Biol 319 exam 2
1/447
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
448 Terms
Culture media
nutrient media that enhances the growth of microorganisms
solid medium
has agar and other solidifying agents
liquid medium
contains specific nutrients but has no gelling agents
minimal medium
chemically defined medium
- you know how much of each is present in each individual component
-Components are limited to only those nutrients that the organisms needs in order to grow
complex medium
chemically non-defined medium
- don't really know what the complex medium is made up of
heterotrophs
rely on other organisms to make the organic compounds that they use as carbon sources
autotrophs
use the CO2 discarded by heterotrophs to make complex cel constituents made up of C, H, and O, such as carbohydrates
phototrophs
extract energy from absorption of light
chemotrophs
extract energy from oxidation-reduction reactions that remove electrons from high energy compounds to produce lower energy compounds
- lithotrophy
- organotrophy
obtaining nitrogen
-Nitrogen is a critical component of proteins, nucleic acids, and other cellular constituents and is required in large amounts by living organisms
-N2 makes up nearly 79% of Earth's atmosphere, but the nitrogen in N2 is unavailable for use by most organisms
-Nitrogen from N2 must be "fixed" or converted to ammonium ions (NH4+) through the nitrogen cycle
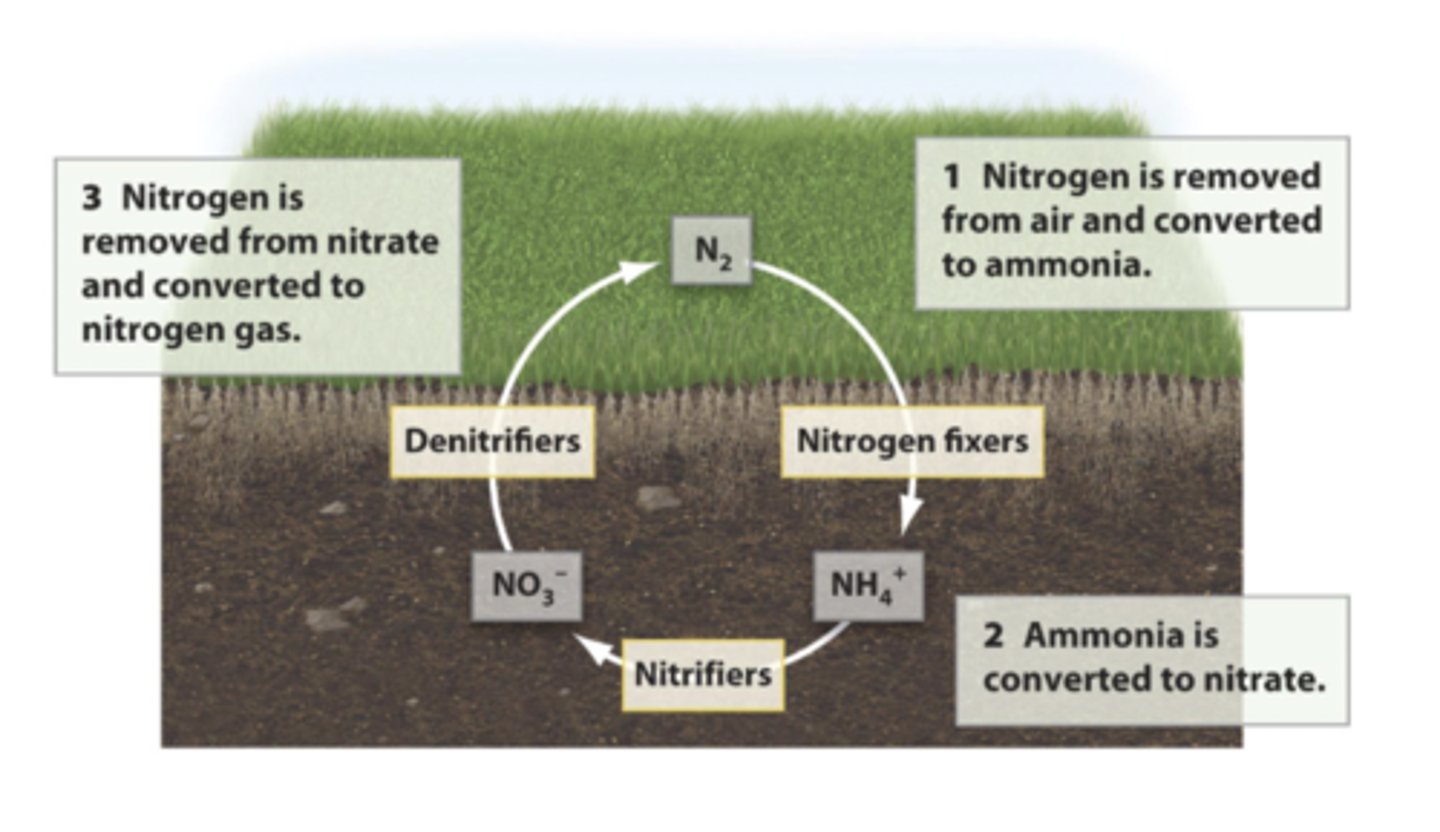
nitrogen cycle
1. nitrogen is removed from air and converted to ammonia
2. Ammonia is converted to nitrate
3. Nitrogen is removed from nitrate and converted to nitrogen gas
nitrogen fixation
Bacteria can't use N2 alone they have to go through nitrogen fixation to form NH4+ (ammonium ions)
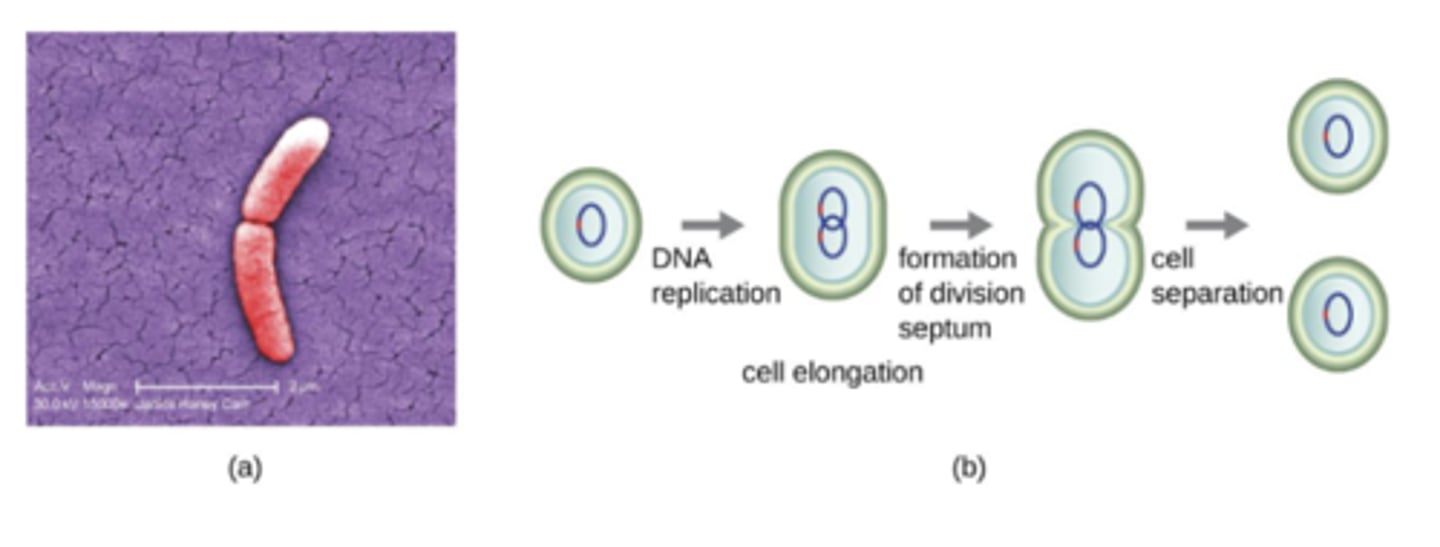
Binary fission
one parent cell splits into 2 equal daughter cells
-most bacteria grow by binary fission
budding in a planctomyces species
-form of asexual reproduction
-growing a smaller new cell (called a bud) off the side of the parent
-doesn't split in two equal halves like most bacteria
budding process
1. Bud formation
2. DNA transfer: parent DNA --> Bud
3. growth of the bud
4. separation: free independent daughter cell
Epulopiscium
a giant bacterium that has a viviparous life cycle
viviparous lifecycle
it gives birth to live offsprings
the Epulopiscium life cycle
1. epulopiscium forms one or two daughter cells inside its own cytoplasm
-- they grow while inside the other cell
-- mother cell nourishes the offspring
2. once the offsprings are fully developed, the mother cell dies and lyses (breaks open)
-- this releases the daughter cell into the environment (gut)
3. the process repeats
batch culture system
