Exam 1 Review
0.0(0)
Card Sorting
1/188
Earn XP
Description and Tags
Prep for Exam #1 - origin of the universe, types of minerals, and type of rocks.
Last updated 2:07 AM on 2/28/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
189 Terms
1
New cards
big bang theory
massive explosion occurring 15 bya from singularity
marks beginning of the universe
marks beginning of the universe
2
New cards
singularity
incredibly dense, energetic, yet very small concentration in space
part of the big bang
part of the big bang
3
New cards
The big bang theory describes…
the origin of the universe
4
New cards
matter, right after the big bang
very hot
has cooled down as time went on
has cooled down as time went on
5
New cards
evidence of the big bang
red shift / Doppler effect
background microwave radiation
background microwave radiation
6
New cards
red shift / Doppler effect
fast moving objects going away from us elongates light waves, “shifting” objects to become closer to the lower frequency red range
7
New cards
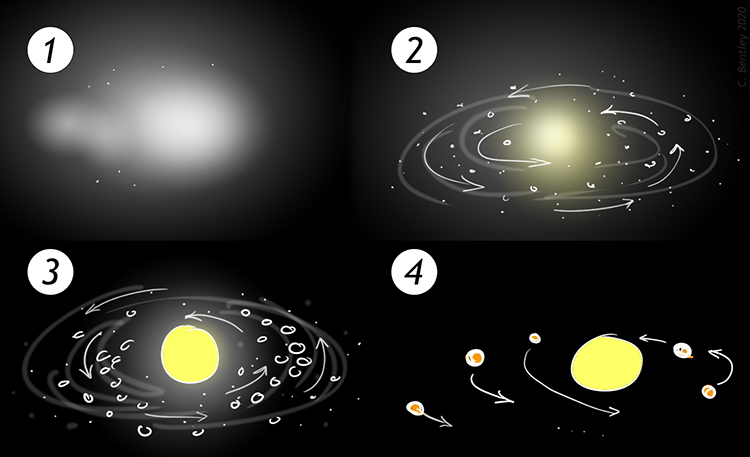
nebular theory
planets were formed from nebular clouds
contraction began \~ 5 bya
produced gravitational collapse of the nebular, causing heat, molten rock, and “dirty orbits” that haven’t defined themselves yet
contraction began \~ 5 bya
produced gravitational collapse of the nebular, causing heat, molten rock, and “dirty orbits” that haven’t defined themselves yet
8
New cards
nebula
cloud of gas and space dust
9
New cards
The nebular theory describes…
the origin of the solar system
10
New cards
inner planet traits
none or few moons
smaller
rocky
denser
hotter
smaller
rocky
denser
hotter
11
New cards
outer planet traits
many moons
larger
gaseous
less dense
colder
larger
gaseous
less dense
colder
12
New cards
Earth’s place in the solar system
part of the inner planets
13
New cards
Proto-Earth characteristics
larger in size than today’s Earth
Had lots of volcanic activity
HOT
no continents or oceans
no life
still clearing its orbit
Had lots of volcanic activity
HOT
no continents or oceans
no life
still clearing its orbit
14
New cards
density stratification
creation of layers from lower density material rising above the sinking, higher density material
15
New cards
layered Earth was formed by…
density stratification
16
New cards
layers of Earth (internally)
inner core (solid iron)
outer core (liquid iron)
mantle
crust
outer core (liquid iron)
mantle
crust
17
New cards
proto-Earth’s atmosphere
hydrogen
helium
helium
18
New cards
source of proto-Earth’s atmosphere
density stratification
19
New cards
What happened to proto-Earth’s atmosphere?
