S3.2-3.3-3.1 ions, metallic, structure, alloys
1/106
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
107 Terms
Empirical formula vs molecular
empirical is the simpelest whole number ratio of atoms it contains (limited use as it doesnt tell us the actual number of atoms in the molecule)
molecular is the actual number of atoms of each element present (multiple of empirical formula)
If we have a ration between molar mass of empirical and molecular formula
then that is the same ratio for number of atoms in either formula, so if there were 100g/mol of empirical formula Hg4Fe3, and 300 g/mol of molecular formula, the molecular formula would be Hg12Fe9
Structural formula
A representation of the molecule showing how the atoms are bonded
Full structural formula- shows every bond and every atom
Condensed structural formula- omits bonds where they can be assumed, groups atoms together
Full structural formula would be the C-C with six H-s around it
condensed would be CH3CH3
Stereochemical formula
Shows the relative positions of atoms and groups around a central carbon in 3 dimensions
Dotted line goes back behind paper
solid line is in the plane of the paper
Thick triangle line come forward off the paper
Skeletal formula
shorthand representation of a structural formula, shows all bonds present in the molecule, except C-H bonds, omits C and H symbols
C4H10
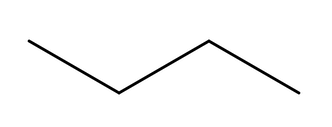
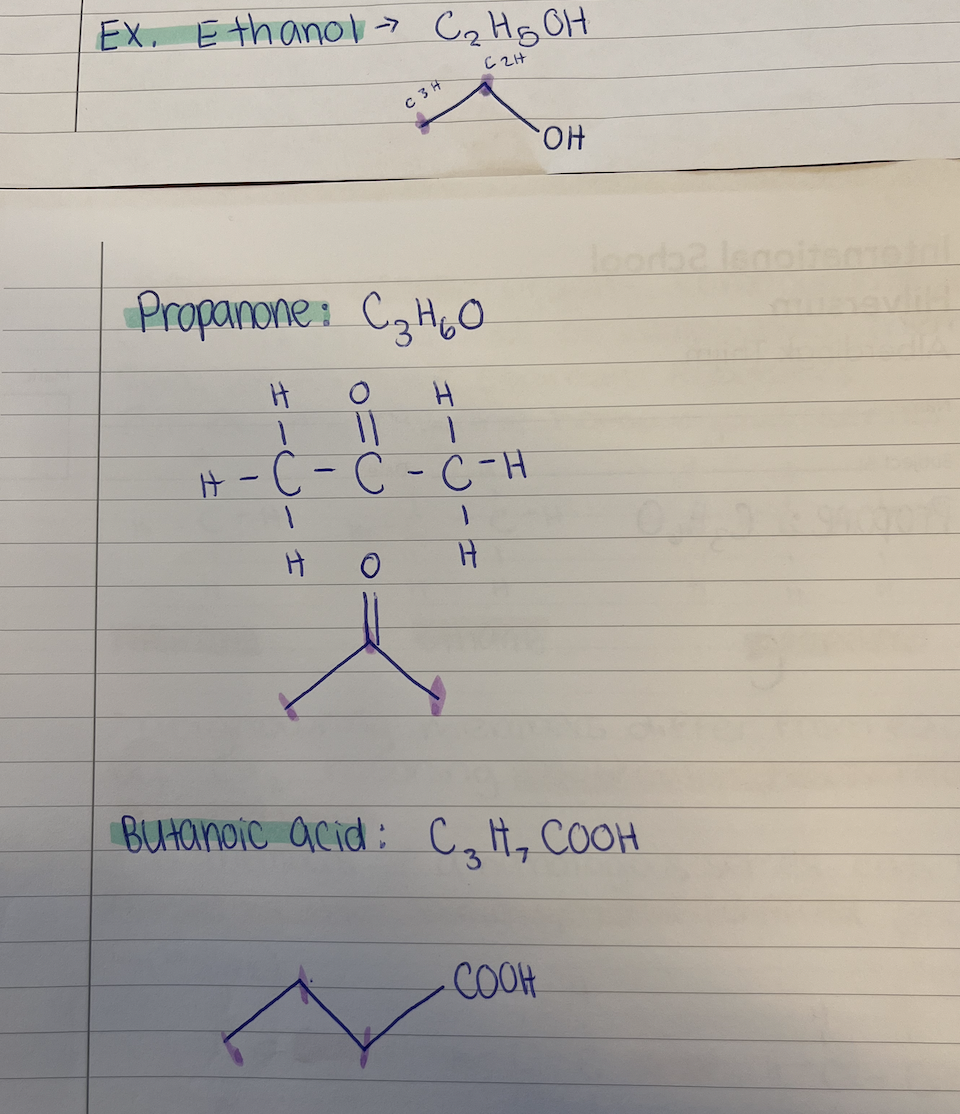
Examples ethanol structural formula (C2H5OH), propanone (C3H6O), butanoic acid (C3H7COOH)
first sketch out how it should look normally, and then make skeletal formula, draw each C spike, and the extra things are another line coming off of it
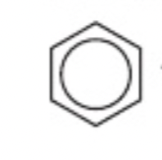
Molecules containing a benzene ring
containing C6H6 we use the benzene ring to show these aromatic compounds
Catenation
Carbon is able to link to itself and form chains of bonded carbon atoms (why we have so many organic compounds)
Carbon atoms are able to form a variety of compounds containing single double or triple bonds
Functional groups
Atoms or groups of atoms that are present in organic compounds- responsible for a compound’s physical/chemical properties
compounds that contain the same functional group belong to the same class
ex class of carboxilic acids contain the same -COOH functional group
Common classes of organic compounds
Alkane-
suffix: -ane
ex ethane
general formula CnH2n+2
Alkene-
functional group- alkenyl
suffix-ene
ex ethene
CnH2n
Alkyne-
functional group- alkynyl
suffix-yne
ethyne
CnH2n-2
Alcohol-
funcitonal group- hydroxyl OH
suffix- anol
C2H5OH ethanol
General formula CnH2n+1OH
Ether
R-O-R’
functional group- alkoxy
suffix- oxyalkane
H3C-O-C2H5 methoxyethane
general formula- CnH2n+2+O
Aldehyde-
Functional group- carbonyl (aldehyde)
suffix- anal
ex of compound- C2H5CHO propanal
CnH2nO R-CHO
always at end
Ketone-
functional group- ketone
suffix- anone
ex. propanone CH3COCH3
general formula- CnH2nO R-CO-R’
always in middle
Carboxylic acid-
Carboxyl functional group
suffix- anoic acid
ex. of compound- C2H5COOH propanoic acid
general formula- CnH2n+1COOH
Ester-
Functional group- carboxyl (ester)
suffix-anoate
ex of compound- C2H5COOCH3 methylpropanoate
general formula - CnH2nO2
R-COO-R’
Amide-
Functional group- amido
suffix- anamide
ex. C2H5CONH2 propanimide
general formula CnH2n+1CONH2

Naming esters and ethers
Esters
Name by prefix being the compound single bonded to the O and the stem being the compound bonded double to the O
Ethers
2 alkyl chains linked by an oxygen atom, longer chain will be the stem and shorter chain is regarded as a substieunt and has prefix alkox, shorter chain-oxy-longer chain

naming amine and arene
Amine-
functional group- amino
suffix- anamine
C2H5NH2, ethylanamine
general formula-CnH2n+1NH2
Arene-
Functional group- phenyl
suffix benzene
C6H5CH3, methylbenzene, C6H5 is benzene, whatever added on becomes the prefix
Naming summary
Prefix- position, number, and name of substituents
Stem- Number of longest chain of C atoms
Suffix- Class of compound determined by functional group
saturated vs unsaturated compounds
Saturated contains only single bonds
Unsaturated contains double or triple bonds
Reaction pathway
Synthesize target compounds by organising several reactions in a sequence so the product of one is the reactant of the next, each step involves a functional group interconversion
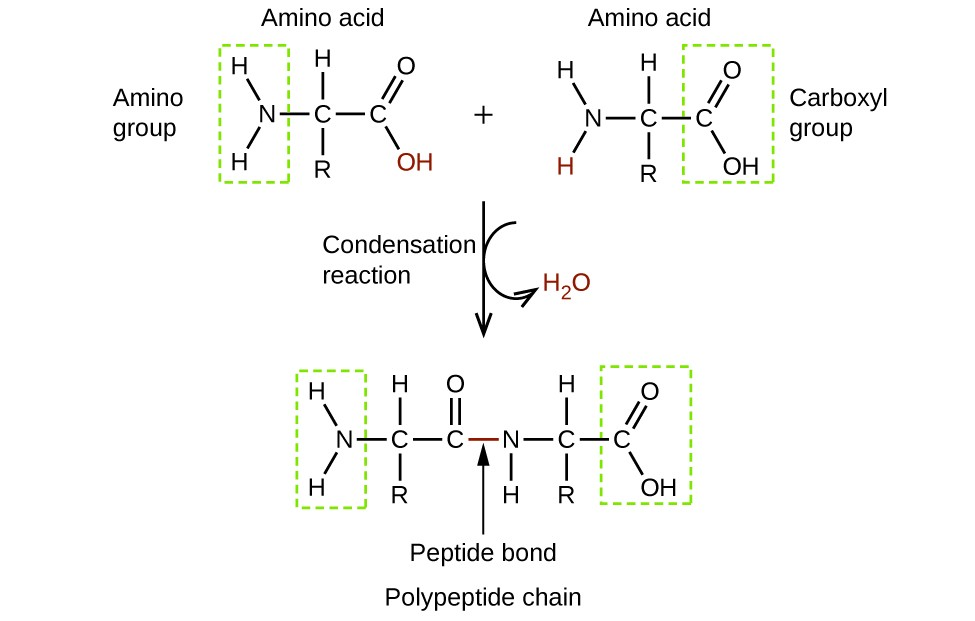
ex. the reaction of 2 amino acids shows how functional groups on different molecules can react to give a new class of product