the growth curve of a bacterial culture is represented by the logarithm of the number of live cells plotted as a function of time the graph can be divided into four phases according to the slope, each of which matches events in the cell
-Lag
-log
-stationary
-death
lag phase
no increase in number of living bacterial cells
log
exponential increase in number of living bacterial cells
stationary
plateau in number of living bacterial cells; rate of cell division and death roughly equal
death
exponential decrease in number of living bacterial cells
exponential growth
continuous culture
in open systems where fresh medium is continually added to a culture and an equal amount of culture is constantly siphoned off, bacterial populations can be kept in exponential phase at a constant cell mass for long periods of time
-culture media is constantly added (feed) and constantly removed (effluent)
chemostat
a continuous culture system in which the diluting medium contains a growth-limiting amount of an essential nutrient
chemostat biofilms
Complex and dynamic ecosystems that common form on surfaces
biofilm growth occurs in 5 stages
1. Cellular flagella attach to the monolayer
2. Microcolonies form
3. Cells produce exopolysaccharides (EPSs)
4. The biofilm matures
5. The biofilm dissolves and cells disperse
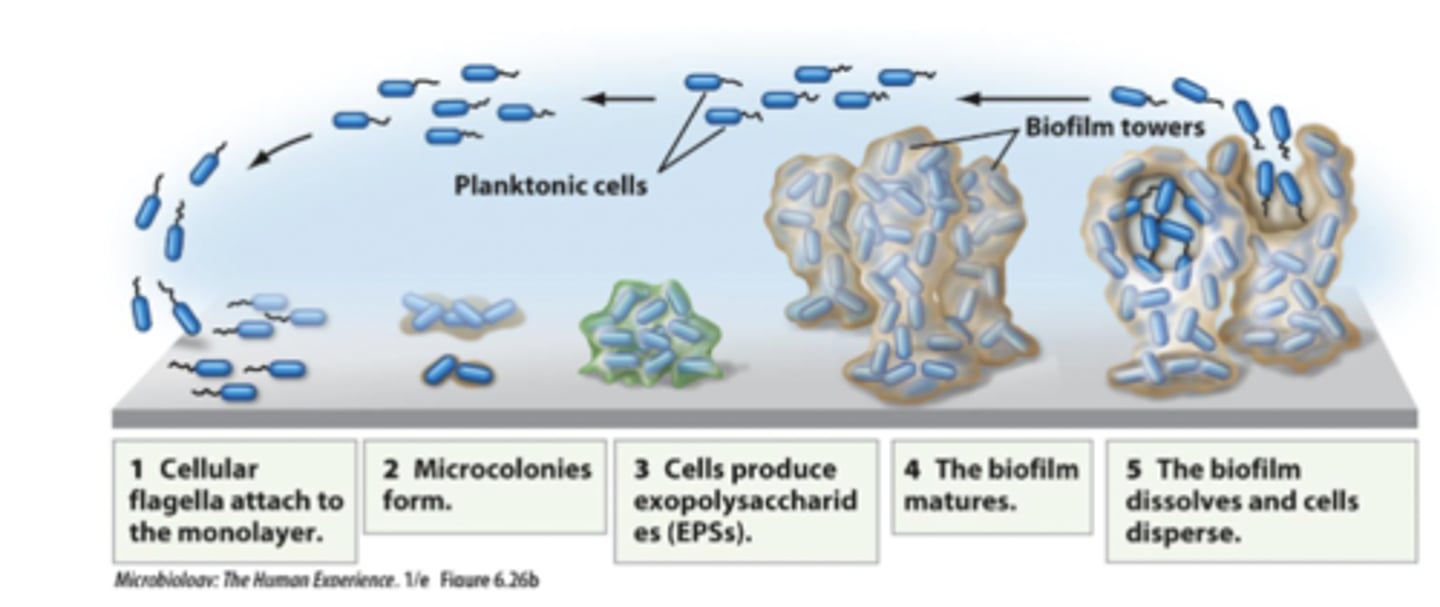
biofilm
grow in continuous culture system while attached to a surface and their growth consists of 5 developmental stages
biofilm structure
-extracellular polymeric substances (EPS)