burnt off immediately because it was still so hot
20
New cards
early Earth’s atmosphere 5 bya
***water vapor***
***nitrogen***
***carbon dioxide***
carbon monoxide
ammonia
methane
sulfur dioxide
NO FREE OXYGEN
***nitrogen***
***carbon dioxide***
carbon monoxide
ammonia
methane
sulfur dioxide
NO FREE OXYGEN
21
New cards
source of early Earth atmosphere
volcanic out-gassing
22
New cards
present day Earth atmosphere
\~3/4 nitrogen
\~1/4 oxygen
\~1/4 oxygen
23
New cards
source of present day Earth atmosphere
volcanic out-gasing
photosynthesis
photosynthesis
24
New cards
origin of free oxygen gas in present day atmosphere
life evolving \~3.8 bya performing photosynthesis
25
New cards
Earth’s spheres
atmosphere
hydrosphere
cryosphere
biosphere
geosphere/lithosphere
hydrosphere
cryosphere
biosphere
geosphere/lithosphere
26
New cards
atmosphere
thin gaseous envelope surrounding Earth
27
New cards
hydrosphere
water layer dominated by the oceans
28
New cards
source of hydrosphere (oceans)
H2O vapor and other gases condensed into clouds when planet began to cool down, raining down into the ocean
29
New cards
cryosphere
ice layer, including ice caps and glaciers
30
New cards
biosphere
all life on earth
31
New cards
The biosphere mostly resides…
just above or at sea level
32
New cards
geosphere/lithosphere
earth’s rocky surface, that is layered internally
33
New cards
internal sources of energy for Earth
radioactive decay (potassium, uranium, thorium)
decay releases heat energy
decay releases heat energy
34
New cards
external sources of energy for Earth
driven by the light energy of the sun
absorbed by atmosphere, oceans, and continents → converted to heat
drives circulation, head transfer, evaporation, and condensation
absorbed by atmosphere, oceans, and continents → converted to heat
drives circulation, head transfer, evaporation, and condensation
35
New cards
mineral
natural, inorganic crystalline solid that have exact chemical composition with an orderly internal arrangement of atoms (has a structure)
formed generally by inorganic processes
formed generally by inorganic processes
36
New cards
ionic bonds
formed by force from ions of opposite charges
usually formed by elements far apart on the periodic table
usually formed by elements far apart on the periodic table
37
New cards
covalent bonds
sharing of electrons to get noble gas structure
38
New cards
metallic bonds
electrons move freely from atom to atom, being constantly shared
weak bond form, but conduct electricity well because of the free electron movement
weak bond form, but conduct electricity well because of the free electron movement
39
New cards
chemical bonds, ranked from strongest to weakest
covalent
ionic
metallic
ionic
metallic
40
New cards
states of matter
solid
liquid
gas
liquid
gas
41
New cards
types of solid matter
crystalline
amorphus
amorphus
42
New cards
crystalline solid
atoms bond together in a regular, orderly pattern
43
New cards
amorphous solid
atoms bonded together in a random pattern
44
New cards
liquid
atoms or molecules tightly packed, but allowed to slip past each other in random motion
45
New cards
gas
particles in random motion at high speeds, separated by empty space
46
New cards
\n The orderly internal arrangement of atoms in a mineral \n causes it to…
exhibit characteristic physical properties that allow the mineral to be identified
47
New cards
polymorph
minerals with the same chemical composition, but different internal structure
48
New cards
example of a polymorph mineral
diamond and graphite
both made of carbon, however, graphite is made in a 3D formation, while graphite is made of sheets.
both made of carbon, however, graphite is made in a 3D formation, while graphite is made of sheets.