still has functional group on both sides so condensation can happen again to form a tripeptide and eventually a polypeptide
Homologous series
Organic compounds that are classified into families of compounds, members of each series have common features, for example the alkane homologous series
methane, ethane, propane
all members of a homologous series can be represented by the same general formula
some homologous series are characterized by the presence of a functional group for ex. alcohols shown below
methanol (CH3OH), ethanol (C2H5OH), propanol (C3H7OH)
homologous series properties
neighbouring members differ from each other by CH2 in alkane example, meaning molecular mass increases by a fixed mass
successive members have successively longer carbon chain causing a gradual trend in physical properties of members
the effect of the length of the carbon chain on the boiling point; gets higher as you get longer carbon chain length
same with melting point, density (stronger van der waal forces as induces more induced and instantaneous dipoles so more energy needed to break bonds present, stronger intermolecular forces )
Effect of polar vs non polar bonds/ intermolecular forces on physical properties
IUPAC nomenclature
Rules 1) identify the longest straight chain of carbon atoms
1- meth
2-eth
3-prop
4-but
5-pent
6-hex
2)identify the functional group- Determines the suffix to the name, replaces the “ane” in the parent alkane
class- refers to the type of compound
functional group- site of reactivity in the molecule
position of the funcitonal group is shown by a number inserted before the functional group ending
the chain is numbered starting at the end that will give the smallest number ot the functional group
ex. Propan-2-ol CH3CHOHCH3
but-1-ene- CH3CH2CHCH2
sometimes you dont need the number for ex. carboxilic acid is always at end of chain
3-identify side chains, side chains or functional groups are known as substieunts and are given the prefix check table
NH2 appears as a suffix and a prefix, when its the only functional group it will take the suffix (anamine), but if it is one of two or more it will be a prefix (amino)
if there are more than one substituent groups we use commas between the numbers of where they are and the prefixes di, tri, tetra, if there is more than one subtituent we place them in the name in alphabetical order ex. CCLH2CBrClCH3- 2,bromo,1,2,dichloropropane
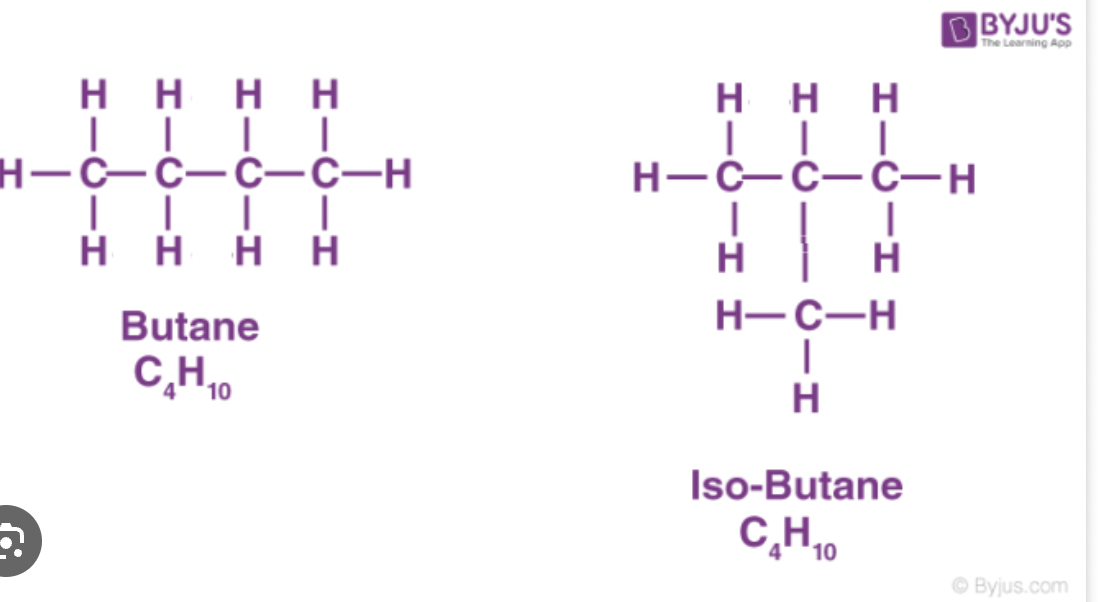
Organic compounds with the same molecular formula can have different structures (not same name, same molecular formula)
ex. C4H10 can be butane or 2-methylpropane, because of this they will have different properties
Same molecule, different arrangements of atoms are structural isomers
each isomer is a distinct compound with unique physical and chemical properties
number of isomers for a molecular formula increases with the size of the molecule, ex pentane has pentane, 2methylbutane, 2,2,dimethylpropane
the more branching, the lower the boiling point so pentane has the highest, 2methylbutane middle and 2,2dimethylepropane has lowest
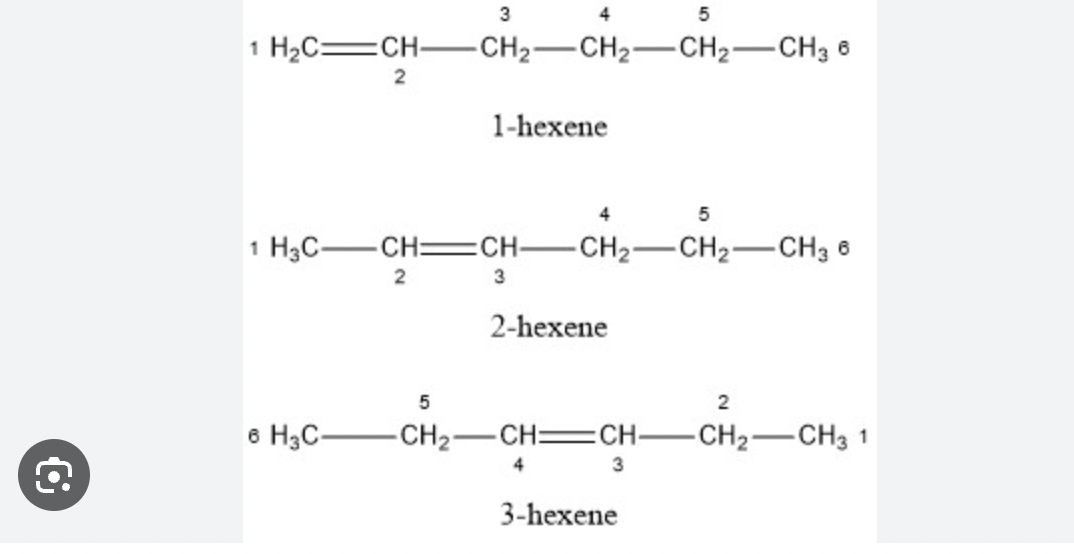
Alkenes can have structural isomers
contain atleast 1 carbon carbon double bond, different type of isomer, not due to branching like alkanes but due to position of the double bond (functional group)
ex C6H12 hexene
also applicable for alkynes with atleast one triple bond and its possible positions
structural isomers can have functional groups attatched but they must be in the same place
positional isomers
have the same functional group but at different positions 1bromopropane vs 2bromopropane
metal atoms
lose electrons to form positive ions (cations)
non metal atoms
gain electrons to form negative ions
atomic number of an element is defined in, valence electrons vs protons
number of protons which does not change in the chemical reaction, valence electrons are the furthest from the electrostatic attraction of the nucleus so more open to external influences
Outer electrons of metal atoms experience a smaller effective nuclear charge than the outer electrons of non metals
nuclear charge given by atomic number which increases across a period
valence electrons which determine chemical properties do not experience full attraction of the charge as they are shielded from nucleus/repelled by inner electrons, presence of innershielding reduces attraction of the nucleus for outer electrons and effective charge experienced by valence electrons is less than full nuclear charge
across period ENC increases as same shielding but increase in proton number
