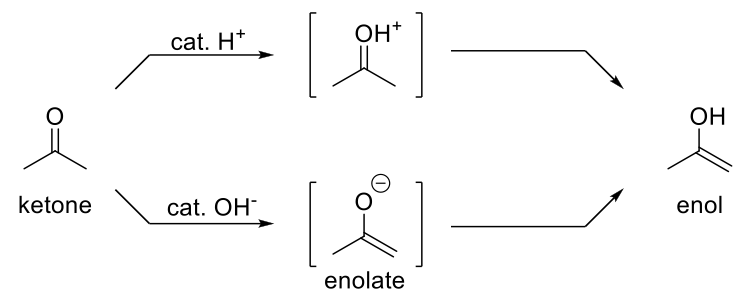
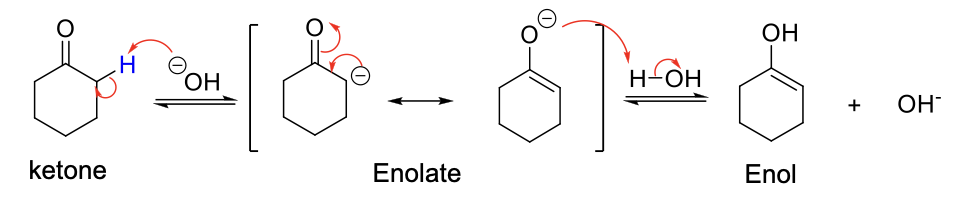
Basic Keto-Enol Tautomerization
Mechanism:
1) Elimination.
2) Resonance structure.
3) C-O- is protonated,
Reversible and Irreversible Enolate Formation Bases
If Pka = 19/20 (Next to one ketone)
Reversible = NaOR, T-BuOk
Irreversible = LAH, NaH
If Pka = 9/10 (Next to two ketones
Irreversible = NaOR, T-BuOk, LAH, NaH
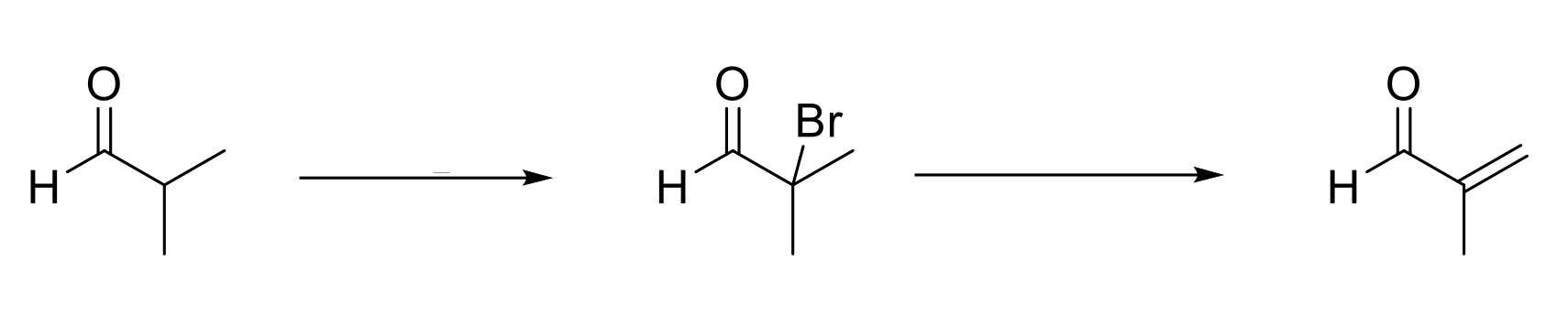
Alpha Halogenation of Enols & Enolates (Acidic Conditions)
Mechanism:
1) Enol forms.
2) Formation of C=O bond, double bond attacks bromine, Br2 bond breaks.
3) C=OH+ deprotonated by water.
4) Adding a base undergoes elimination.
Notes:
Alpha bromination on aldehydes and ketones only.
Major product is transition state with more substituted (more stable) alkene/enolate.
Adding a base forms α,β-unsaturated aldehyde/ketone.