49
New cards
________________ may occur, causing small variations in composition
ionic subsitution
50
New cards
ionic subsitution
the replacement of ions with similar charges in a formula
ex. biotite - K*(Mg,Fe)*3AlSi3O10(OH)2
ex. biotite - K*(Mg,Fe)*3AlSi3O10(OH)2
51
New cards
native element
type of mineral, only composing of one element
ex. gold - Au
ex. gold - Au
52
New cards
physical properties of minerals
crystal form/habit
color
streak
luster
cleavage
density
hardness
color
streak
luster
cleavage
density
hardness
53
New cards
crystal form/habit
shape in which individual crystals or aggregates grow
54
New cards
streak
color of powdered mineral
usually shown on an unglazed porcelain tile
usually shown on an unglazed porcelain tile
55
New cards
luster
how the mineral looks with the reflection of light
56
New cards
types of luster
metallic
nonmetallic
vitreous
pearly
resinous
greasy
nonmetallic
vitreous
pearly
resinous
greasy
57
New cards
cleavage
planes along which the mineral breaks easily
58
New cards
hardness
how easily a mineral can scratch (or be scratched) by another mineral
59
New cards
If no space and time restrictions are present, crystals will…
be well developed (their ideal form)
60
New cards
The size of the atoms determines the…
architecture of the crystal structure.
61
New cards
If space and/or time restrictions are present, crystals will…
not be grown in their ideal form
will grow to completely fill their space
will grow to completely fill their space
62
New cards
cleavage types
1 direction
2 directions at 90 degrees
2 directions NOT at 90 degrees
3 dimensions at 90 degrees
3 dimension NOT at 90 degrees
fracture / no cleavage
2 directions at 90 degrees
2 directions NOT at 90 degrees
3 dimensions at 90 degrees
3 dimension NOT at 90 degrees
fracture / no cleavage
63
New cards
1 directional cleavage
sheds off in sheets due to weak bonds holding together strong silicate sheets
ex. Micas
ex. Micas
64
New cards
2 dimension at 90 degrees cleavage
break off in 2 dimensions (but not in a cube - has a rounded dimension), forming 90 degree corners
ex. feldspars and pyroxene
ex. feldspars and pyroxene
65
New cards
2 dimension NOT at 90 degrees cleavage
break off in 2 dimensions (but not in a cube - has a rounded dimension), forming 60/120 degree angles (or other angles not at 90 degrees)
ex. amphiboles (hornblende)
ex. amphiboles (hornblende)
66
New cards
3 dimension at 90 degrees cleavage
cube-like shapes at 90 degrees
ex. halite
ex. halite
67
New cards
3 dimension NOT at 90 degrees cleavage
rhombohedral-shape, forming 60/120 degrees (or other degrees instead of 90)
ex. calcite
ex. calcite
68
New cards
fracture
no cleavage
break off into shards
ex. quartz, obsidian
break off into shards
ex. quartz, obsidian
69
New cards
What is the cause of fracturing minerals?
have equally strong bonds in all directions, cannot find regular path of breaking
70
New cards
Is cleavage different than crystal form?
Yes, it is.
Crystal form is the ideal growth given no space or time restrictions,
and cleavage is the result of breaking with the same geometry (or lack of, if it fractures)
Crystal form is the ideal growth given no space or time restrictions,
and cleavage is the result of breaking with the same geometry (or lack of, if it fractures)
71
New cards
Moh’s hardness scale
arbitrary, exponential scale to compare hardness from 1 (talc) to 10 (diamond)
72
New cards
hardness scale tests
2\.5 - fingernail
3\.5 - copper coin
4\.5 - paper clip
5\.5 - Knife blade, glass plate
7\.5 - steel file
3\.5 - copper coin
4\.5 - paper clip
5\.5 - Knife blade, glass plate
7\.5 - steel file
73
New cards
Four unknown minerals labeled A, B, C, and D are needing to be tested for hardness. You do not have a hardness testing kit, and you just cut your nails, so you decide to test the hardness of each mineral relative to each other. After some tests, you conclude the following:
Mineral A can scratch Mineral B.
Mineral A cannot scratch Mineral C.
Mineral B can scratch Mineral D.
Mineral D cannot scratch Mineral A, B, or C.
Rate the hardness of the minerals from softest (closest to 1) to hardest (closest to 10).
Mineral A can scratch Mineral B.
Mineral A cannot scratch Mineral C.
Mineral B can scratch Mineral D.
Mineral D cannot scratch Mineral A, B, or C.
Rate the hardness of the minerals from softest (closest to 1) to hardest (closest to 10).
