Exothermic and Endothermic Reactions
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
What can’t energy do?
be created or destroyed
What is an exothermic reaction?
Energy from the reacting chemicals transferred to the surroundings (often increases temp)
What are examples of exothermic reactions?
combustion
neutralisation
oxidation
hand warmers
What are endothermic reactions?
energy from surroundings is transferrred to the reacting chemicals (causing temp to decrease)
What are examples of endothermic reactions?
thermal decomposition
reaction between citric acid and baking soda
sports injury packs
What are the 2 main requirements for a reaction to happen?
collisions- reacting particles must collide
activation energy- collisions must take place with sufficient energy
Diagram of an endothermic reaction?
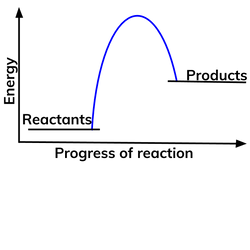
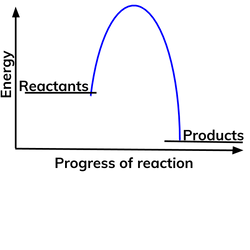
Diagram of an exothermic reaction?
What is the catalyst?
Increases reaction rate by decreasing the activation energy