down a group increase in nuclear charge from increase proton number is offset by increase in inner shielding remains the same
Metal atoms form positive ions as they (cations)
have low ionization energies (little energy needed to remove outermost electron)
first ionization energies increase across a period due to increase in ENC
increased ENC, harder to remove electron
more likely to lose an electron and form a positive ion if it is a metal on left of periodic table, low ionization energy
ionization energy decrease down a group metals on bottom left have greatest tendancy to lose electrons and form ionic compounds
Non metal atoms form negative ions as they have (anions)
a high effective nuclear charge
electron transfer will be more likely to occur if non metals attract the transferred electrons more strongly
non metal top right highest ENC (most atomic number and least inner shielding layers) and smalledt atomic radii
once electron transfer is complete
electrostatic attraction between oppositely charged ions pull the ions together
most vigorous reactions occur
between elements furthest apart in the periodic table
caesium is the most reactive alkali metal, bottom group 1, lowest ionization energy
flourine is the most reactive non metal, smallest atomic radius, attracts transferred electrons the strongest
increased force of attraction ionic
greater charge, more electrons transferred
halogens will attract electrons the strongest as they have only one vacancy left to fill their outer shells
formation of Si4- ion not feasible because
the addition of electrons becomes more difficult with increasing negative charge of the ion due to increased electron electron repulsion
High first ionization energies
(lot of energy needed to remove electron), noble gases, nucleus holds tightly to outer electrons, not available for chemical activity, complete outershell, added electrons would occupy an empty outer energy level, ENC of zero
Transition metals form
ions of different charge, iron (Fe2+ or Fe3+) or copper (Cu+ or Cu2+)
the iron and copper ions of different charge form compounds with different properties like color
Naming for transition metals using oxidation number
only necessary when an element has more than one oxidation state, ex Na2O only shows one in compounds
Formula compound- FeO, oxidation state Fe2+, name using oxidation number Iron (II)oxide
Cu2O, Cu+, Copper(I)oxide
CuO, Cu2+, copper (II)oxide
MnO2, Mn4+, manganese (IV)oxide
ionic compound is generally nuetral, how to balance negative and positive charge
look at charge of both, and see what you need to multiply one or both by to get the lowest same whole number ratio
Polyatomic ions
made up of more than one atom together have lost or gained an electron, found in salts formed from common acids
NO3- HNO3 acid
NO3- Nitrate
SO42- sulfate
PO43- phosphate
OH- hydroxide
CO32- carbonate
HCO3- hydrogen carbonate
NH4+ ammonium
CHC3OO- ethanoate
bonds holding polyatomic ions together are covalent bonds, but once they join together, the bonds that joins two polyatomic ions are ionic bonds
Ionic compounds have a lattice structure, ionic bond, geometry
once ions are formed by electron transfer they are pulled together by electrostatic attraction which is the ionic bond
many cations and anions arrange themselves in a 3D lattice structure held together by ionic bonds between oppositely charge ions
geometry varies for each compound depends on sizes of ions, involves a fixed arrangement of ions based on a repeating unit cell
consists of a very large number of ions, can grow indefinitely
ionic compounds do not
have a fixed number of ions so their formulas are ratios of ions present , empirical formula known as the formula unit
formation of the ion energetically feasible
energy output when ion is formed (neg enpalthy) is offset by energy required to form the ion (pos enpalthy)
Lattice enpalthy
the measure of strength of the ionic bond in different compounds influenced by ion radius and charge (amount of energy to seperate one mole of solid ionic compound into consituent gaseous ions)
enpalthy change can be calculated by
the ionic model which assumes that the crystal made up of spherical ions which only interact by electrostatic forces
increase in ionic charge increases the attraction between ions and increases lattice enpalthy
increase in ionic radius of one of the ions decreases attraction between ions and decreases lattice enpalthy
lattice structure results in