D, B, A, C
74
New cards
special physical properties for specific minerals
taste (halite)
striations (plagioclase feldspars)
magnetism (magnetite)
double refraction (calcite)
chemical tests (limestone)
striations (plagioclase feldspars)
magnetism (magnetite)
double refraction (calcite)
chemical tests (limestone)
75
New cards
___________ is not the best characteristic to ID minerals because…
color
has a great deal of variation for a lot of minerals
ex. rose quartz, smoky quartz, etc.
has a great deal of variation for a lot of minerals
ex. rose quartz, smoky quartz, etc.
76
New cards
In a streak test, if the mineral is harder than the porcelain…
it leaves no streak and scratches it instead
77
New cards
most common elements in crustal rocks by abundance
oxygen
silicon
aluminum
iron
calcium
sodium
potassium
magnesium
silicon
aluminum
iron
calcium
sodium
potassium
magnesium
78
New cards
silicate minerals
most common minerals on earth
make up 95% of crust volume, and 75% of all of Earth’s mass
based on the silica tetrahedron - SiO4^-4
make up 95% of crust volume, and 75% of all of Earth’s mass
based on the silica tetrahedron - SiO4^-4
79
New cards
2 ways silica tetrahedra balance charges
attaching to metals, which usually have + charges
sharing oxygen atoms, which reduces charge on each tetrahedron