Pyridine, or weaker bases, can be used as a base because the formed alkene is conugated.
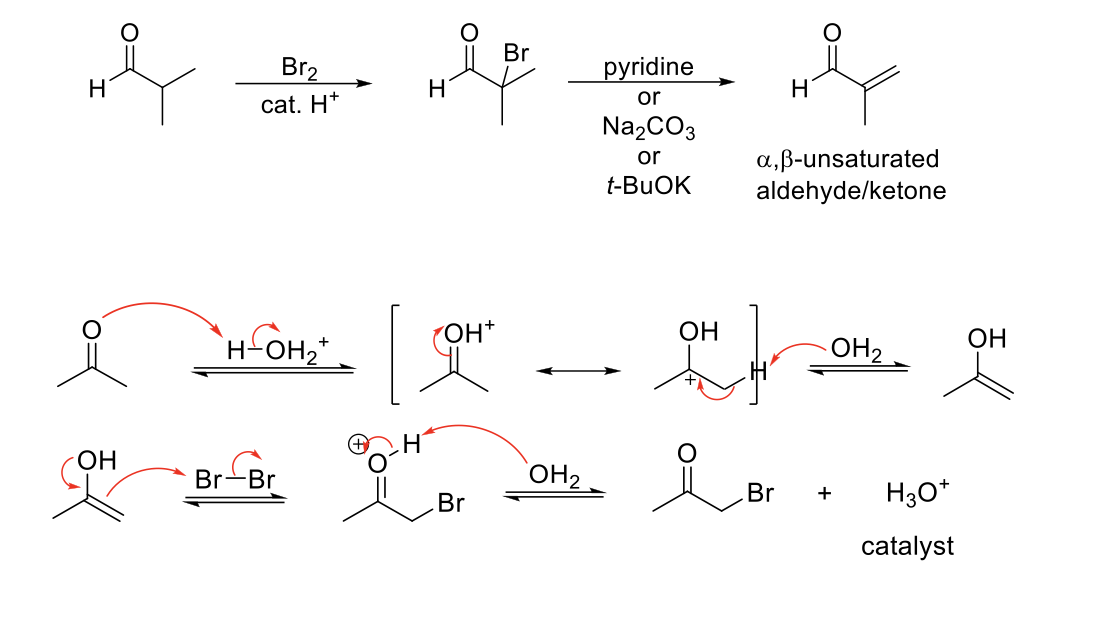
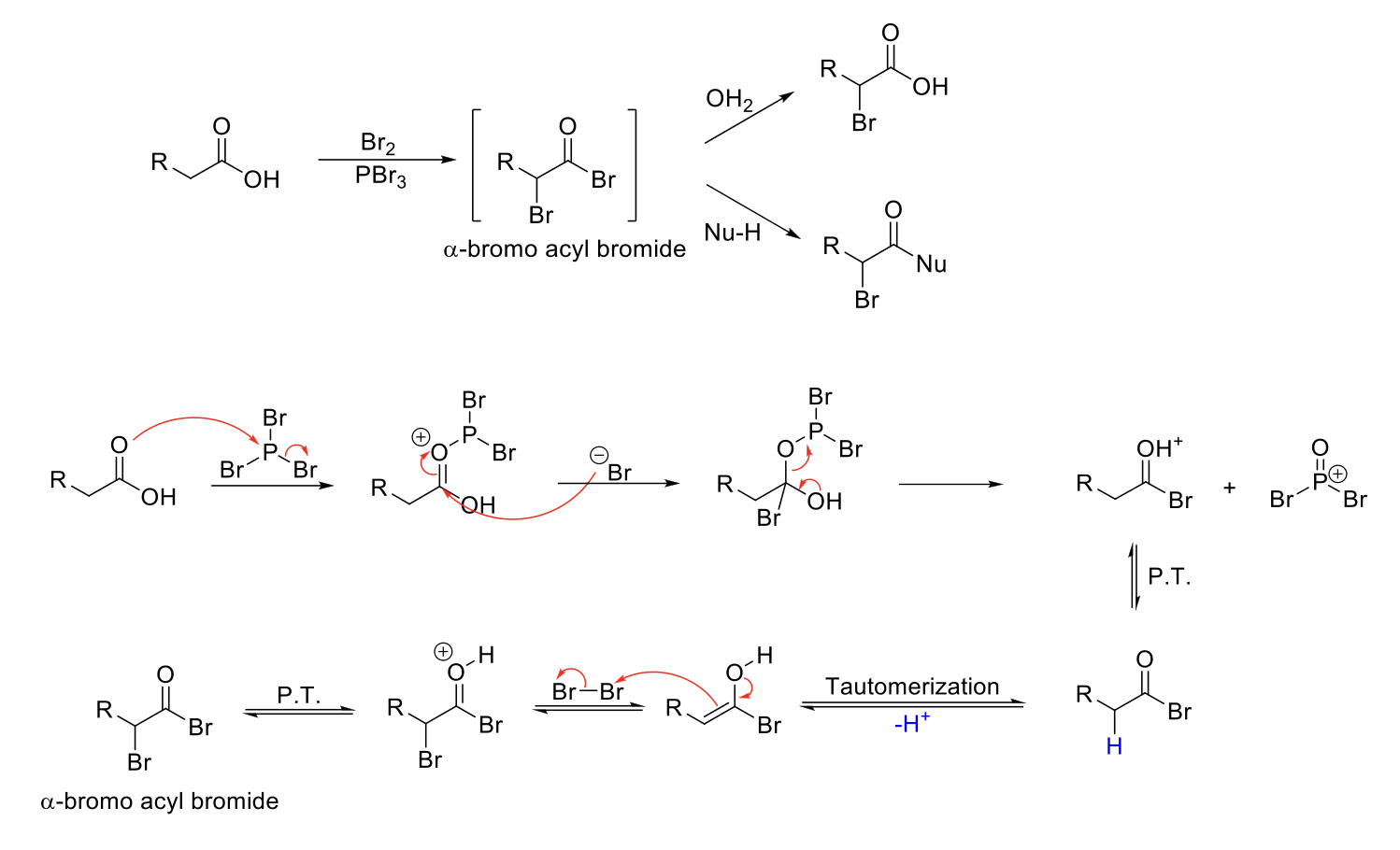
Hell-Volhard-Zelinsky (HVZ) Reaction
Mechanism:
1) C=O bonds to P and kicks out a Br.
2) -Br attacks C=O and double bond removed.
3) Lone pair from OH kicks out OPBr2+.
4) C=OH+ is deprotonated.
5) Tautomerization to enol form.
6) Formation of C=O bond, double bond attacks bromine, Br2 bond breaks.
7) C=OH+ deprotonated.
Notes:
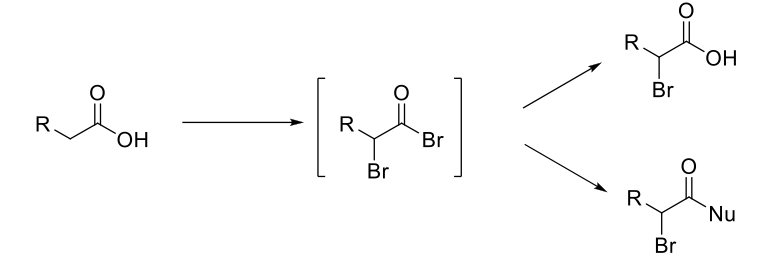
Alpha bromination on carboxylic acids, esters, and amides.
Br2 and PBr3 forms an α-bromo acyl bromide.
α-bromo acyl bromide can be reacted with a nucleophile to replace acyl bromide.
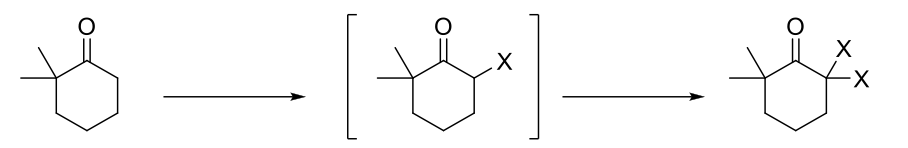
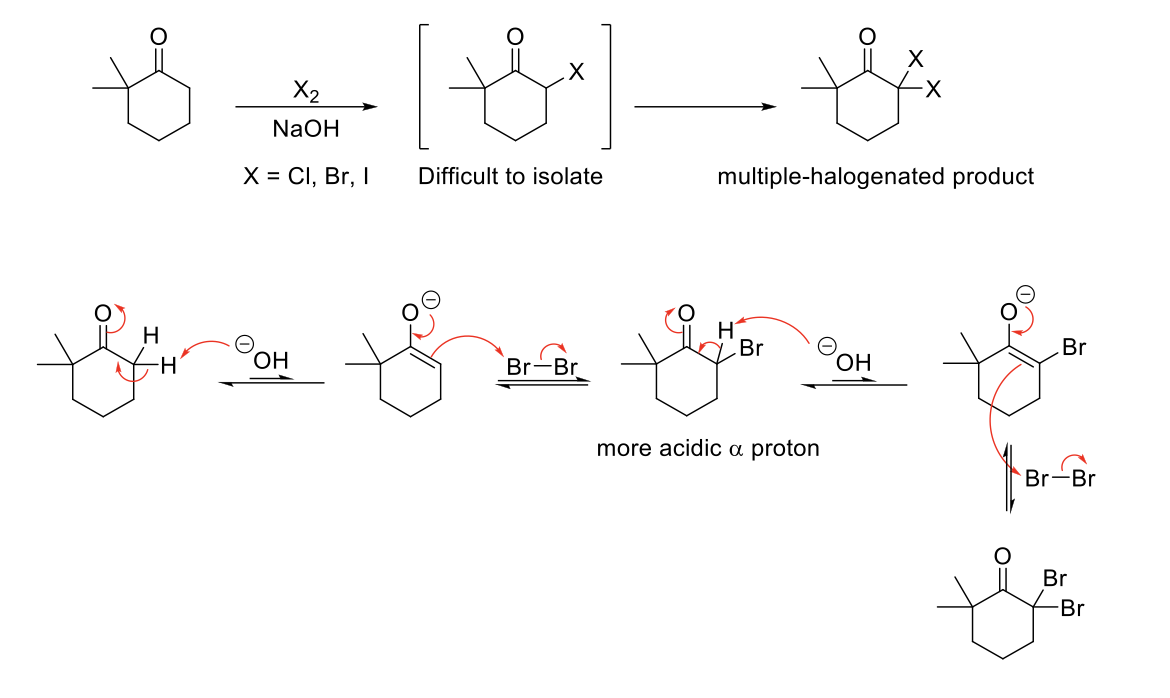
Alpha Halogenation of Enols & Enolates (Basic Conditions)
Mechanism:
1) Enolate formation.