-channels
-micro-colonies
-streamers
physical requirements on microbial growth
-pH
-temperature
-moisture
-hydrostatic pressure
-osmotic pressure and radiation
chemical requirements on microbial growth
-availability of carbon
-nitrogen
-sulfur
-phosphorus
-trace elements
-organic compounds
-oxygen
classification of growth temperatures
-psychrophiles
-mesophiles
-thermophiles
-hyperthermophiles
psychrophiles
microorganisms that can grow at cold temperatures from -5℃-20℃
mesophiles
microorganisms that can grow in temperature from 15℃-45℃
theermophiles
microorganisms that can grow in temperature from 45℃-80℃
hyperthermophiles
microorganisms that can grow in temperatures from 65℃-105℃
psychrotrophs
microorganisms that can grow at cold temperatures 0-30° (best at 20-30°) which is not too different from psychrophiles which can grow at 0-20° (best at 10°)
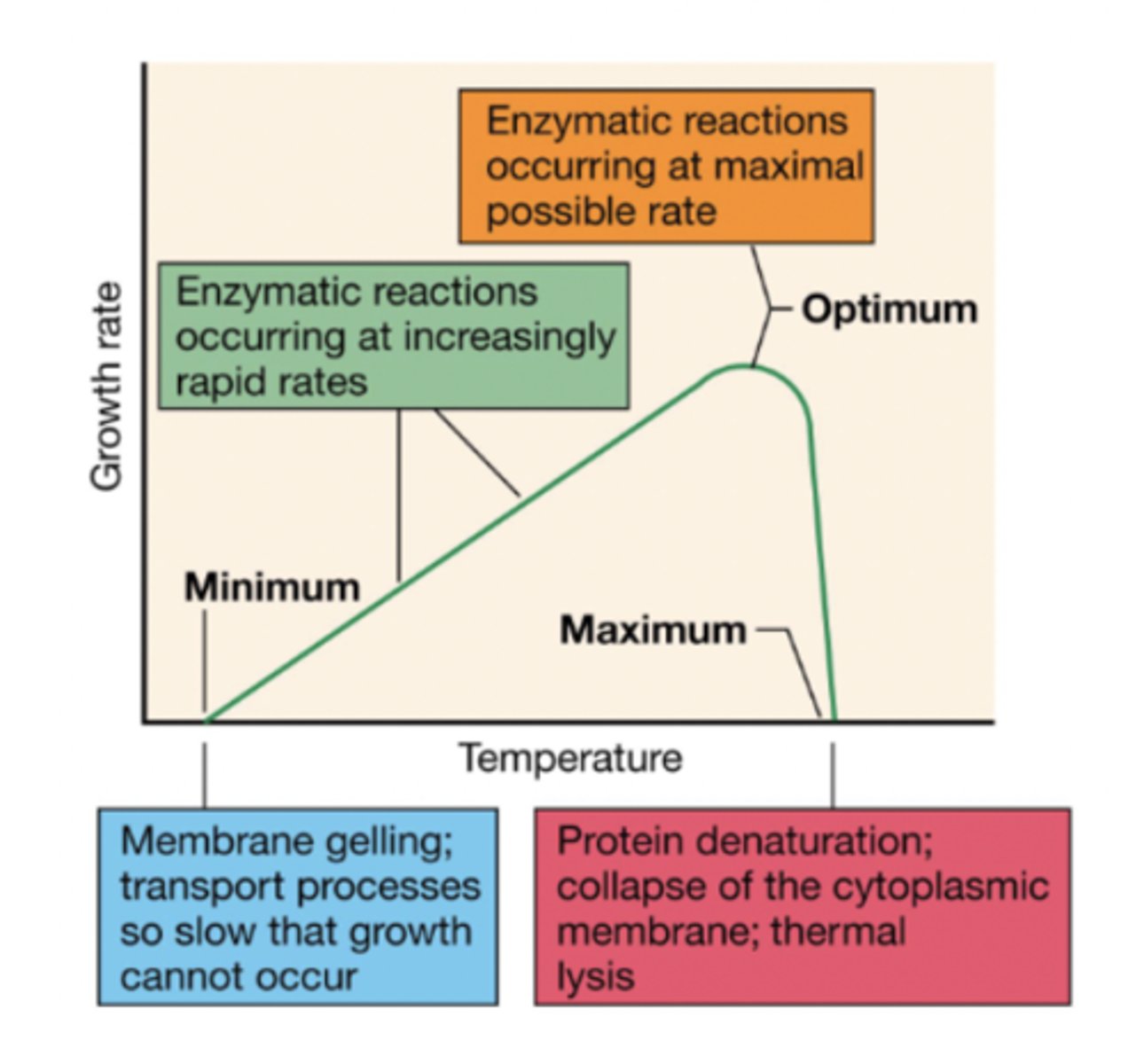
temperature and growth rate
whether or not a cell can survive in a temperature depends on the enzymatic activity
-enzymatic activity is low at lower temps and high at optimal temps
barophiles (weight loving) or piezophiles (pressure loving)
organisms that have adapted to grow at high pressures
water activity
water availability is measured as water activity which is a quantity approximated by concentration
-Interactions with solutes (such as NaCl) lower water activity. The more solutes in a solution, the less water is available for microbes to use for growth
