Thermodynamics (Temperature and Heat)
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
Temperate
It is a measure of the hotness or coldness of an object
What is another definition for Temperature?
[T] = K
Heat
It is a form or energy that causes a rise in temperature when added or a fall in temperature when withdrawn
What is Heat measured in?
Joules (J)
What is the Definition of the Degree Celsius?
θ (°C) = T (K) - 273.15
Theta = Temperature in Degrees Celsius = Temperature in Kelvins
Establishment of a Temperature Scale
To establish a temperature scale, you need 3 things.
The first thing to establish a temperature scale
A thermometric property. It is a physical property that changes measurably with temperature
Examples of thermometric properties
length of liquid column
emf of thermocouple
pressure of a gas at constant volume
volume of a gas at constant pressure
resistance
colour
Thermocouple
Two junctions of two different metal if held at two different temperatures, will produce emf (electromotive force ie. a voltage)
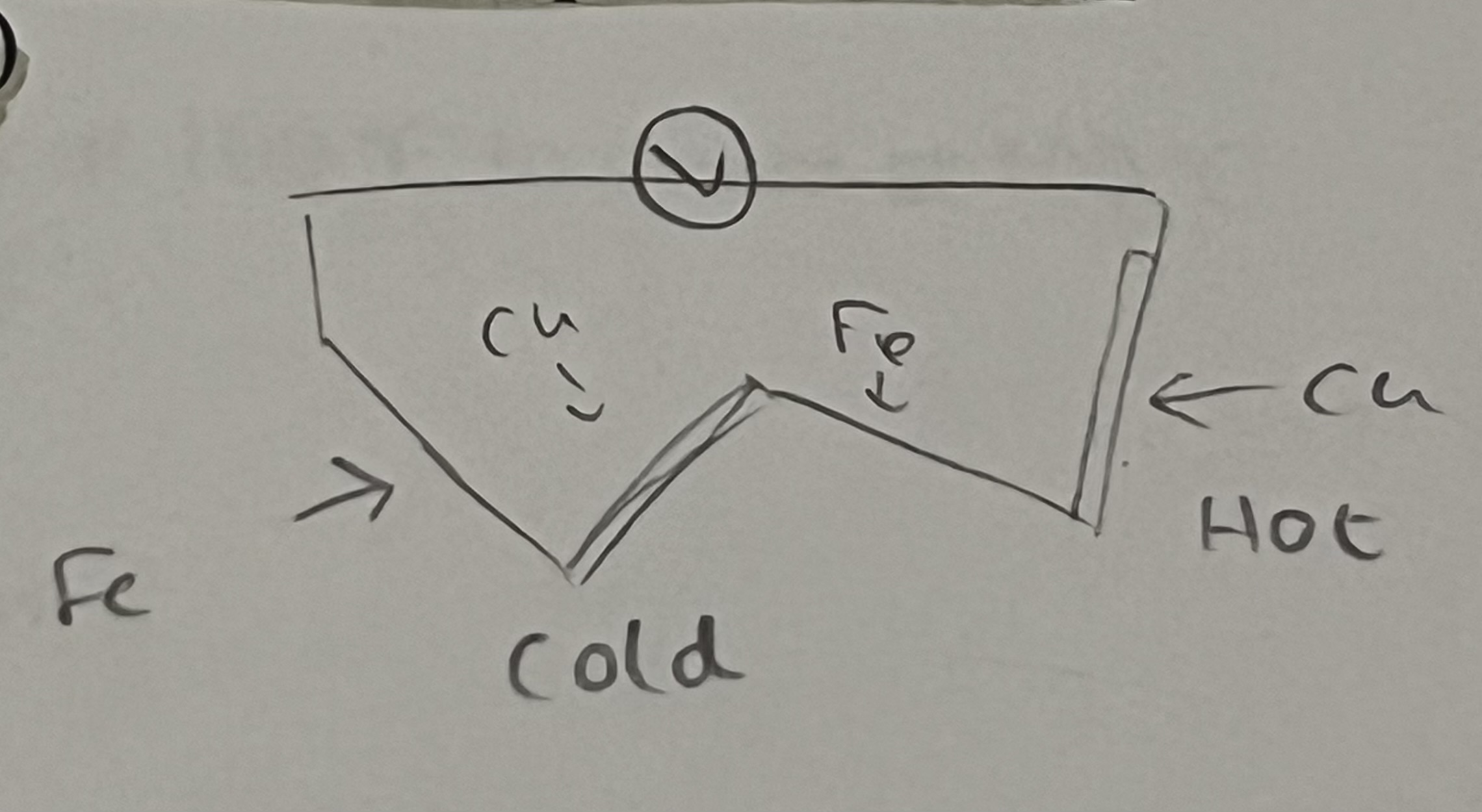
The second thing you need to establish a temperature scale
Two fixed points.
eg. the freezing point of water & the boiling point of water or absolute zero & the triple point of water
The third thing you need to establish a temperature scale
A scale eg. 0 to 100 or 0 to 273.16