ionic compounds being crystalline solids at room temperature, high meleting and boiling points (large amount of energy needed to seperate the ions in the lattice), and low volatility
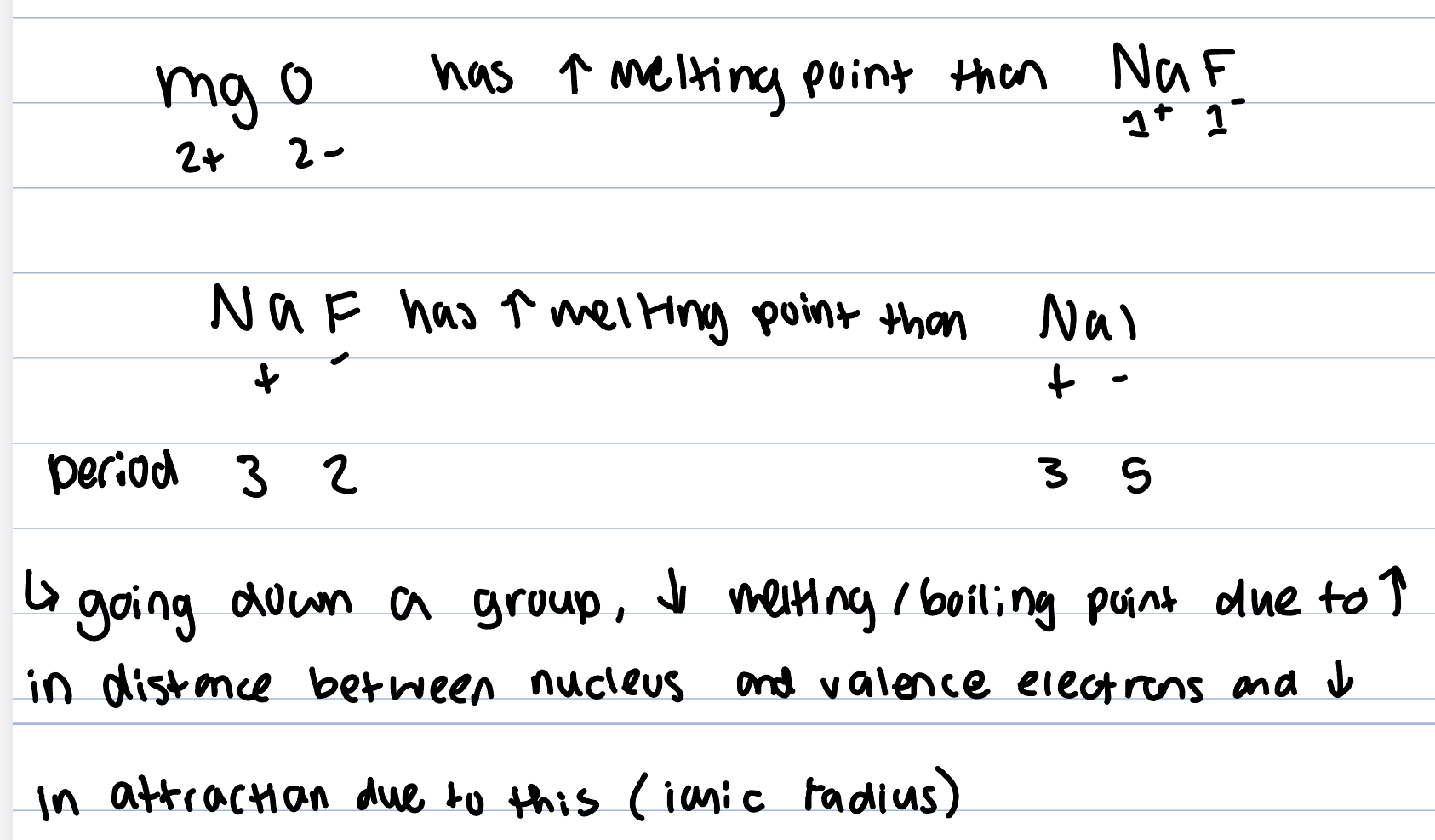
ionic solubility
high solubility in water as water is polar and the partial positive charges on H attract negative ions and the partial negative charges on O attract positive ions
at the contact surface of a crystal, attraction of ions to partial charges in water molecule pulls ions away from their lattice positions as those ions are now seperate they are surrounded by water molecules and are hydrated and dissolved
if liquid is non polar there is no attraction between liquid molecules and ions as there are no partial charges so ions remain in lattice and solid is insoluble
exceptions like calcium carbonate not soluble in water
solvents other than water, solute ions are said to be
solvated
ionic compounds electricity conductors
not able to conduct electricity in their solid state as the ions are now fixed within their lattice but they can conduct in the liquid state as the ions are mobile (can carry pos or neg charges from one area to another)
ionic compounds are brittle
the crystal structure shatters with shear force as ions within the lattice are displaced resulting in ions of the same charge being positioned alongside each other and repulsive forces between the pos-pos and neg-neg ion cause the lattice to split
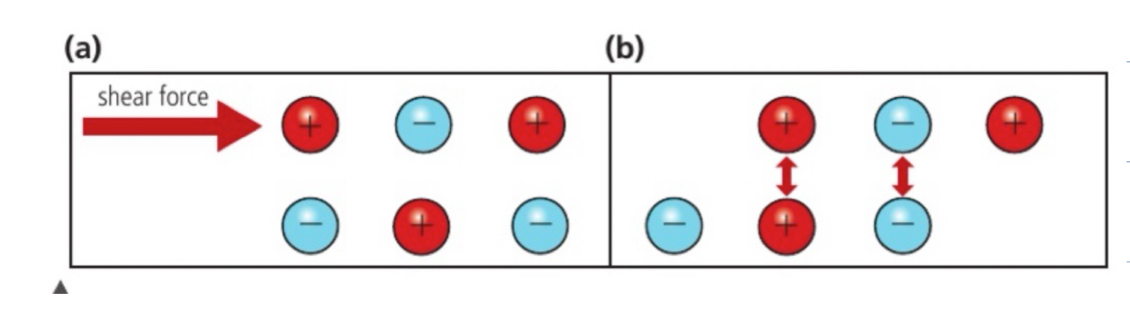
when two elements react to form an ionic compound they show different tendancies to lose or gain electrons
metals on the bottom left lose electrons most easily, non metals on top right gain electrons most easily, caesium and flourine are the compound with the most ionic character
down a group, in first group most reactive metal, electrons furthest away from nucleus so most reactive and most tendancy to form positive ion, electron can be removed easiest
across a period, increasing tendancy to form negative ions, most reactive non metal group 17 period 1, electrons are closest and ionization energy is the highest and electron affinity is the highest
period 3 chloride compounds
less ionic across a period and more covalent, meaning lower melting point as turns more covalent (molecular covalent), and electrical conductivity in molten state gets lower (ions can carry pos or neg charge and move in ionic compound, atoms are nuetral and no charged particles free to move in molecular covalent), ionic bonds stronger than weak intermolecular forces
A covalent bond forms by
Atoms sharing electrons
Atoms of two non metal reacting together
Both seeking to gain valence electrons to achieve the stable electron structure of a noble gas
share electron pair to do this
shared pair of electrons is concentrated in the region between the two nuclei and is attracted to them both
electrostatic attraction between the shared pair of electrons and positively charged nuclei holds atoms together- covalent bond
why the atoms are held at a fixed distance apart in covalent bond
system containing the two atoms is stabilized when the forces of attraction between the nuclei and shared electrons are balanced by the forces of repulsion of the two nuclei
covalent bonds form at the point
of lowest energy as two atoms approach each other

octet rule
8 electrons for a full outer shell to predict stable arrangements in covalent bond
ex. cl needs to gain 1 electron because group 17 have 7 valence electrons, so both will share 1 electron
ability of two identical atoms to form a covalent bond
is due to the similair strength with which they attract valence electrons
atoms of group 18
have a complete octet without needing to share electrons, low levels of reactivity, do not form covalent bonds (except helium)
Incomplete octet
Be beryllium and B boron forms stable molecules in which central atoms have less than 8 valency electrons (small atoms)- limits number of atoms that can get close enough to share required number of electrons for complete octet
Be has 4
B has 6
Lewis structure
Describes the structure of covalent molecules
1- calculate total number of valence electrons in the molecule
2- look how many time each one has to bond (how many extra electrons it needs to gain)
3- all other electrons are lone pairs
4-check that all atoms have a full valency shell and that the total number of valency electrons is correct
if there are ions, if it is negative, you add an electron if positive you remove one, see where it fits best so that there are lone PAIRS of electrons and not an uneven number, and that there are still full valency shells and the right number of electrons