osmolarity
a measure of the number of solute molecules in a solution
-The more particles in a solution, the greater the osmolarity and the lower the water activity
-Most microorganisms are relatively tolerant of the external environment relative to the osmolarity or tonicity. Halophiles grow only in high salt concentrations
plasmolysis
hypertonic environments, or an increase in a salt or sugar, cause plasmolysis
halophiles
grow in high salt concentration
extreme or obligate halophiles
require high osmotic pressure
facultative halophiles
tolerate high osmotic pressure
hypersaline habitats
Hypersaline habitats for halophilic Archaea. Aerial view near San Francisco Bay, California, of a series of seawater evaporation ponds where solar salt is prepared. The red-purple color is predominantly due to bacterioruberin and bacteriorhodopsin in cells of Halobacterium

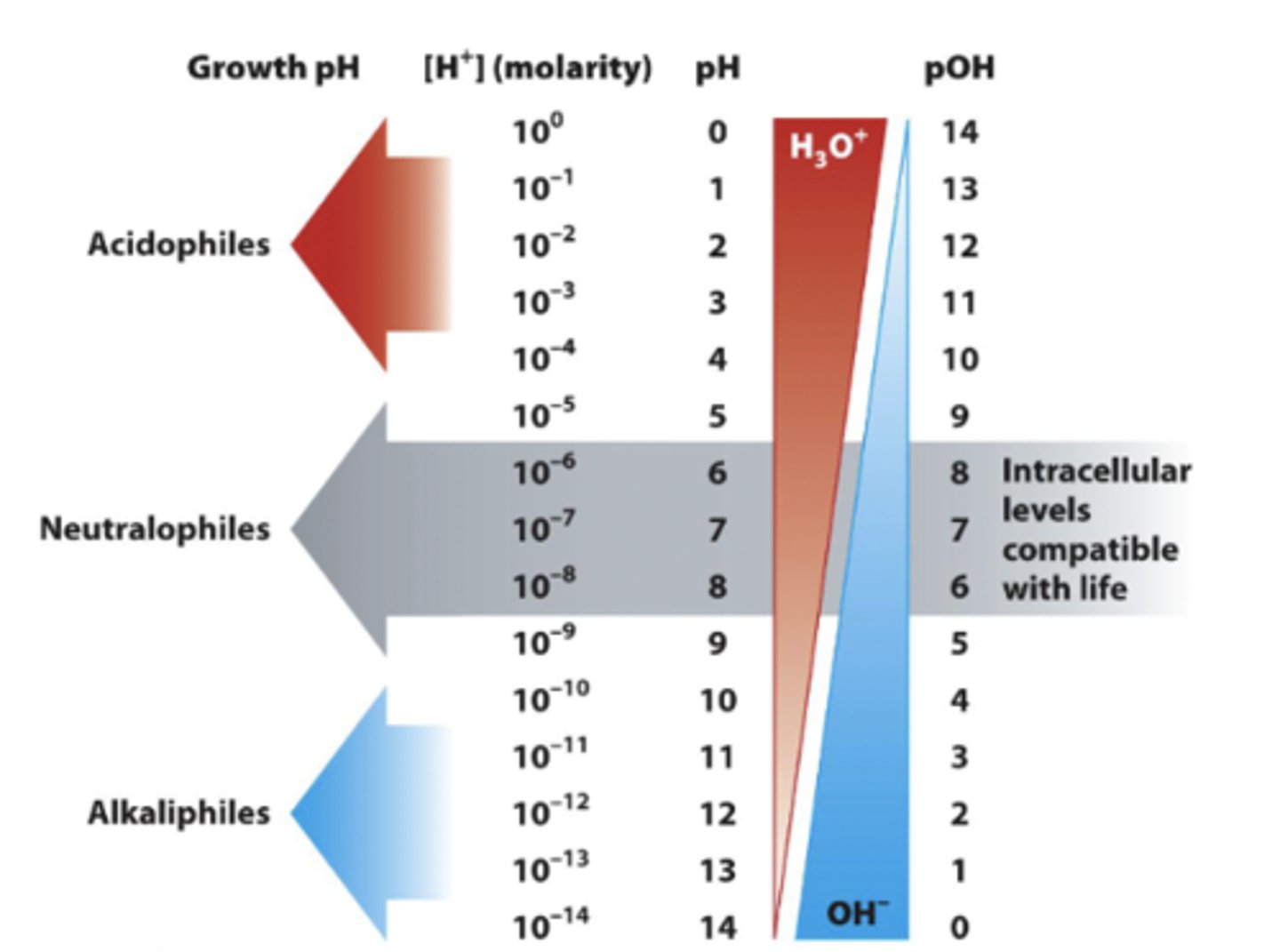
microbial responses to changes in pH
-pH influences growth by altering protein shape, which in turn changes protein activity
-Extreme concentrations of either hydrogen or hydroxide ions in solution will limit growth
-Concentration of hydrogen ions [H+] also have a direct effect on the cell's macromolecular structures
-Microbes have adapted to inhabit diverse pH environments from 0-11.5
strategies for pH homeostatis in bacterial
