Chap 7A - Gaseous state
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Compare
Volume |
Shape |
Compressibility |
Density |
Arrangement |
Intermolecular forces |
Vibration, rotation and translation |
of solids, liquids and gas
Solid | Liquid | Gas | |
Volume | Fixed | Fixed | Takes volume of container, greatly influenced by temperature and pressure |
Shape | Fixed | Takes shape of container | Takes shape of container |
Compressibility | 0 | 0 | High |
Density | High | Moderate to high | Low, influenced by temperature and pressure |
Arrangement | Particles are very close together, arranged in an orderly manner of long distance | Particles are close together, arranged in fairly orderly manner but there is no long range order | Particles are far apart, mainly empty spaces between particles |
Intermolecular forces | Strong | Strong (not as strong as solids) | Very weak |
Vibration, rotation and translation | Particles vibrate and rotate about fixed positions but are not free to move throughout solid | Particles vibrate, rotate and move throughout the liquid | Particles move very freely throughout container |
State assumptions of an ideal gas
Gas particles are of negligible size and volume compared to volume of container
Gas particles have negligible intermolecular forces of attraction
Collisions between gas particles are perfectly elastic (gas particles bounce apart with no loss in kinetic energy on collision)
Gas particles are in continuous, rapid, random and linear motion
Average kinetic energy of gas particles is proportional to temperature on the kelvin scale
State and explain conditions for ideal gasses
At low pressures
The gaseous molecules relatively far apart + volume of the molecules themselves is negligible compared to the volume of the container -> real gas molecules at low pressure can be approximated to have negligible volume
Also, intermolecular forces are negligible as the particles are far apart -> their behaviour at low pressures would approach that of ideal gases
At high temperatures
Gas particles have enough kinetic energy to overcome intermolecular forces, which can thus be considered insignificant
As such, the behavior of real gases approach ideal gas behavior at high temperatures
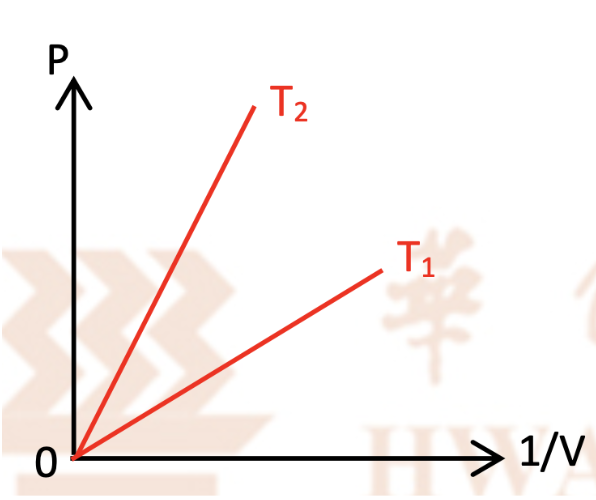
Which temperature is higher , T1 or T2? Explain
Rearrange PV = nRT and make P the subject of the equation, we will get P = (nRT)(1/V) Therefore, plotting P against 1/V will give us a straight line that passes through the origin with gradient = nRT Since the graph with T2 is a steeper line than that of T1, that means nRT2 > nRT1. This is only possible if T2 > T1. Hence T2 has a higher temperature than T1 |
State properties of real gasses
Gas particles have significant volume compared to volume of the container
There are significant intermolecular forces of attraction between gas particles
State the conditions when gases would deviate from ideal conditions
Real gases would deviate from ideal behavior at high pressures and low temperatures as they are most likely to condense into a liquid -> behave ideally at high temperatures and low pressure
They do not obey ideal gas equation at very high pressures and very low temperatures as they are most likely to condense