2) Formation of C=O bond, double bond attacks bromine, Br2 bond breaks.
3) Repeats if α proton available.
Notes:
Halogenation is repeated because once a bromine is added, the remaining proton is more acidic than the starting material due to resonance and induction.
Number of halogenations = number of α protons.
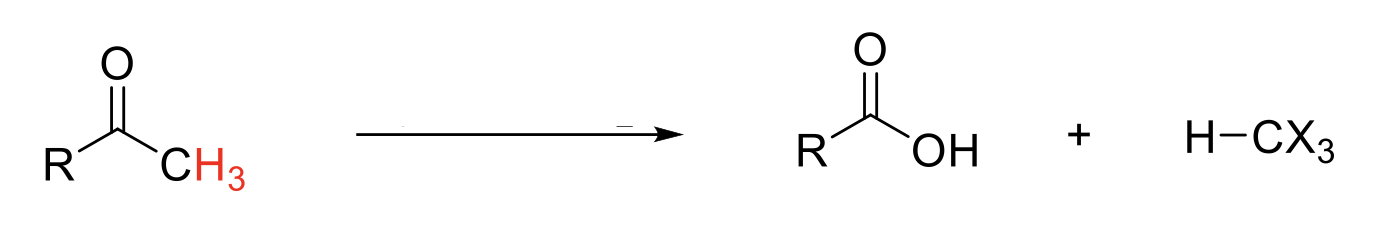
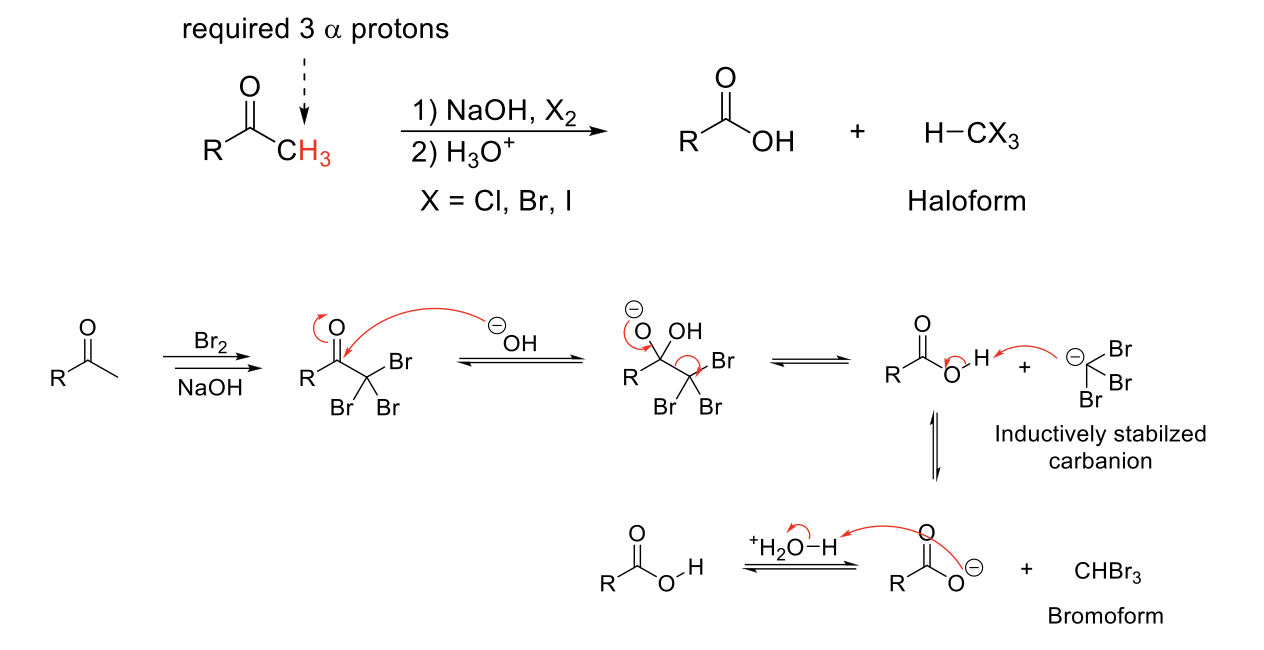
Haloform Reaction
Mechanism:
1) Enolate formation.
2) Formation of C=O bond, double bond attacks bromine, Br2 bond breaks.
3) Repeats if α proton available.
4) -OH attacks C=O.
5) Reformation of C=O bond kicks out -CX3.
5) -CX3 deprotonates carboxylic acid.
6) Quenching step protonates carbonate ion.
Notes:
Special case of alpha halogenation on an aldehyde/ketone when there are 3 α protons.
-CX3 (X = Br, Cl, I) is a good leaving group due to induction.
In basic conditions, -CX3 will deprotonate carboxylic acid. A quenching step is required.
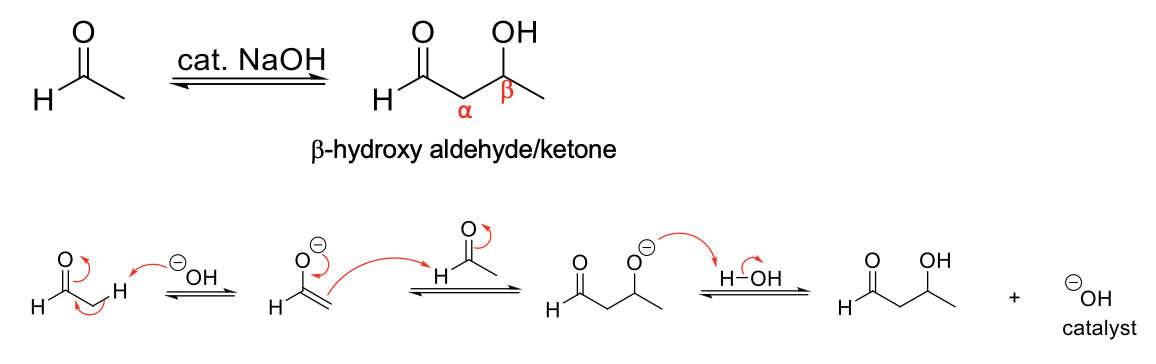
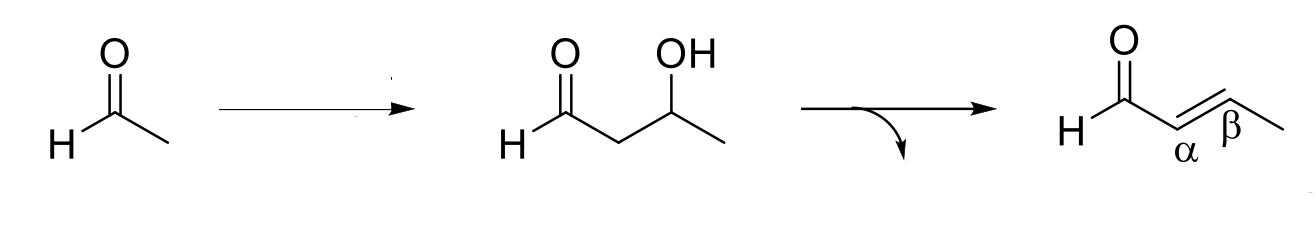
Aldol Reaction
Mechanism:
1) Enolate formation.