-Increased metabolic acid production through amino acid deaminases and sugar fermentation
-Increased ATP synthase that couples H+ entry to ATP generation
-Changes in cell surface properties
-Increased expression and acidity of monovalent cation/proton antiporters.
-Among these strategies, monovalent cation/proton antiporters play an essential and dominant role in alkaline pH homeostasis in many bacteria in addition to roles in Na+ and volume homeostasis
most bacteria grow..
between pH 6.5 and 7.5
molds and yeast grow ...
between pH 5 and 6
acidophiles
grow in acidic environments. they are often chemoheterotrophs that oxidize reduced metals and generate strong acids such as sulfuric acid
-grow below pH 3
alkaliphiles
occupy the opposite end of the pH spectrum, growing best at values ranging from pH 9 to pH 11
neutrophiles
bacteria that generally grow between pH 6 and pH 8 and include most humans pathogens
pH homeostasis mechanisms
-E.coli can reverse proton influx by importing a variety of cations such as K+ and Na+
-Under extremely alkaline conditions the cells can use Na+/H+ antiporters to recruit protons into the cell in exchange for expelling Na+
-Some organisms can change the pH of the medium by using various amino acid decarboxylases and deaminases, producing alkaline and acidic products, respectively
hot, acidic, and rich in sulfur
Habitats of hyperthermophilic archaea.