or a combination of both
sharing oxygen atoms, which reduces charge on each tetrahedron
or a combination of both
80
New cards
types of silicate linkages
single tetrahedron
single chains
double chains
2D sheets
3D frameworks
single chains
double chains
2D sheets
3D frameworks
81
New cards
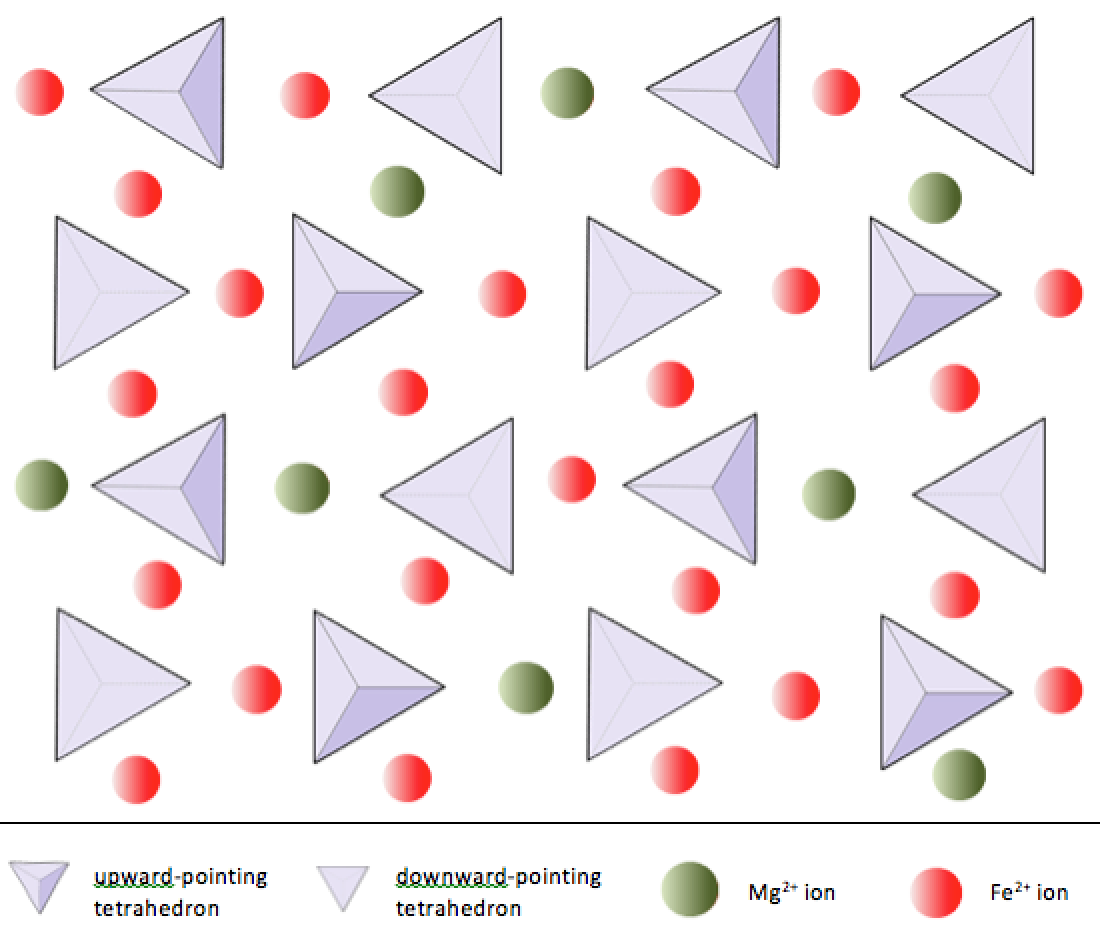
single tetrahedrons silicates
isolated tetrahedra
need to be bonded with cations
need to be bonded with cations
82
New cards
single tetrahedrons silicates example
olivines
83
New cards
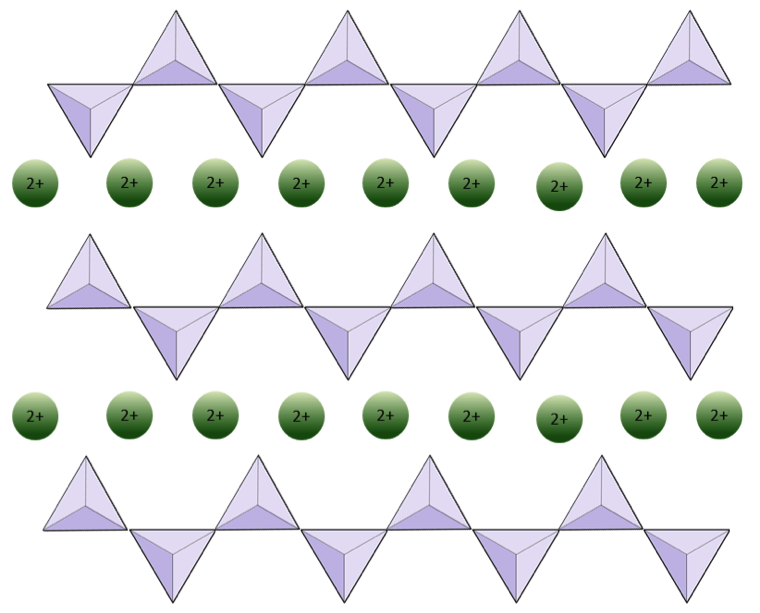
single chain tetrahedra silicates
share a single oxygen atom to reduce overall negative charge - as a result, reduce the need for cations between each tetrahedra
cations hold the chains together
cations hold the chains together
84
New cards
single chain tetrahedra silicates examples
pyroxene group, like augite
combines with iron and/or magnesium
combines with iron and/or magnesium
85
New cards
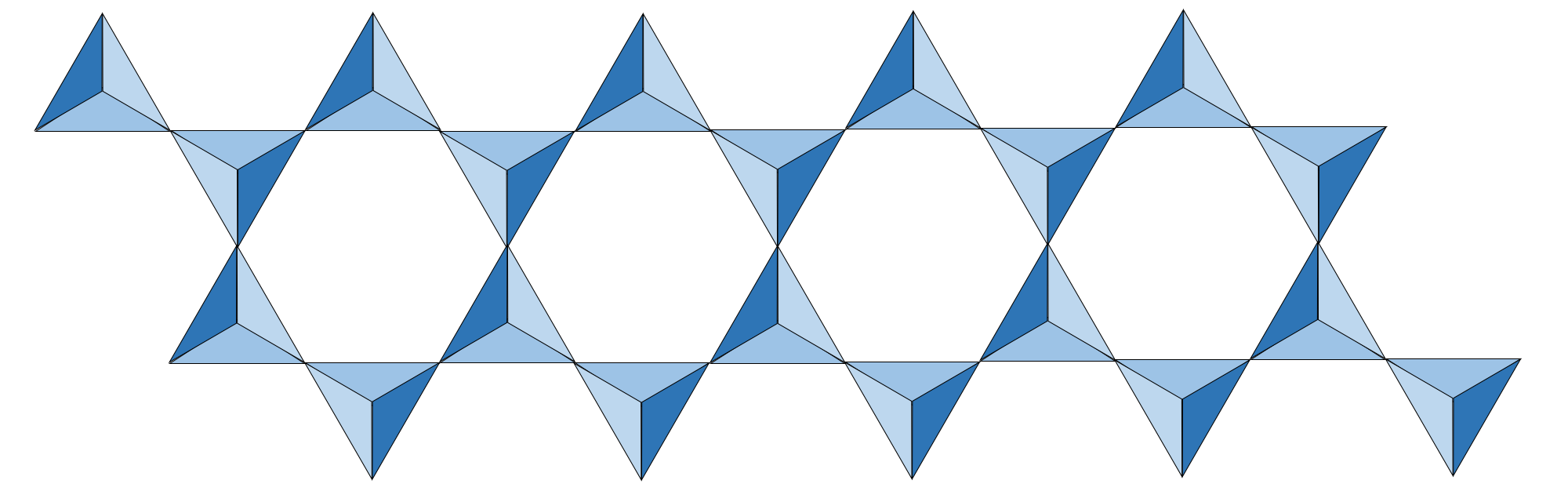
double chain tetrahedra silicates
two parallel chains
every other tetrahedron along the chain shares one oxygen atom with an adjacent chain
like single chains, reduces the overall negative charge and need for cations further
every other tetrahedron along the chain shares one oxygen atom with an adjacent chain