diatomic molecules
normally bond with double or triple bonds which are hard to break making the molecule very stable (not reactive)
multiple bonds have a greater number of shared electrons and a stronger force of electrostatic attraction to bonded nuclei, greater pulling power between atoms brings them closer together resulting in shorter and stronger bonds than single bonds
3x bonds is most 1x bonds is least strong
1x bonds is greatest length 3x bonds is least
oxygen, nitrogen, hydrogen, flourine, chlorine, bromine, iodine
Covalent bond characterized by
bond length- distance between 2 bonded nuclei
bond strength- measure of energy required to break the bond (enpalthy)
atomic radius increases down a group
more shells, then the bond length increases
bond enpalthy decreases because the shared electron pair is further from the pull of the nuclei in the larger molecule, so bond is weaker and takes less energy to break
Coordination bond, ex NH4+, H3O+, CO
A coordination bond is a covalent bond in which both shared electrons come from same atom
an arrow on the head of the bond is used to show coordination bond
once coordination bonds are formed, they are no different from other covalent bonds
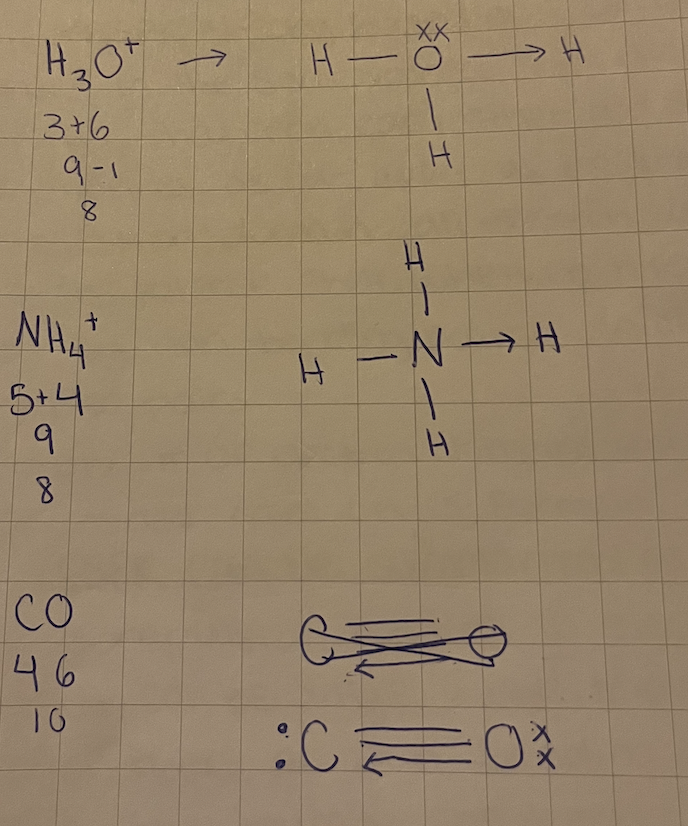
shape of a molecule is determined by
repulsion between electron domains around central atoms
based on VESPR theory-
Because electron pairs in the same valence shell carry the same charge they will repel each other and spread out as much as possible
the repulsion applies to electron domains, which can be single, double, triple bonding electron pairs, or non bonding pairs of electrons
the total number of electron domains around the central atom determines the arrangement of the electron domains
the shape of the molecule is determined by the angles between the bonded atoms
non bonding pairs and multiple bonds cause slightly more repulsion than a bonding pair because a non bonding pair has a higher concentration of charge than bonding pairs, as they are not shared between 2 atoms and multiple bonds have a higher concentration of charge as they contain 2 or 3 pairs of electrons
electron domain
all electron locations in the valence shell, whether they are occupied by the non bonding pairs, single, double, triple bonded pairs
total number of electron domains and this can be determined from Lewis formula determines shape
repulsion force
single bond<double or triple bond<lone pairs
molecules with lone pairs or multiple bonds on central atom have some distortions in their structure that reduce the angle between the bonded atoms
electron geometry vs molecular geometry
electron geometry is based on the lone pairs and the bonding pairs, lone pairs is only on central atoms
molecular geometry is only the bonding pairs
2 electron pairs
Positioned 180 degrees from each other, linear shape
if there are two bonding pairs and two lone pair, molecular geometry is v shaped and electron geometry is tetrahedral (104.5 degrees if there is two lone pairs )
if there are two bonding pairs and one lone pair, electron domain is triangular planar, molecular geometry is v shaped (117 degrees)
3/4 electron domains
positioned 120 degrees from each other, triangular planar shape as electron domain geometry
when you have a double bond, bond angles will be slightly distorted due to increased repulsion from double bond, so less bond angles
if there are three bonding pairs and one lone pair, electron domain is tetrahedral and molecular geometry would be triangular pyrimidal, 107 degrees
tetrahedral is 109.5 degrees
4 electron domains
positioned 109.5 degrees from each other, tetrahedral electron domain geometry, same for molecular geometry if all 4 are bonding
Polar bonds occur from
If electron pairs spend more time with one atom than the other, they are not equally shared occuring when there is a difference in electronegativity of bonded atoms
electronegativity- ability of an atom to attract electrons in a covalent bond
as the more electronegative atom exerts a greater pulling power on the shared electrons, it gains more posession and electron distribution is uneven- polar bond
Bond dipole
This type of bond has two partially seperated opposite electric charge
The more electronegative atom with greater share of electrons is partially negative, less electronegative becomes partially positive
extent of polarity in a covalent bond varies depending on how big a difference between electronegativity values between bonded atoms
electronegativity periodic trends
increases across a period, more protons, same inner shielding, more ENC
