02 - physical and chemical properties of water
1/45
Earn XP
Description and Tags
finished
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
46 Terms
how can we understand biological molecules and the reactions they can undergo?
in the context of their aqueous environment
how do most biological molecules assume their 3d shape
in response to the properties of surrounding water
what do reactants and products of a reaction depend on water for?
transport within and between cells
why don’t atoms share electrons equally?
due to the differences in electronegativity
why is water polar?
because the oxygen side has a partial negative charge and the hydrogens have a partial positive charge
what is a dipole moment? where does it come from?
measure of the polarity of a molecule which comes from the unequal sharing of electrons in chemical bonds
what is water’s shape? where does it come from?
bent or V-shape and it comes from the arrangement of its electrons and the repulsion between electron pairs
what specifically on the water molecule causes its shape? what is the angle of the H-O-H bond?
the stronger repulsion from the lone pairs results in the bent molecular geometry; the angle is 104.5 degrees
what are hydrogen bonds?
intermolecular association between the oxygen atom of one water molecule and the hydrogen of the other
how do neighboring water molecules orient themselves?
so that the positive end of one points towards the negative end of the other water molecule
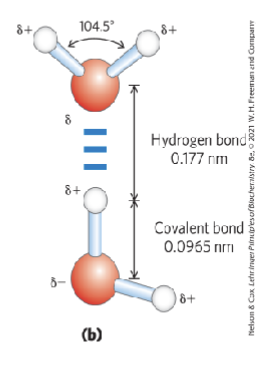
what type of attractions are crucial to the properties of water itself and its role as a biochemical solvent?
electrostatic attractions
what is one characteristic of carbon when it comes to hydrogen bonds?
C-H groups lack polarity and do not H bond
when are hydrogen bonds the strongest?
when they are in a straight line
are hydrogen bonds strong or weak?
weak
what elements form hydrogen bonds?
nitrogen, oxygen, fluorine
what type of forces do hydrogen bonds make between water molecules that make water a liquid at room temperature and ice when highly ordered at cold temperatures?
cohesive forces
describe why the bent geometry of water allows it to interact with polar molecules?
because of the dipole moment of the hydrogens which allows the lone pairs on the oxygen to be more accessible
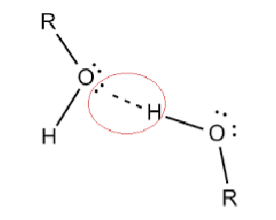
which is the hydrogen bond donor and which is the acceptor?
oxygen is the acceptor
and hydrogen is the donor (hydrogen is being donated)
what is the other common electronegative atom often
forms hydrogen bonds in biological systems?
nitrogen
what type of bonds do alcohols, aldehydes, ketones, and compounds containing N-H bonds form?
hydrogen bonds
what are hydrogen bonds crucial for?
maintaining biological structure and facilitating molecular recognition
define hydrophilic
describes compounds that dissolve easily in water; generally charged or polar
define hydrophobic
nonpolar molecules such as lipids or waxes
define amphipathic
contain regions that are polar (or charged) and regions that are nonpolar
how does water dissolve salt and charges biomolecules?
by screening electrostatic interactions (solvation layer)
what is largely responsible for the ease of dissolving salts in water?
the increase in entropy
what is the ordering of water molecules around non polar solutes (like oil)? does this increase or decrease entropy? what happens to the non polar solute?
water molecules form a highly ordered, cage like shell around each solute molecule which decreases entropy; this tends to force non polar solute together in blobs
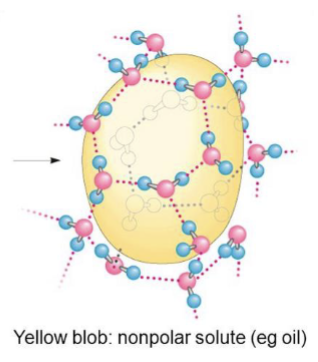
how do amphipathic compounds interact in aqueous solutions?
polar, hydrophilic region interacts favorably with water and tends to dissolve while the non polar hydrophobic region tends to avoid contact with water and cluster together
what is the hydrophobic effect?
the observed tendency of non polar substances to aggregate in an aqueous solution and exclude water molecules
what happens to the non polar regions in the hydrophobic effect?
the non polar regions cluster together to maximize interactions with each other and and avoid the water
does less order of water increase or decrease entropy?
increase
what is the hydrophobic effect driven by?
the increase in entropy of the water molecules
If you shake a mixture of oil and water, the oil will initially disperse as small droplets but will eventually combine into a larger aggregate. Explain to someone near you this phenomenon in terms of the hydrophobic effect and the energetic drive of the water molecule.
the oil will first have a large cage which means a decrease in entropy but then the oil will aggregate together which also decreases entropy but then the oil will create a solvation layer and a smaller cage which would increase entropy
how is a hydrgen ion formed?
when a hydrogen atom loses an electron and becomes positively charged
what is responsible for determining the pH of a solution?
[H+]
what is the equation for pH?
pH=-log[H+]
what is an equilibrium constant (Keq)?
gives the position of equilibrium
what is the equilibrium constant formula of water?
Keq=[H+][OH-] \ [H2O]
define neutral pH
exactly equal concentrations of H+ and OH-
what is the pH of pure water?
7
what is the concentration of H+ in a solution of 0.01 M NaOH
Kw=[H+][OH-] = ion product of water
1.0×10-14 = [H+][0.01]
[H+] = 1×10-12
What is the pH of a 0.0001 M solution of hydrochloric acid?
4
what is a characteristic of strong acids when calculating pH?
they completely dissociate
what is the equation for the ion product of water?
Kw = [H+][OH-]
what is Kw constant?
1×10-14 M2
formula for pOH
pOH = -log[OH-]