like single chains, reduces the overall negative charge and need for cations further
86
New cards
double chain tetrahedra silicates examples
amphiboles group, like hornblende
combines with iron and/or magnesium
combines with iron and/or magnesium
87
New cards
sheet tetrahedra silicates
each tetrahedron shares 3 oxygen atoms, forming a sheet
cations hold the sheets together
cations hold the sheets together
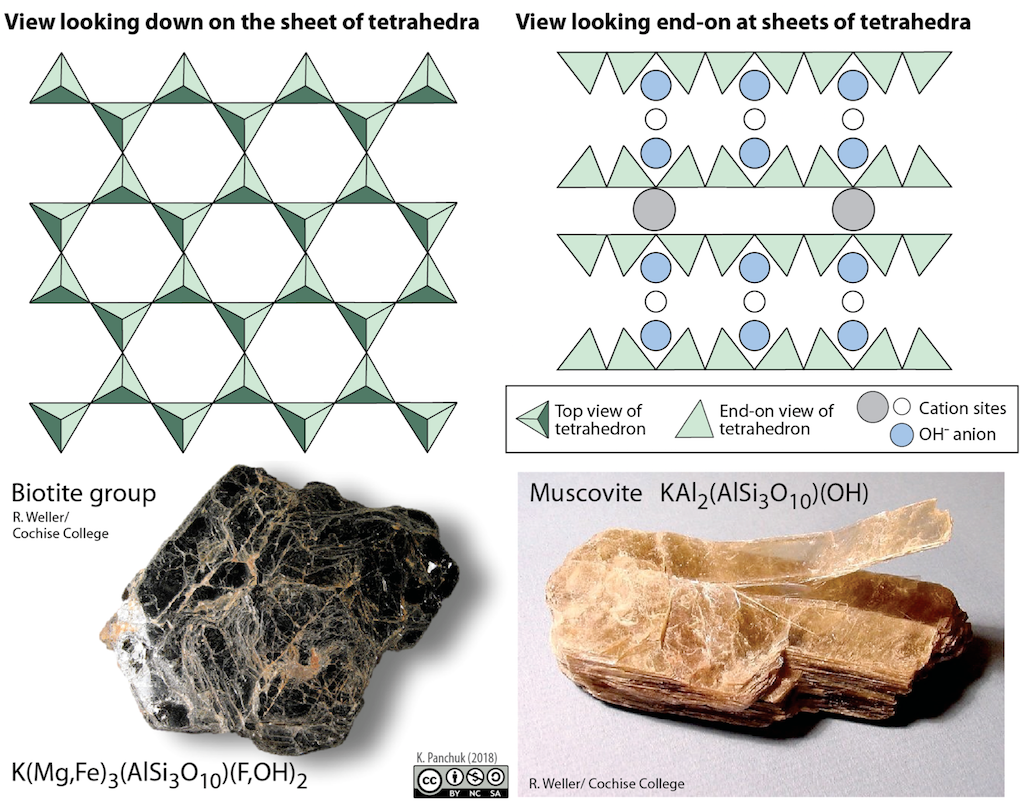
88
New cards
sheet tetrahedra silicates examples
mica group, including clays, biotite, muscovite
can be seen combined with iron and/or magnesium (biotite)
or aluminum and/or potassium (muscovite)
can be seen combined with iron and/or magnesium (biotite)
or aluminum and/or potassium (muscovite)
89
New cards
framework silicates
all 4 oxygen atoms shared by adjacent tetrahedrons
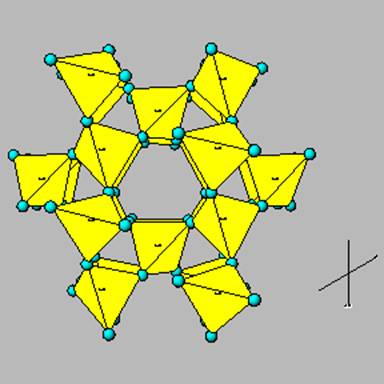
90
New cards
framework silicates examples
quartz and feldspars
91
New cards
ratio of silica to oxygen in silicate linkages
less Si: more O in simpler linkages, like isolated tetrahedrons
more Si: less O in complex linkages, like framework
more Si: less O in complex linkages, like framework
92
New cards
density in silicate linkages
most dense in simpler linkages, like isolated tetrahedrons
least dense in complex linkages, like framework