-Sulfur-rich hot spring, a habitat containing dense populations of Sulfolobus. The acidity in solfataras and sulfur springs comes from the oxidation of H2S and SO to H2SO4 (sulfuric acid) by Sulfolobus and related prokaryotes
facultative anaerobes
posses the enzymes to detoxify oxygen radicals and also the machinery for both fermentation and aerobic respiration
-growth with or without oxygen
strict aerobe
microorganism that grow only in oxygen
strict anaerobe
microorganisms that grow only without oxygen
aerotolerant anaerobes
use only fermentation to provide energy but contain superoxide dismutase and catalase or peroxidase to protein them from ROS
Microaerophiles
will grow only at low oxygen concentrations. they possess a decreased level of superoxide dismutase and/or catalase
- superxode dismutase and catalase are 2 enzymes required for a microorganism to be able to grow and survive under oxygenated conditions. so microaerophiles has low levels of these since they only grow in low oxygen
capnophiles
microorganisms that thrive in the presence of high concentrations of carbon dioxide. species of Campylobacter are bacterial capnophiles that are more easily identified because they are also microaerophiles, organisms that can grow in high carbon dioxide as long as a small amount of free oxygen is present but at a dramatically reduced concentration
problem with oxygen
-Oxygen is a powerful oxidant and can oxidize numerous chemicals in the cell, leading to cell injury or death
enzymes that protect the cell from toxic forms of oxygen
1. Catalase
2. Peroxide
3. Superoxide Dismutase
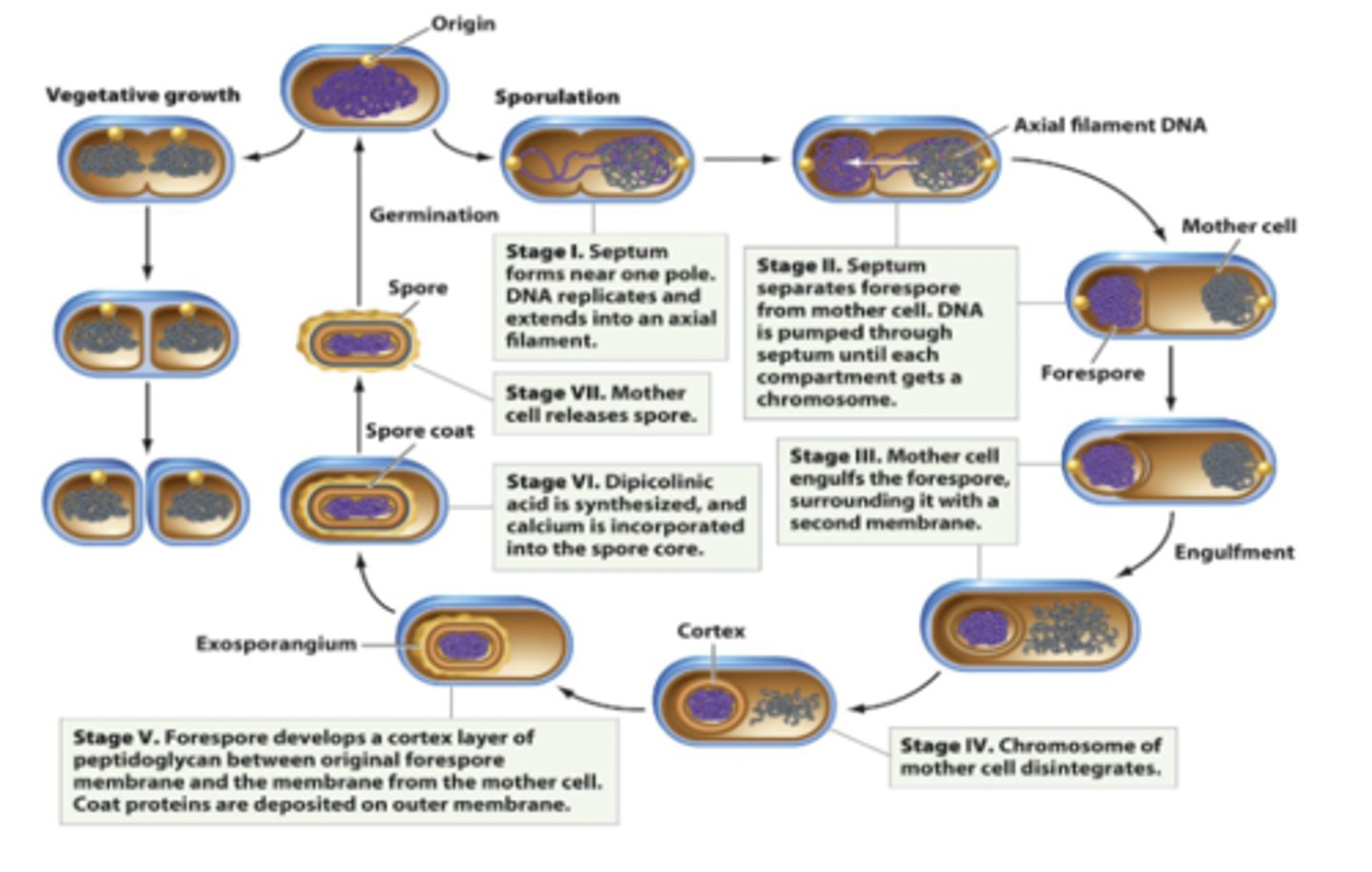
Dipicolinic acid (DPA)
-helps the endospore by:
1. removing water from core: makes the spore super dry, which protects proteins & DNA
2. stabilizes DNA which prevents damage from heat or radiation
3. increases resistance to chemicals, heat, & other environmental factors
agar
-complex polysaccharide
-used a solidifying agent for culture media
-in Petri plates, slants, and deeps
-generally not metabolized by microbes
-liquefies at 100°C
-solidifies at ~40°C
-agar itself has no nutritional function or value
selective media
Encourage the growth of certain organisms and discourage others (e.g. Media containing antibiotics)