Liquefaction is a key property of real gases that is not predicted by the kinetic theory of gases, as it requires the action of intermolecular forces (which the theory assumes to be negligible) in order to occur -> ideal gases never condense into liquids
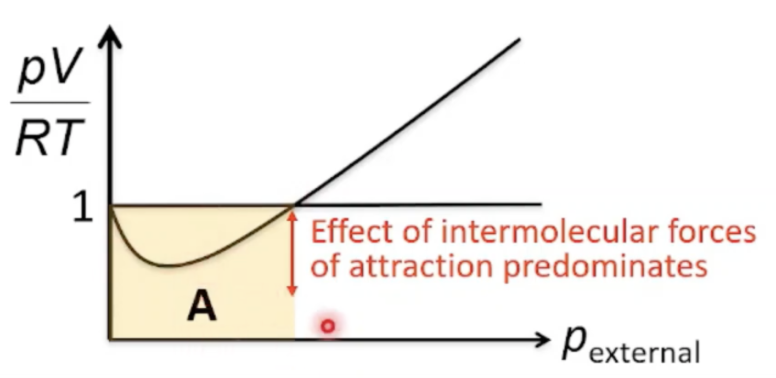
Explain pV value at moderately high pressure
At first blue bracket of graph, there is negative deviation (pressures up to 400atm), values of pV/RT are lower than ideal (< 1) due to significant intermolecular forces of attraction
Molecules that are about to strike the walls of the container experience intermolecular forces of attraction -> attractive forces decrease force of collisions between molecules and walls -> impact on walls reduced -> pressure exerted less than expected Pobserved < Pideal -> pV smaller than ideal value
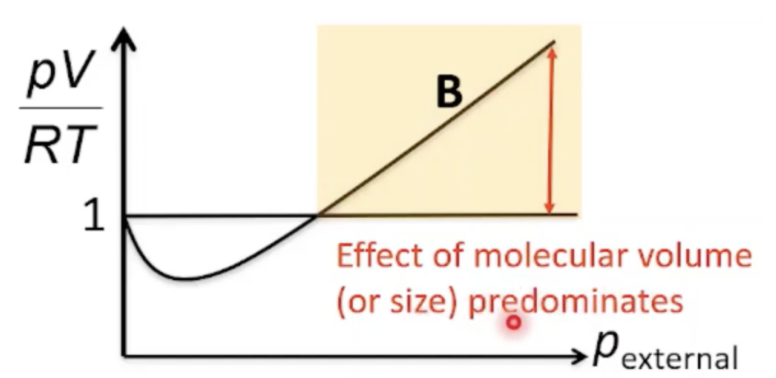
Explain pV value at very high pressure
At 2nd blue bracket of graph, there is positive deviation where values of pV/RT are greater than ideal (> 1)
At high pressures, the volume of the container decreases
The molecules are pushed closely together and take up a significant portion of the container volume, resulting in less space in which the molecules can move
Particles occupy a significant volume compared to the container volume
Gas is less compressible and volume of gas observed is greater than ideal volume in which molecules are move about -> pV higher than ideal value
Since the gas particles are close together, they tend to interact with one another, hence intermolecular attractions are not negligible
Explain how real gases deviate from ideal behaviour at low temp
As temperature is lowered, the kinetic energy of the gas particles decreases, causing them to move more slowly and intermolecular forces to become more significant
This also causes collisions to become inelastic (such that assumption 3 is no longer valid either) -> it reaches a point where the particles can no longer overcome the intermolecular forces, causing:
A molecule striking the wall of container experiences a force of attraction from other molecules and is pulled back -> lesson impact of wall -> pressure less than ideal OR
Real gases liquefy (condense to form a liquid) when cooled to below its boiling point
Describe the other effects causing real gasses to deviate from ideal conditions
At very high pressures where effect of molecular size or volume predominates, larger molecules experience greater deviation
At moderately high pressure where intermolecular forces of attraction predominates, molecules with stronger intermolecular forces have greater deviation
Eg. CO2 has a larger electron cloud and hence stronger dispersion forces than N2, hence CO2 deviates more from ideal behaviour.
For molecules with similar electron cloud size, other intermolecular forces besides dispersion forces need to be considered. NH3, with stronger hydrogen bonding, should deviate more from ideal behaviour than CH4, with only intermolecular dispersion forces
The H2 and H2 curves do not show the typical dip at moderate pressures
The IMF are so weak that the effect caused by molecular size or volume predominates at all pressures
State the type of molecules that deviate the most
Molecules that deviate the most:
Polar gases due to strong pd-pd or hydrogen bonds
Polar molecules (Eg. NH3) with hydrogen bonding deviate more than those with id-id (Eg. N2)
Large and heavy molecules due to significant molecular size or volume