2) Formation of C=O bond (of enolate), double bond attacks C=O bond of original form, C=O bond broken (of aldehyde/ketone).
3) Protonation of O- by H2O.
Notes:
Forms a β-hydroxu aldehyde/ketone.
If aldehyde/ketone is in solution with its enolate, they will react.
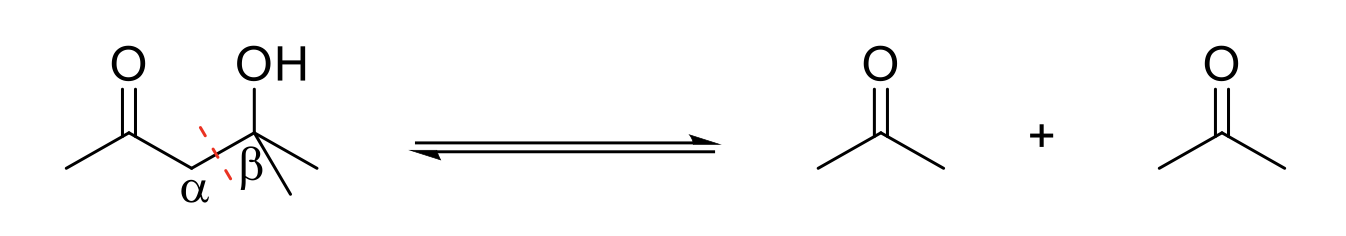
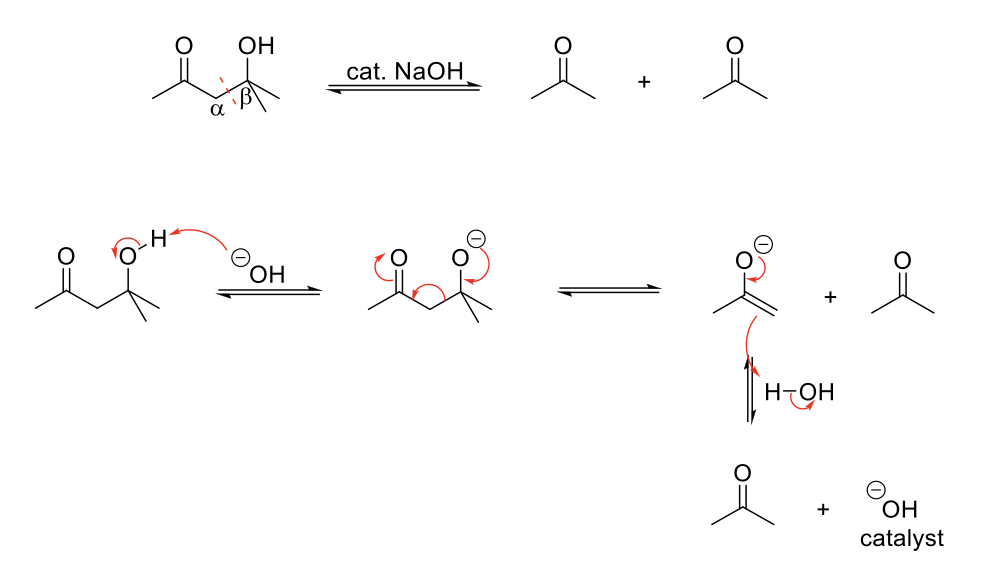
Retro-Aldol Reaction
Mechanism:
1) Deprotonation of alcohol.
2) Formation of C=O bond, single bond breaks and C=O bond breaks. Forms an enolate and the aldehyde/ketone.
3) Enolate: Formation of C=O bond and double bond is protonated.
Notes:
Reverse of aldol formation.
2 equivalents of aldehyde/ketone formed.
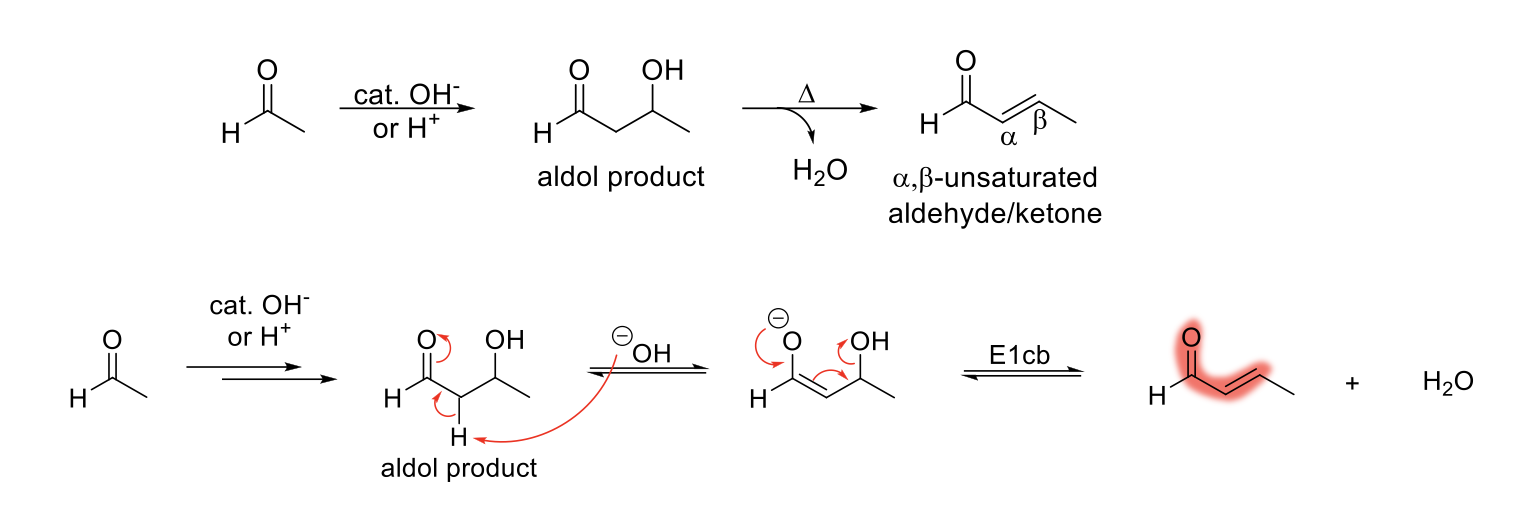
Aldol Condensation Reaction
Mechanism:
1) Aldol product formation mechanism.
2) Aldol product is deprotonated to form an enolate.
3) Reformation of C=O bond, movement of double bond, and loss of leaving group (OH).
Notes:
Forms a α,β-unsaturated aldehyde/ketone.
Condensation reaction: two small molecules are combined with a loss of a small molecule like H2O, CO2, or N2.
Condensation reaction requires two α protons.
Driving force is highly conjugated system and the formation of water.
Trans is major product over minor product (steric hindrance).
E1cb reaction requires presence of EWG to stabilize the conjugate base and a relatively poor LG to be expelled by the lone pair.