least dense in complex linkages, like framework
93
New cards
types of minerals based on chemical composition
carbonates
oxides
native elements
sulfides
sulfates
silicates
oxides
native elements
sulfides
sulfates
silicates
94
New cards
carbonates
minerals with CO3^-2
ex. calcite (CaCO3)
ex. calcite (CaCO3)
95
New cards
oxides
being composed of oxygen (O^-2)anions and metallic cations
rusts
ex. hematite (Fe2O3)
rusts
ex. hematite (Fe2O3)
96
New cards
sulfides
minerals that have sulfide anions (S^-2) and metallic cations
ex. pyrite
ex. pyrite
97
New cards
sulfates
minerals that have sulfate (SO4^-2) anions and metallic cations
ex. gypsum, anhydrite
ex. gypsum, anhydrite
98
New cards
types of silicates based on silica content
felsic/silcic
intermediate
mafic
ultramafic
intermediate
mafic
ultramafic
99
New cards
felsic/silicic silicate minerals
high Si (>75-65%)
has Al or other metals other than Fe or Mg
relatively low crystallization temperatures
less dense
major minerals - mostly light in color
has Al or other metals other than Fe or Mg
relatively low crystallization temperatures
less dense
major minerals - mostly light in color
100
New cards
intermediate composition
intermediate Si (55-65%)
equal parts mafic and silicic
ex. andesite
equal parts mafic and silicic
ex. andesite