Increases up a group, less inner shielding, more ENC
flourine is most electronegative atom, will have greatest electron density when covalently bonded to another element
only bonds that are non polar
are bonds between the same atoms, such as F2, O2, H2, difference in electronegativity is zero so it is pure covalent
C-H very low polarity, determines properties of organic compounds
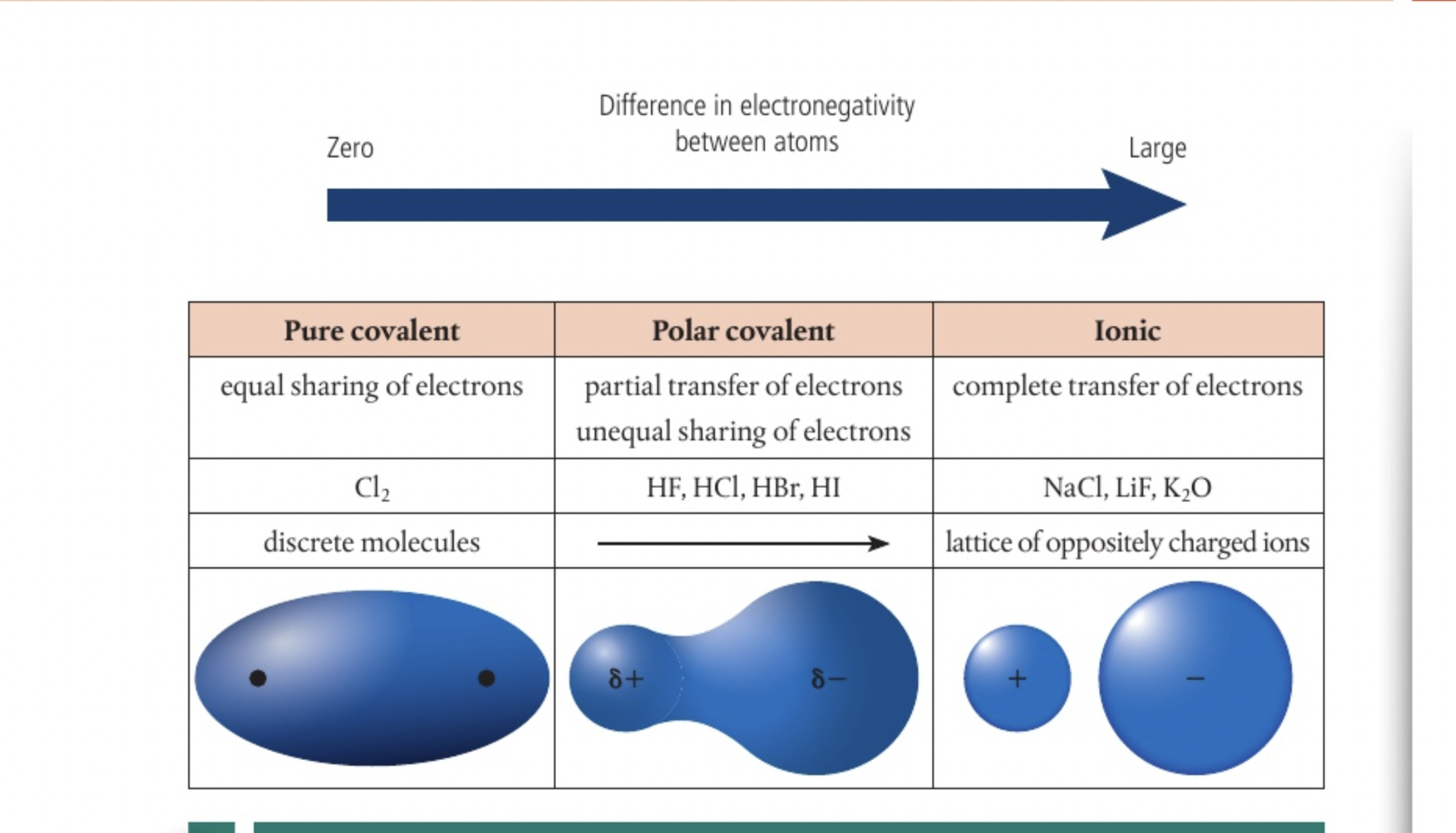
polar vs covalent vs ionic
the more polar the bond the greater the seperation of charges, more like an ionic compound, so polar bonds act like an intermediate between pure covalent and ionic bonds which is why we see an overlap in properties of ionic and covalent substances
more than 1.7 ionic
more than 0.4 POLAR
Molecular polarity depends on
the polar bonds it contains
the way which such polar bonds are orientated with respect to each other; molecular geometry
vs. bond polarity depends on charge seperation between its two bonded atoms
if bonds are of equal polarity and are arranged symmetrically, their charge seperations will oppose/cancel each other out, the molecule will be non polar but contain polar bonds
ex. CO2
notation for a dipole that results from the pull of electrons in bond towards more electronegative atom is the arrow with a line
If either the molecule contains bonds of different polarities or its bonds are not symettrically arranged
dipoles will not cancel out and molecule will be polar and will have a net dipole
find which molecules are polar or non polar by
first draw lewis structure
find electronegativities of each molecule in data booklet
find differences in electronegativities and write them next to the bond
largest difference will pull most in that direction and have a partial negative charge
most covalent structures exist as
discrete molecules with a finite number of atoms
covalent network structure
some substances have a crystalline lattice structure in which the atoms are linked together by covalent bonds , single molecule with a regular repeating pattern of covalent bonds, has no finite size, have different properties than other smaller covalent molecules
Allotropes
have different bonding and structural patterns of the same element in the same physical state- different chemical and physical properties ex. molecular oxygen and ozone both exist as gases are examples of allotropes of oxygen
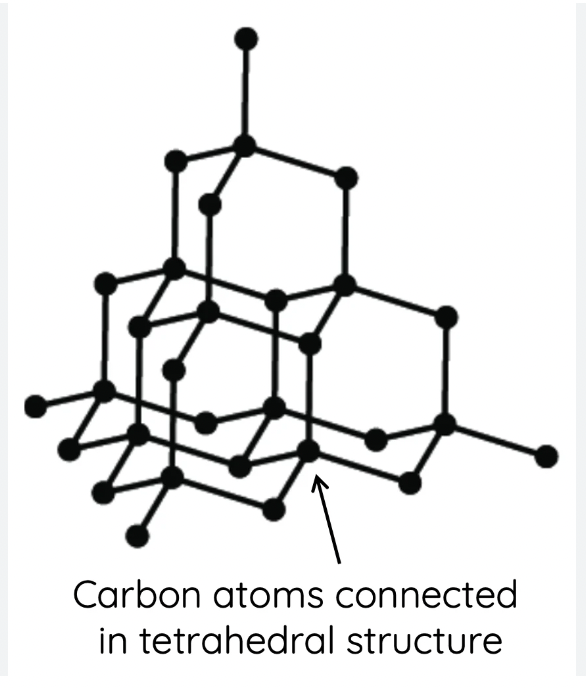
Carbon’s allotropes- diamond
structure- Each C atom is covalently bonded to 4 others, tetrahedral, repetitive pattern, 109.5 degrees bond angles
non conductor of electricity- all electrons bonded so non mobile
thermal conductivity- very efficient better than metals (strong covalent bonding)
appearance- transparent
physical/chemical- hardest natural substance, brittle, very high melting point
uses- tools for cutting glass or jewelery
Graphite
Structure- each C atom covalently bonded to 3 others, hexagons in parallel layers, bond angles in 120 degrees, valence electrons remaining delocalized and can move freely across layers held together by weak london dispersion forces so they can slide over each other
electrical conductivity good, contains one delocalised electron pair per atom giving mobility
thermal conductivity- not good unless heat can be forced to conduct in a parallel direction to crystal layers
appearance- grey crystalline solid
physical/chemical- soft and slippery due to slippage of layers over each other, brittle, high melting point, stable
uses in pencils
functional group isomers
molecules with the same molecular formula, but different functional groups
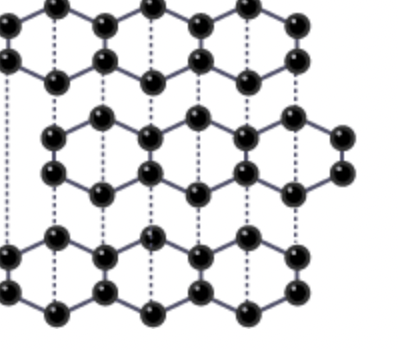
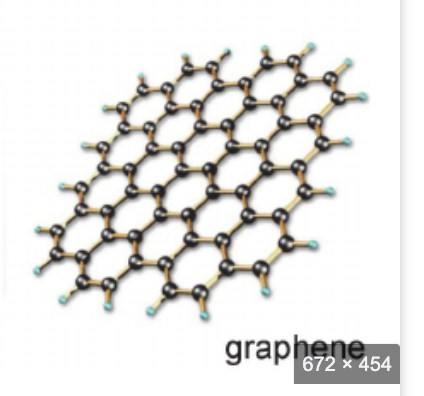
graphene
structure- each C atom is covalently bonded to 3 others, bonding angles of 120 degrees, 2D, remaining electron on each carbon is delocalized
electrical conductivity- very good, one delocalized electron per atom gives electrical mobility
thermal conductivity- best thermal conductivity
appearance- almost transparent
physical/chemical- thickeness of one atom, thinnest material, strongest, flexible, high melting point
uses— transmission electron microscopy
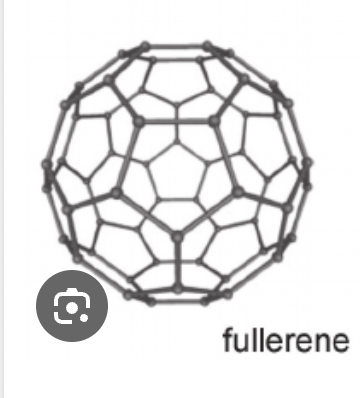
Fullerene
structure is carbon bonded to 3 others, closed spherical cage of 60 carbon atoms
electrical conductivity- poor conductors, little movement of electrons between molecules despite delocalised electrons
thermal conductivity- very low
appearance- black powder
physical/chemical- light strong low melting point
uses lubricants for medical purposes
graphite occurs naturally