Crossed Aldol Reaction
Directed Aldol Reaction
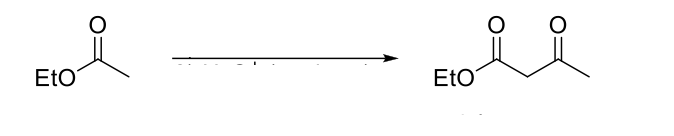
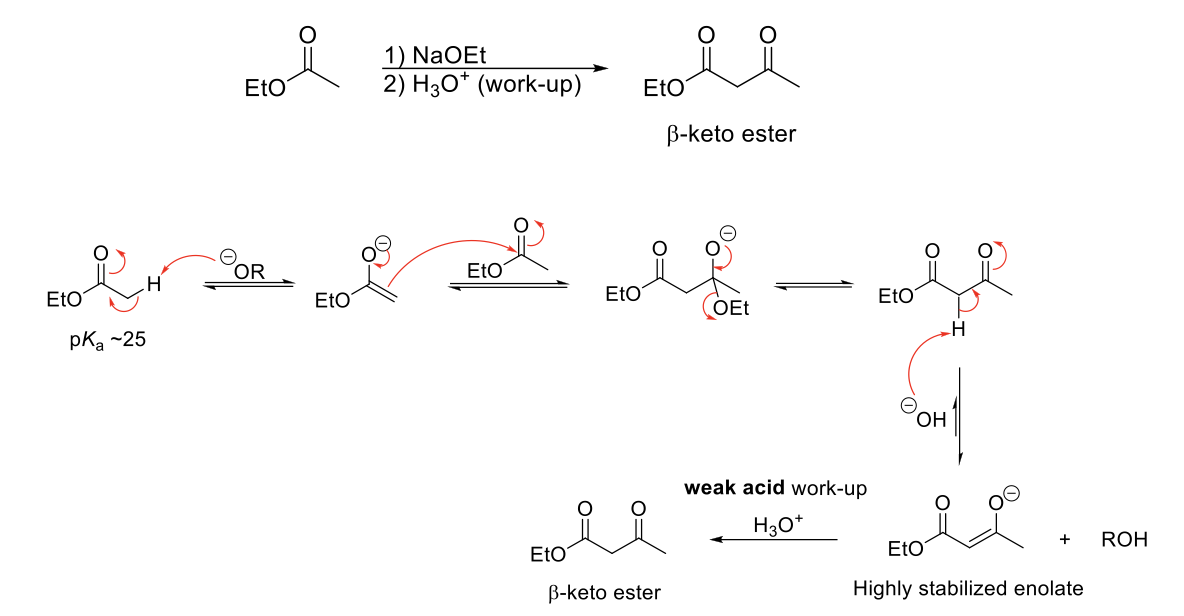
Claisen Condensation
Mechanism:
1) Enolate formation.
2) Formation of C=O bond (of enolate), double bond attacks C=O bond of original form, C=O bond broken (of ester).
3) Formation of C=O bond kicks out -OR group.
4) α proton is removed to form a highly stabilzed enolate.
5) Weak acid work up causes tautomerization to β-keto ester.
Notes:
Forms a β-keto ester.
Requires 2 α protons.
Must use a weak acid (NH4CL) during the acid work-up step because strong acids will hydrolyze esters to carboxylic acids.
Use base (alkoxide) that resembles starting ester to avoid transesterification. Also avoid NaOH (hydrolysis).
Crossed Claisen Condensation
Directed Claisen Condensation
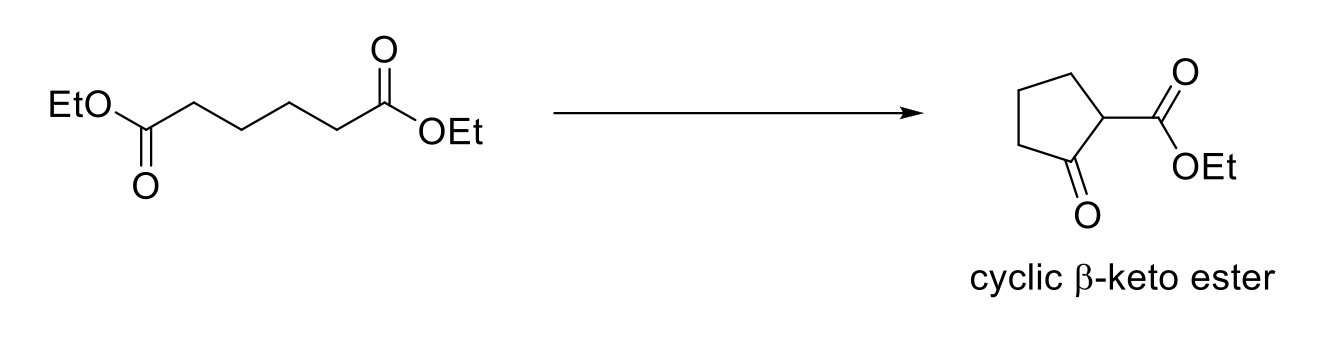
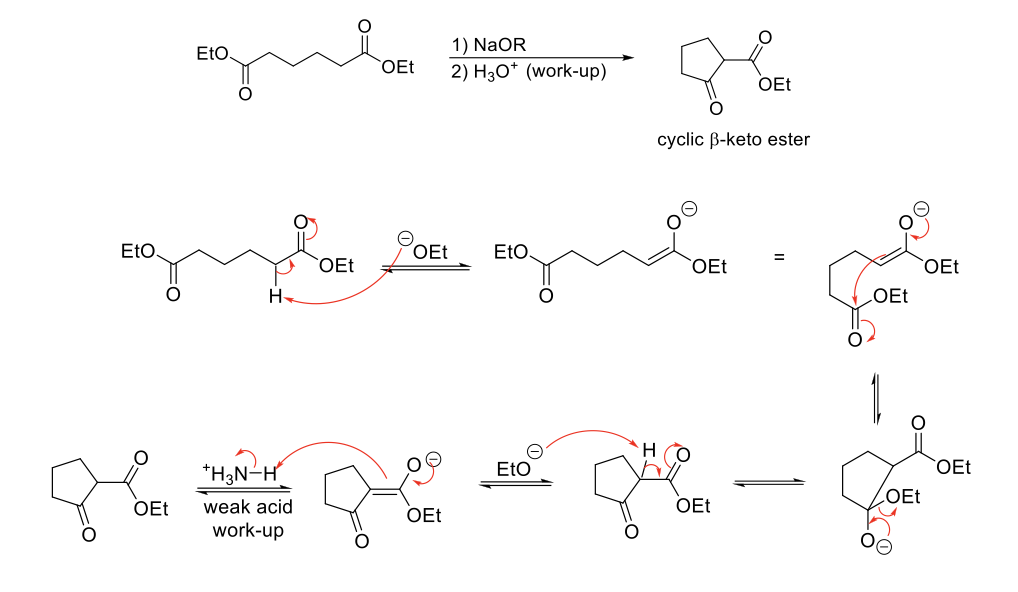
Dieckmann Cyclization (Intramolecular Claisen Condensation)
Mechanism:
1) Enolate formation.
2) Formation of C=O bond (of enolate), double bond attacks C=O bond of other ester, C=O bond broken (of ester).
3) Formation of C=O bond kicks out -OR group.
4) α proton is removed to form a highly stabilzed enolate.
5) Weak acid work up causes tautomerization to β-keto ester.
Notes:
Forms a cyclic β-keto ester.
Requires 2 α protons.
Must use a weak acid (NH4CL) during the acid work-up step because strong acids will hydrolyze esters to carboxylic acids.
Use base (alkoxide) that resembles starting ester to avoid transesterification. Also avoid NaOH (hydrolysis).
