CHEM 344: Quiz 4 Bromination of Acetanilide
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
How many 1H NMR signals for the product?
4
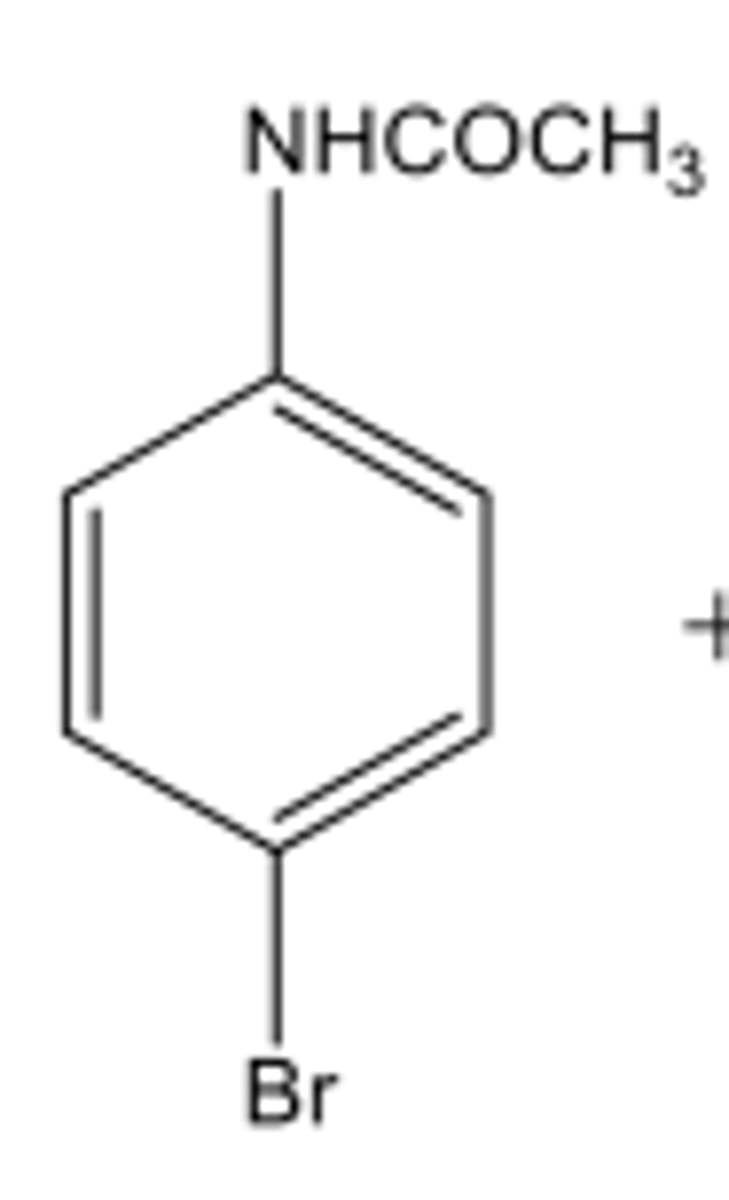
The driving force for losing the proton in the last step of EAS?
To rearomatize the product
How many 13C signals for the product?
8
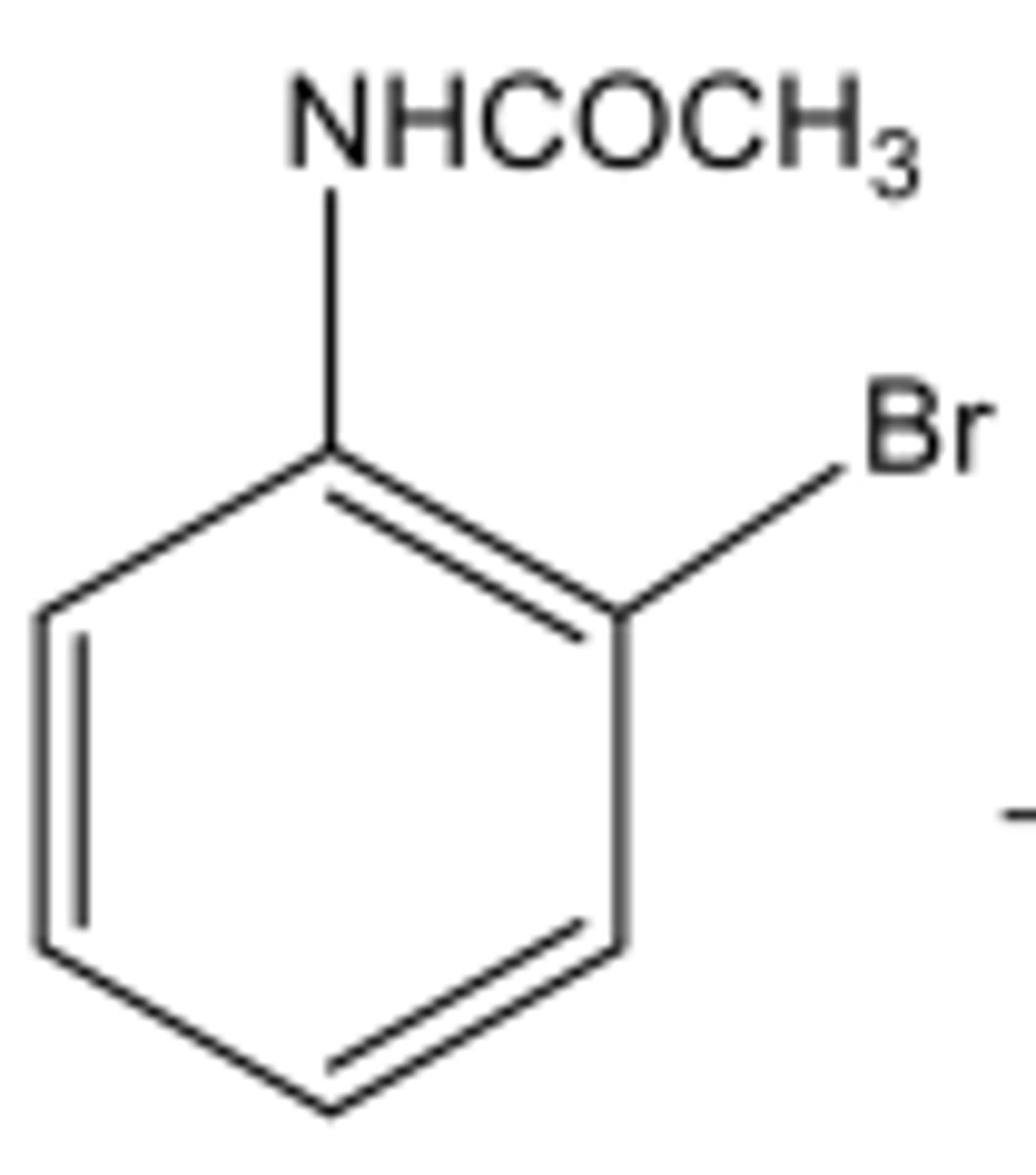
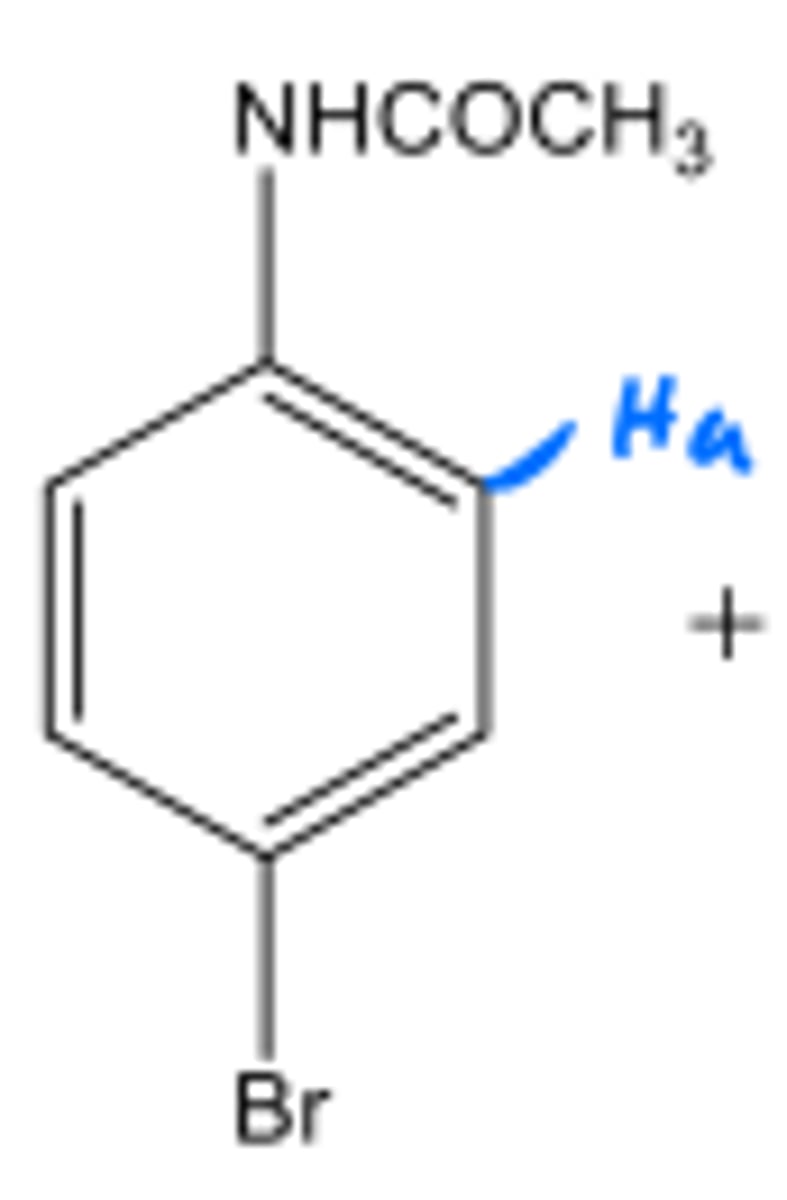
What is the splitting of Ha?
Doublet
What is the nucleophile?
Acetanilide
What is the function of acetic acid?
To act as a solvent and polarize the Bromine bond
What is a good safety measure for this experiment?
Do the experiment in the fume hood
Is acetanilide an activated or deactivated group?
Activated
Why is 30% sodium thiosulfate added?
To quench any unreacted Bromine in th emixture
What is the first step in EAS?
Addition of the electrophile to the aromatic ring
What is the theoretical yield of the brominated product if 600mg of acetanilide and 1.0 mL of 4.1M bromine solution in acetic acid?
878 mg
Bromination of acetanilide. How is the product purified?
Recrystallization