single seperated layer is graphene, rolled up layer of graphene is a nanotube, closed cage is fullerene
development of structures like nanotubes, nanobuds, graphene is part of nanotechnology science through atomic scale and manipulation of matter
silicon
atoms have 4 valence shell electrons
in elemental state each is covalently bonded to four others in tetrahedral, giant lattice structure
silicon dioxide
forms a giant covalent structure based on tetrahedral arrangement
each Si atom covalently bonded to 4 O atoms and each O atom to 2 Si atom
strong,insoluble,high melting point, non conductor of electricity due to strong tetrahedral position, glass, sand
silicon commonly bonds with O and not with itself like carbon, which are stronger than si-si bonds as c-c bonds have a smaller atomic radius, greater electrostatic attraction
in a covalent lattice structure
covalent bonds hold atoms together within the molecule but intermolecular forces exist between the molecules depending on size and polarity of the molecules
strength of intermolecular forces determine physical properties of a substance (volatility, solubility, conductivity)
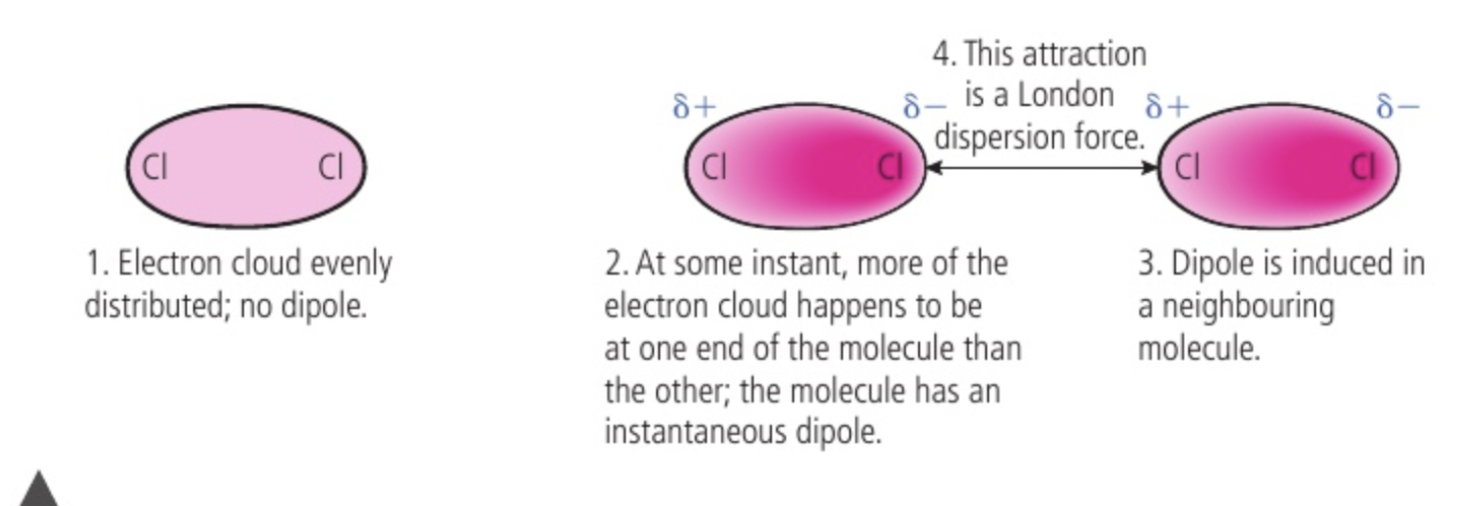
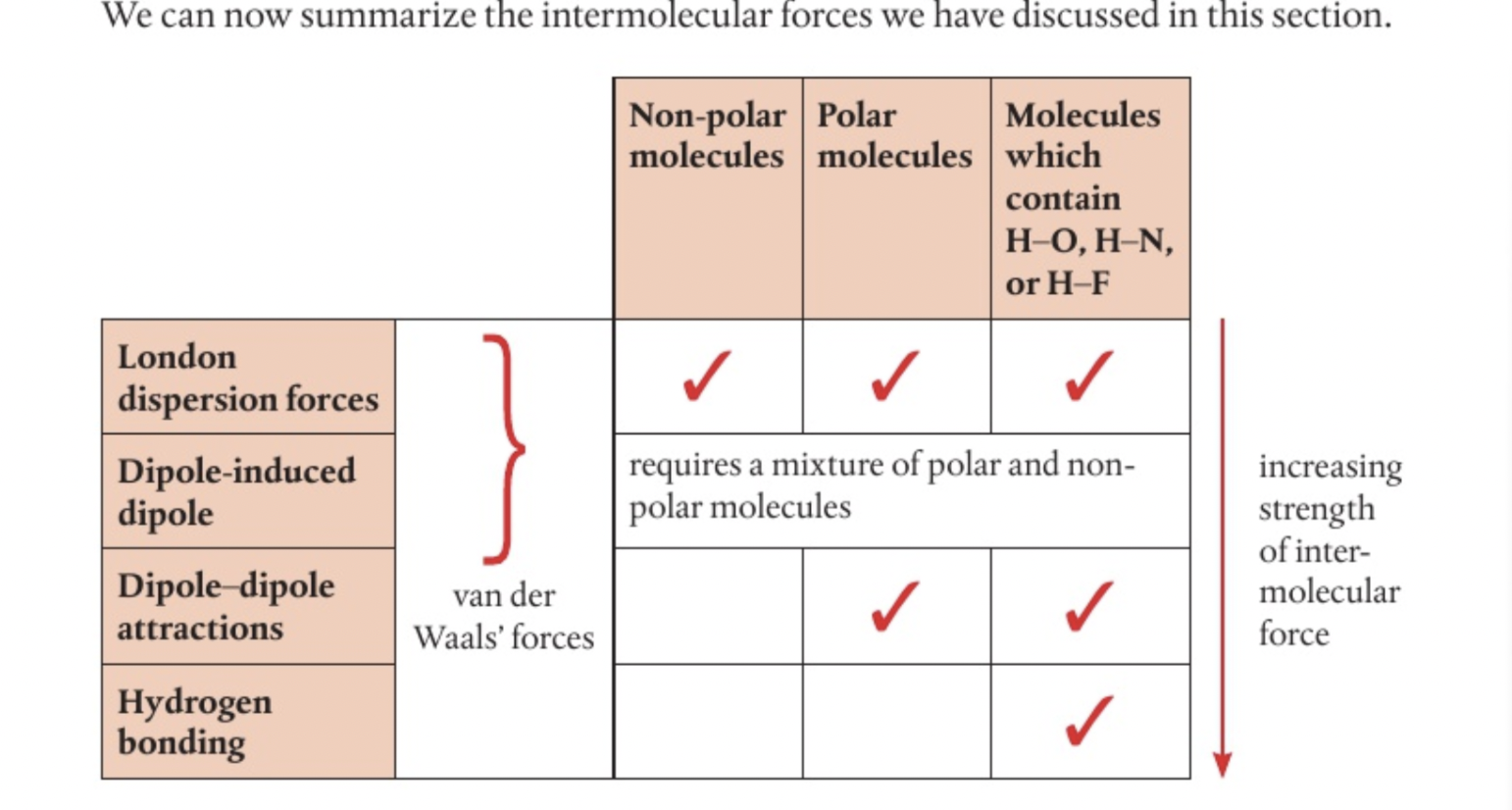
London dispersion forces
non polar molecules like Cl2 have no permanent seperation of charge within their bonds because shared electrons are pulled equally in both directions, no permanent dipole
electrons behave as mobile clouds of negative charge, the density of cloud may be greater over one atom than over another at any moment, when this happens an instantaneous dipole gives some seperation of charge on one atom, only lasts an instant, and may influence electron distribution in bond of a neighbouring molecule, induced dipole (makes the nearby side positive because the negatives repel away from each other)
weak london dispersion forces occur between opposite ends of two temporary dipoles in the molecules, weakest intermolecular force, strenght increases with increasing molecular size as you have a greater number of electrons which increases the probability and magnitude of instantaneous dipole formation
only forces between non polar molecules, low melting and boiling points, little energy required to overcome and seperate molecules
strength increases with size so does melting and boiling point, responsible for the fact that non polar substances can condense to form liquids at low temperatures
can also be components of the forces between polar molecules but get overlooked because other forces are stronger
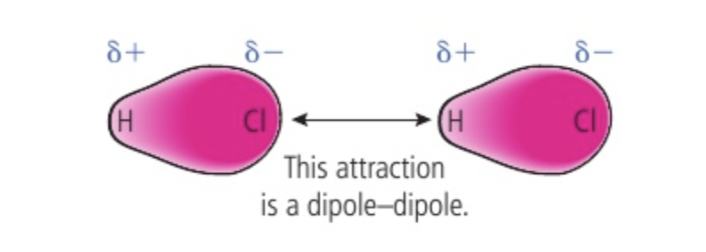
Dipole dipole attraction
Polar molecules like HCl have a permanent seperation of charge (mismatching electronegativities)
one end is partial negative if it is electron sufficient and one end is partial positive if it is electron sufficient so there is a permanent dipole resulting in opposite charges on neighbouring molecules attracting each other
strength of this force will vary depending on distance and relative orientation of dipoles always stronger than london dispersion due to permanent rather than instantaneous dipole, melting and boiling points of polar compounds higher than those of non polar substances of same mass, leads to solubility of polar solutes in polar solvents
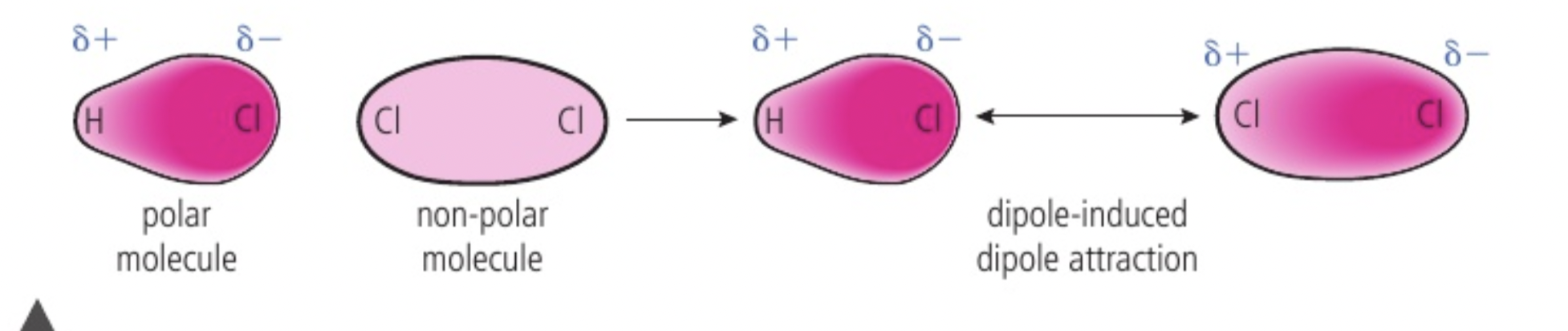
dipole induced dipole attraction
mixture between polar and non polar molecules, the permanent dipole of a polar molecule can cause a temporary seperation of charge on a non polar molecule- force is dipole induced dipole attraction (suddenly because there are so many electrons on one side of a neighbouring molecule, the other side’s electrons will be repelled away and positive charge will be attracted to neighbouring molecule’s negative side)
acts in addition to london dispersion forces between non polar molecules and dipole dipole forces between polar molecules
Van der Waals forces
Includes all three forces, refers to all forces between molecules that do not involve electrostatic attractions between ions
van der waals forces in some cases can occur within a molecule if different groups can position themselves appropriately
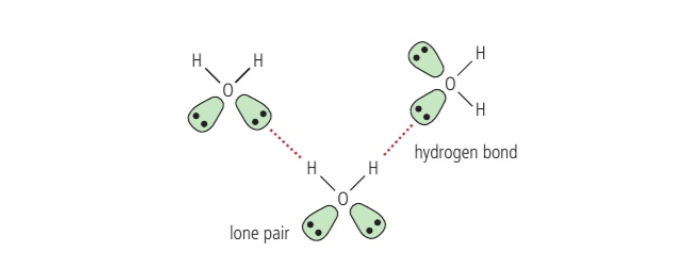
Hydrogen bonding, why ice floats on water, why water is liquid at room temp
Molecule containing hydrogen covalently bonded to a very electronegative atom
strongest intermolecular force