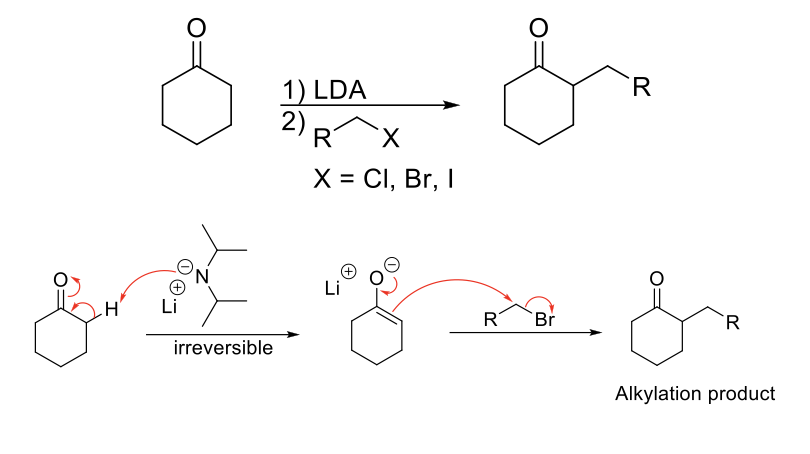
Direct Alkylation at Alpha Position
Mechanism:
1) Enolate formation.
2) SN2 reaction: Formation of C=O, double bond attacks R-X, X leaves.
Notes:
Requires irreversible enolate formation so that aldol side product does not form.
Weaker bases can form aldols or SN2 themselves with R-Xs.
SN2 limitations apply.
Drawbacks include multiple alkylation and competition with elimination products for secondary and tertiary R-Xs.
Kinetic control = short rxn time, low temp (-78 C), large base (LDA) = favors kinetic enolate (less substituted/stable).
Thermodynamic control = long rxn time, high temp (25 C), small base (NaH) = favors thermodynamic enolate (more substituted/stable).
Direct Alkylation at Alpha Position w/ Enamines
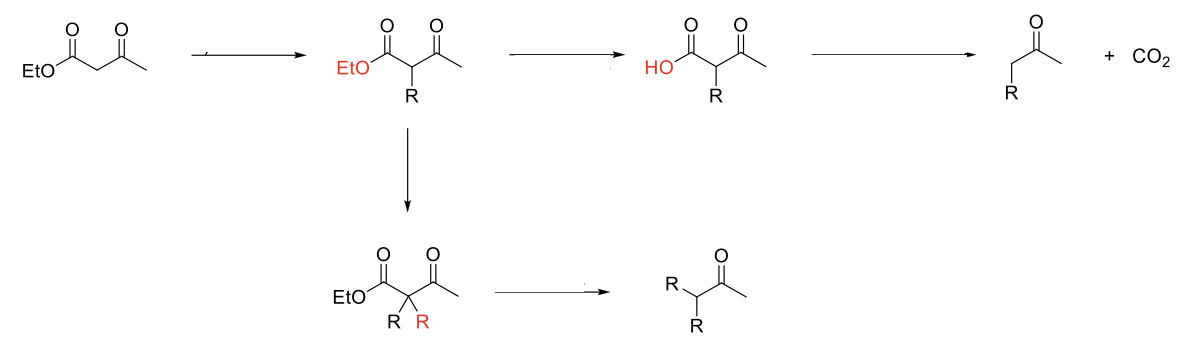
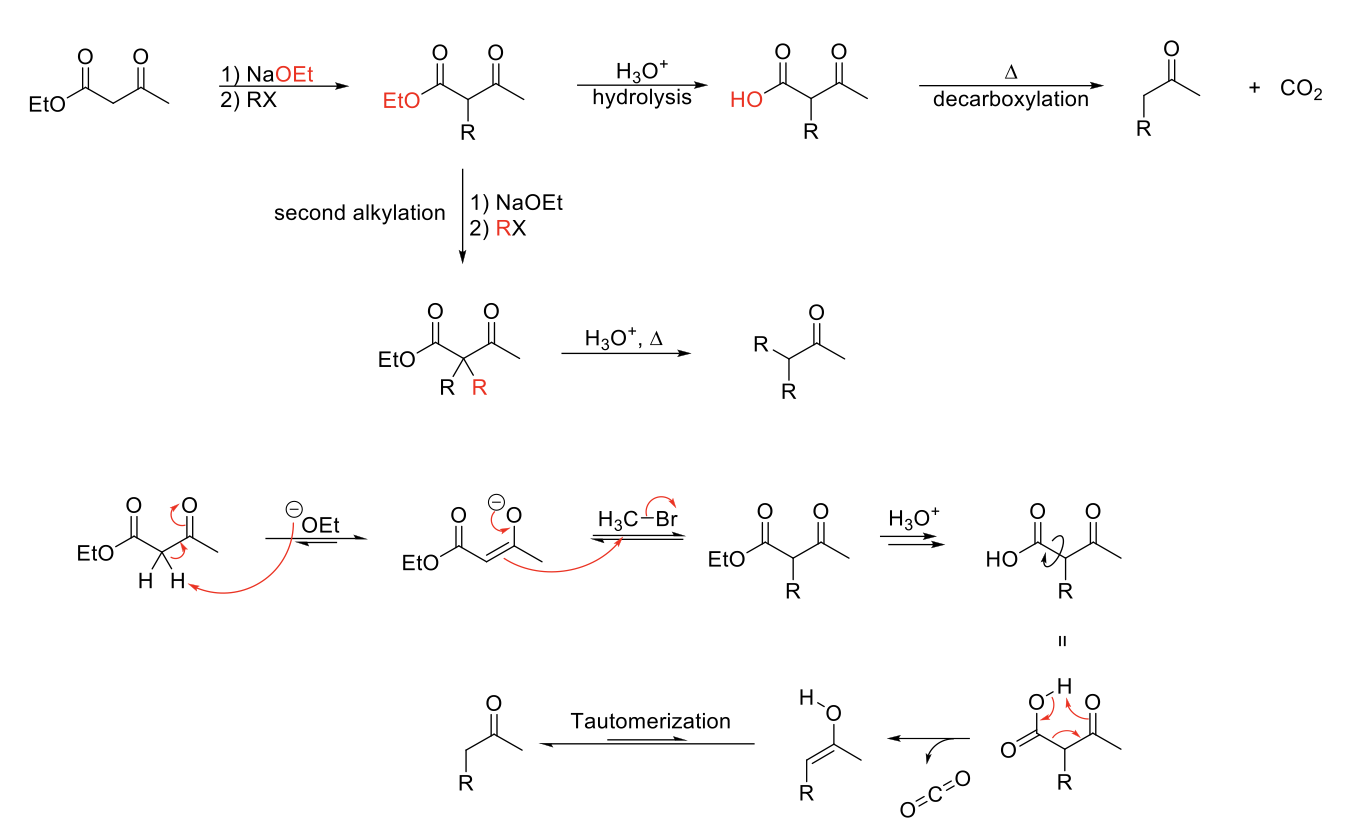
Acetoacetic Ester Synthesis
Mechanism:
1) Enolate formation on ketone of ethyl acetoacetate.
2) SN2 reaction: Formation of C=O, double bond attacks R-X, X leaves.
3) Acid hydrolyizes ester into carboyxlic acid.
4) Heat causes decarboxylation (Ketone grabs H from carboxylic acid, O from alcohol part of carboxylic acid forms CO2 structure, single bond between carboxylic acid breaks to form enol).
5) Enol tautomerizes to acetone.
Notes:
Use to functionalize acetone.
Start with ethyl acetoacetate.
Can do 1 or 2 alkylations.
Weaker base usable since proton of ethyl acetoacetate is more acidic.

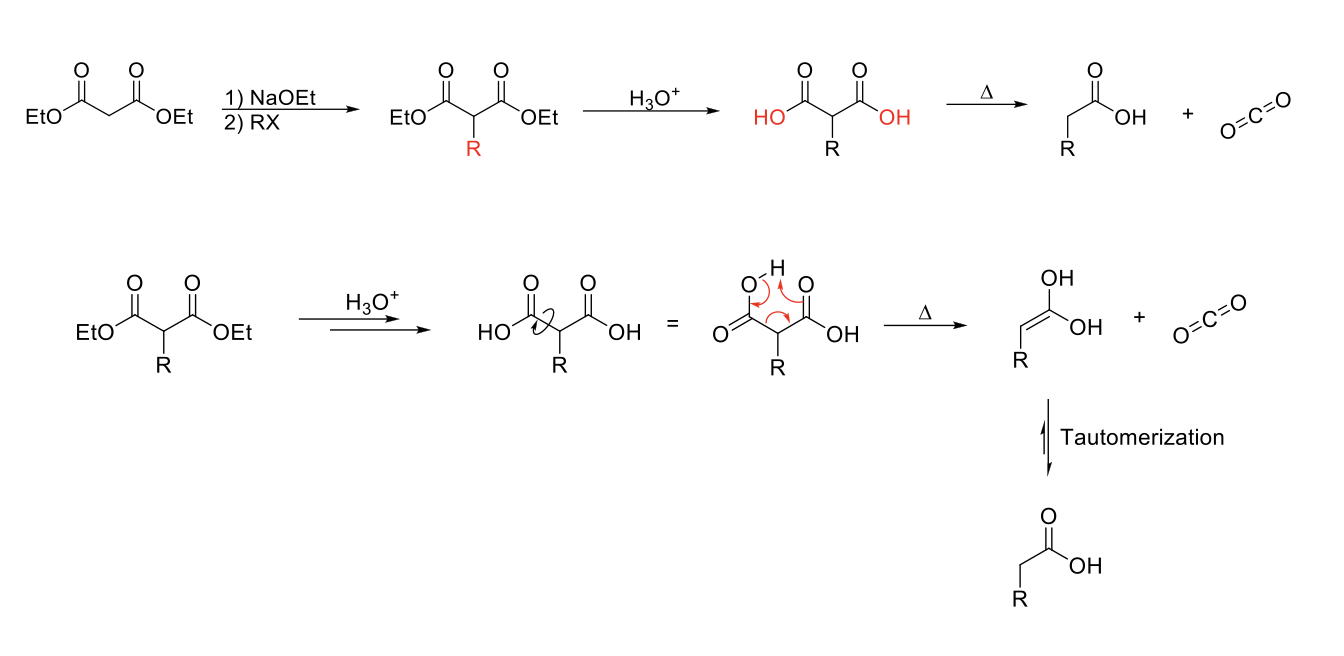
Malonic Ester Synthesis
Mechanism:
1) Enolate formation on one ester of dethyl malonate.
2) SN2 reaction: Formation of C=O, double bond attacks R-X, X leaves.
3) Acid hydrolyizes both esters into carboyxlic acids.
4) Heat causes decarboxylation (C=O grabs H from carboxylic acid, O from alcohol part of carboxylic acid forms CO2 structure, single bond between carboxylic acid breaks to form enol).
5) Enol tautomerizes to carboxylic acids.
Notes:
Use to functionalize acetic acid.
Start with dethyl malonate.
Can do 1 or 2 alkylations.
Weaker base usable since proton of dethyl malonate is more acidic.
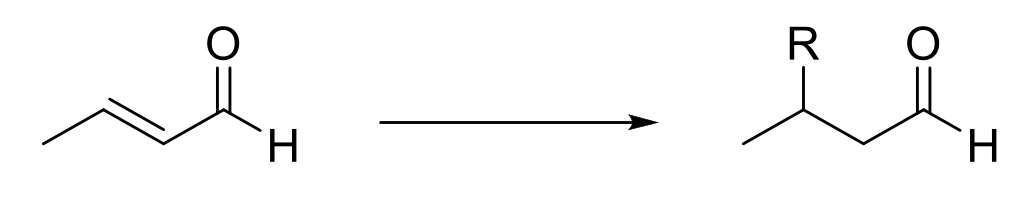
Michael Addition
Mechanism:
1) Michael donor attacks.
2) Protonation
3) Tautomerization
Notes:
After aldol condensation, α,β-unsaturated aldehyde/ketone has two electrophilic centers (carbonyl carbon and farther carbon of alkene).
Hard nucleophiles (1,2-adduct) (attack carbonyl C)
RMgX, LAH, RLi
Borderline nucleophiles (go both)
Enolate, NaBH4
Soft nucleophiles (1,4-adduct) (attact alkene)
R2CuLi, any negative charge stabilized by resonance (resonance stabilized enolates)
Michael Acceptors
α,β-unsaturated aldehyde/ketone/nitrile/ester/amide/nitro group
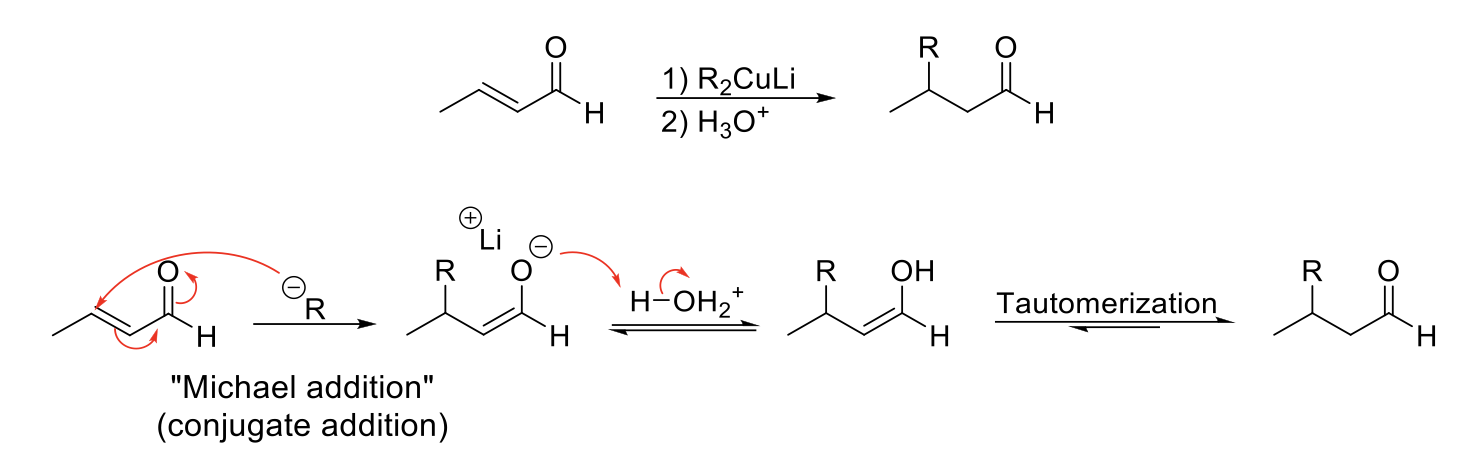
Stork Enamine Synthesis
Mechanism:
1) Enamine formation with secondary amine.