particular case of dipole dipole, large electronegativity difference between hydrogen bonded oxygen for ex which causes electron pair to be pulled towards oxygen, becuase of its small size and having no other electrons to shield its nucleus, hydrogen with a partial positive charge exerts a strong attractive force on negatively charges lone pair in the electronegative atom on the neighbouring molecule with a partial negative charge
higher melting and boiling points than other forces with same mass
if not for hydorgen bonding, water should be a gas at room temperature due to its low mass, but because of two hydrogen atoms and 2 lone pairs, each water molecule can for four hydrogen bonds with neighbouring molecules but liquid will contain less than four
ice contains four and is maximally hydrogen bonded resulting in tetrahedral arrangement holding molecules at a fixed distance apart and less dense than liquid so that way it floats on water
density change means water expands on freezing
hydrogen bonds weaker than
covalent and ionic bonds but stronger than all other intermolecular forces
intermolecular and intramolecular forces both are categories of electrostatic attraction, but significant difference in relative strengths
Intermolecular forces table
melting and boiling points, what are they, covalent vs ionic, covalent giant vs molecular, molecular size and polarity, hydrogen bonding
changing states by melting and boiling involves seperating particles by overcoming the forces between them
the stronger the interparticle forces, the more energy that will be required to break the forces and higher melting or boiling points
covalent substances have lower melting/boiling points than ionic, forces to overcome to seperate molecules are the weak intermolecular forces, easier to break than the electrostatic in ionic lattice
strength of intermolecular forces increases with increasing molecular size (more electrons, more magnitude of intermolecular forces) , also with increase in extent of polarity due to more dipole forces
simple covalent molecules have a small and fixed number of atoms, easier to break intermolecular forces in these than giant molecular structures have stronger covalent bonds (repeating lattice is harder to break), can be as strong as ionic compounds
Hydroxyl functional group leads to formation of hydrogen bonding in acohols
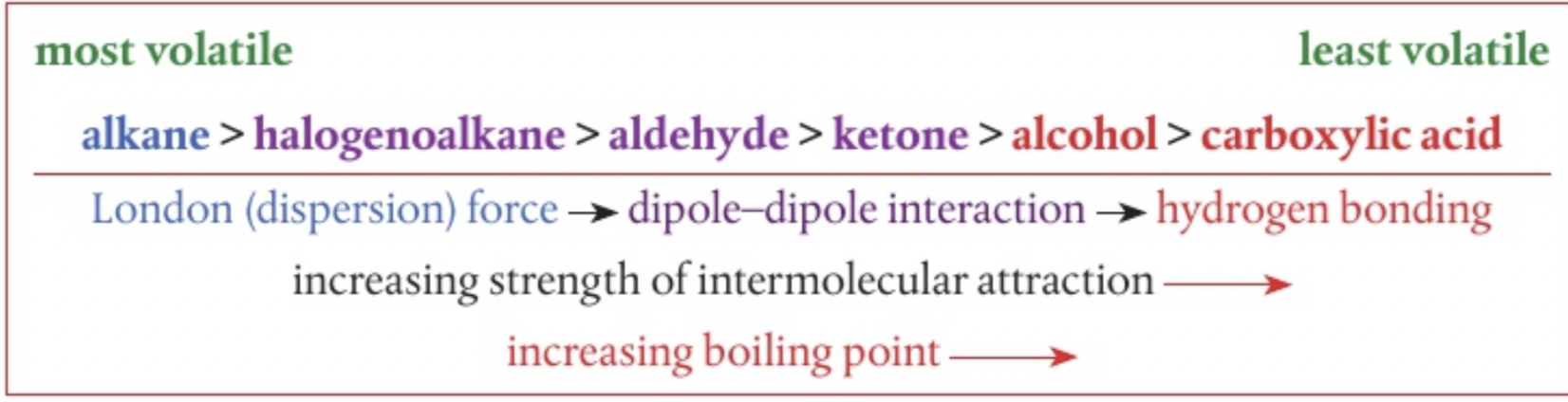
volatility
Tendancy of a substance to vaporize (liquid/solid to vapour)
substance with strong intermolecular forces will have a lower tendancy and vice versa
C3H7OH is less volatile than C4H10 due to strong hydrogen bonding
ionic compounds- low, giant covalent- low polar- high non polar-highest
solubility
non polar substances dissolve in non polar solvents by london dispersion forces between solute and solvent
all halogens which are diatomic and non polar, readily soluble in non polar (parrafin oil solvent)
Polar covalent compounds are soluble in water (polar solvent) interact through dipole interactions and hydrogen bonding ex. HCl in water
solubility of polar compounds is reduced in larger molecules where polar bond is a small part of total structure, non polar parts reduce its solubility ex. C2H5OH is polar, and C7H15OH is non polar
polar susbtances have low solubility in non polar solvents, remain held to each other by dipole dipole attractions and dont interact well with solvent
giant molecular structures insoluble in all solvents as there is too much energy required to break the strong covalent bonds
ionic compounds are polar extremely due to the transfer of electrons so they are soluble in water, non soluble in non polar susbtances
electrical conductivity
covalent compounds do not contain ions and so are unable to conduct electricity, no mobile ions , both non polar covalent and giant covalent
some polar covalent compounds in conditions where they can ionise, will conduct like Hcl in water that dissolves into its H plus ions in water
ionic compounds conduct when molten or dissolved in water
Chromatography
Seperate and identify components of a mixture
components have different affinities for two phases and are seperates as mobile phase moves through stationary phase
levels of solubility of each component dependent on its intermolecular forces
paper containing 10% water is stationary phase
solvent is the mobile phase as it rises up the paper by capillary action dissolving the components of the mixture to different extents carrying them up at different rates
trial multiple solvents to identify which one seperates the components best
polar compounds will stick to stationary phase and not move up with mobile phase when solvent is non polar
non polar substances will move up with non polar solvent mobile phase