2) Michael addition (N=C bond forms, double bond of enamine attacks other double bond, double bond of michael acceptor moves, C=O bond breaks).
3) Hydrolysis of enamine to ketone.
4) Tautomerization of enolate to ketone.
Notes:
Unstabilized enolates are bad michael donors because they can go 1,4-adduct or 1,2-adduct.
Instead, enamines can be used because they are good michael donors and go 1,4-adduct.
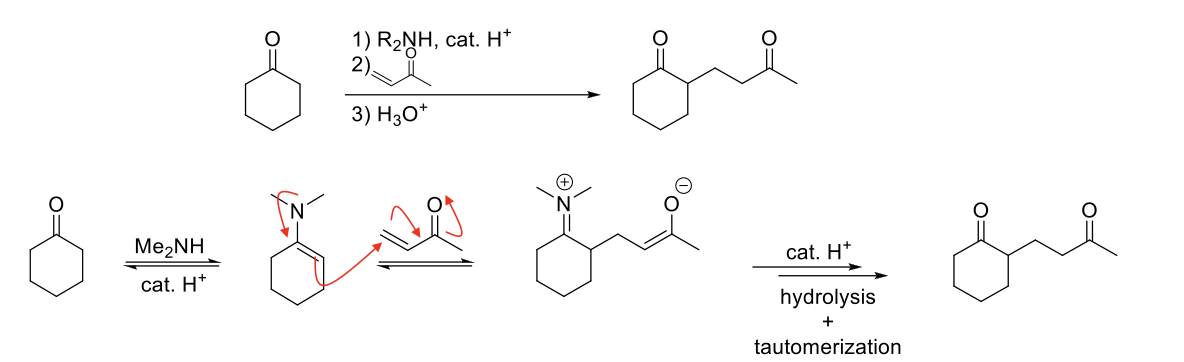
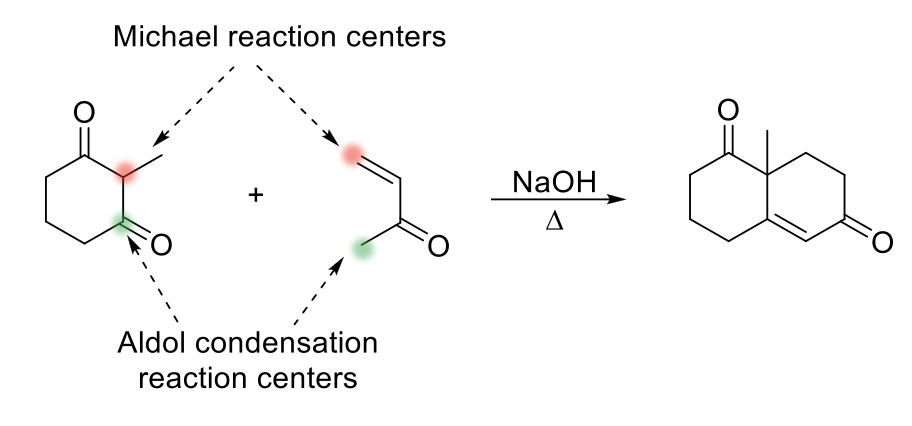
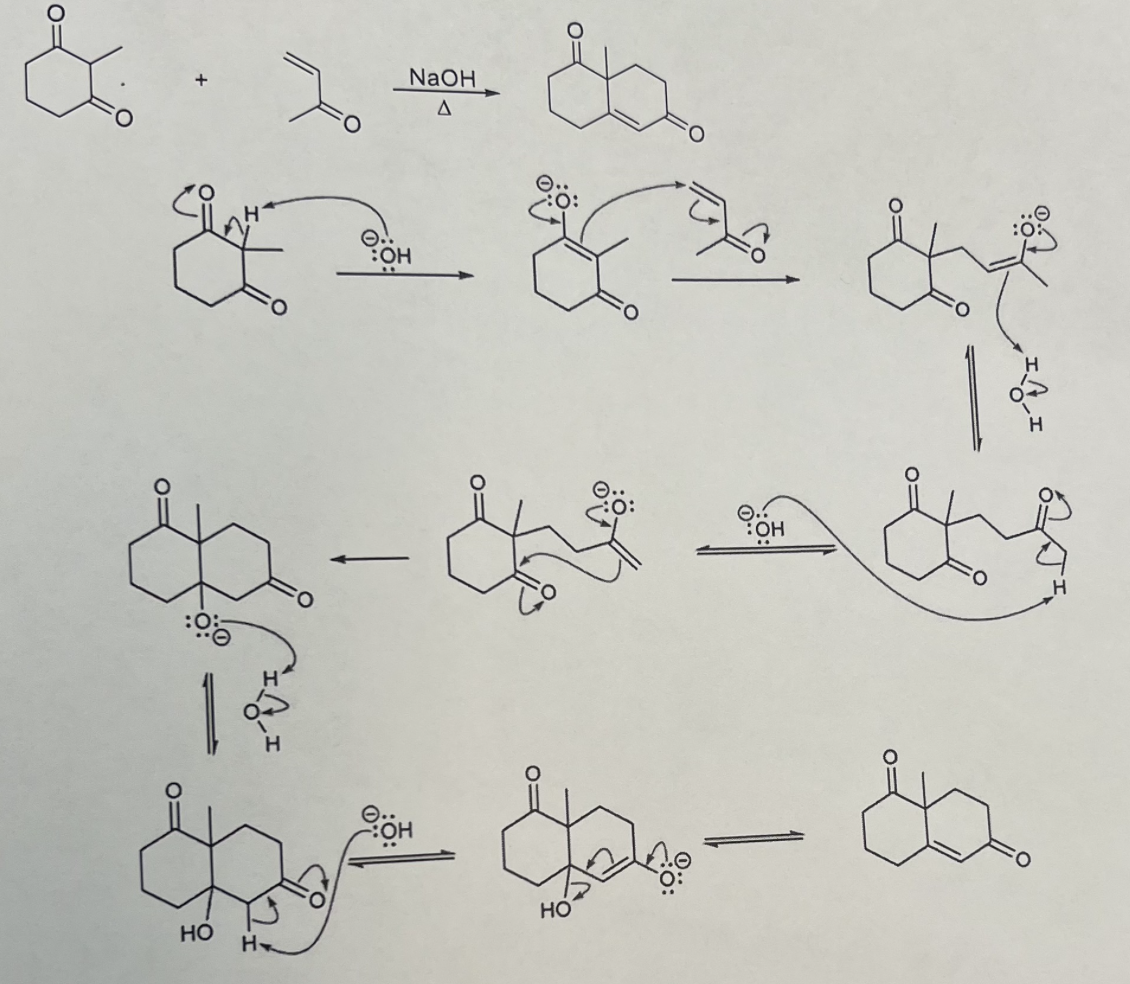
Robinson Annulation Reaction
Mechanism:
1) Enamine formation with secondary amine.
2) Michael addition (N=C bond forms, double bond of enamine attacks other double bond, double bond of michael acceptor moves, C=O bond breaks).
3) Hydrolysis of enamine to ketone.
4) Tautomerization of enolate to ketone.
Notes:
Reaction between α,β-unsaturated aldehyde/ketone and a β-dialdehydeketone
Contains two michael reaction centers and two aldol condensation reaction centers.
Two successive reactions: michael addition followed by aldol condensation.