-contains growth-inhibiting addictive, such as bite salts, crystal violet or an antibiotic, which will limit growth on the medium to only those organisms that are desired
MSA (mannitol-salt agar)
both selective and differential; selects for salt-tolerant organisms and differentiates mannitol fermenters from non-mannitol fermenters
selective media examples
1. Mannitol Salt agar: high concentration of salt (Is selective against Gram-negative and most Gram-positive bacteria)
2. MacConkey Agar: crystal violet, neutral red and bile salts and lactose (Is selective against Gram-positive organisms)
differential media
enable different species to be distinguished from each other (Mannitol Salt Medium)
Enrichment media
encourage the growth of a specific organism (brain Heart Infusion Medium)
Selective vs. Differential
-Selective medium: will have a growth-inhibiting addictive, such as bite salts, crystal violet or an antibiotic, which will limit growth on the medium to only those organisms that are desired
-Differential medium: allows for differentiation of particular chemical reaction yield an observable characteristic associated with the growth a particular organism or group of organisms that are able to grow on the medium
differential medium example
-blood agar
-beta-hemolysis
-alpha-hemolysis
-Y-hemolysis
blood agar
-Blood agar distinguishes bacterial species that can break down the red blood cells included in the blood agar medium. Typically used to differentiate between Streptococcus species
-allows for differentiation of particular chemical reaction yield an observable characteristic associated with the growth a particular organism or group of organisms that are able to grow on the medium
beta-hemolysis
complete lysis, resulting in a clear zone around the colony
-ex. Streptococcus pyogenes
Alpha-hemolysis
partial lysis, resulting in greenish coloration around the colony
-ex. Streptococcus pneumonia
Y-hemolysis
no lysis, no color change
-ex. Enterococcus faecalis
Winogradsky Column
In the latter part of the 19th century, Sergei Winogradsky (b1856-d1953), a Russian microbiologist developed an early form of enrichment culture to study interactions between soil microorganisms
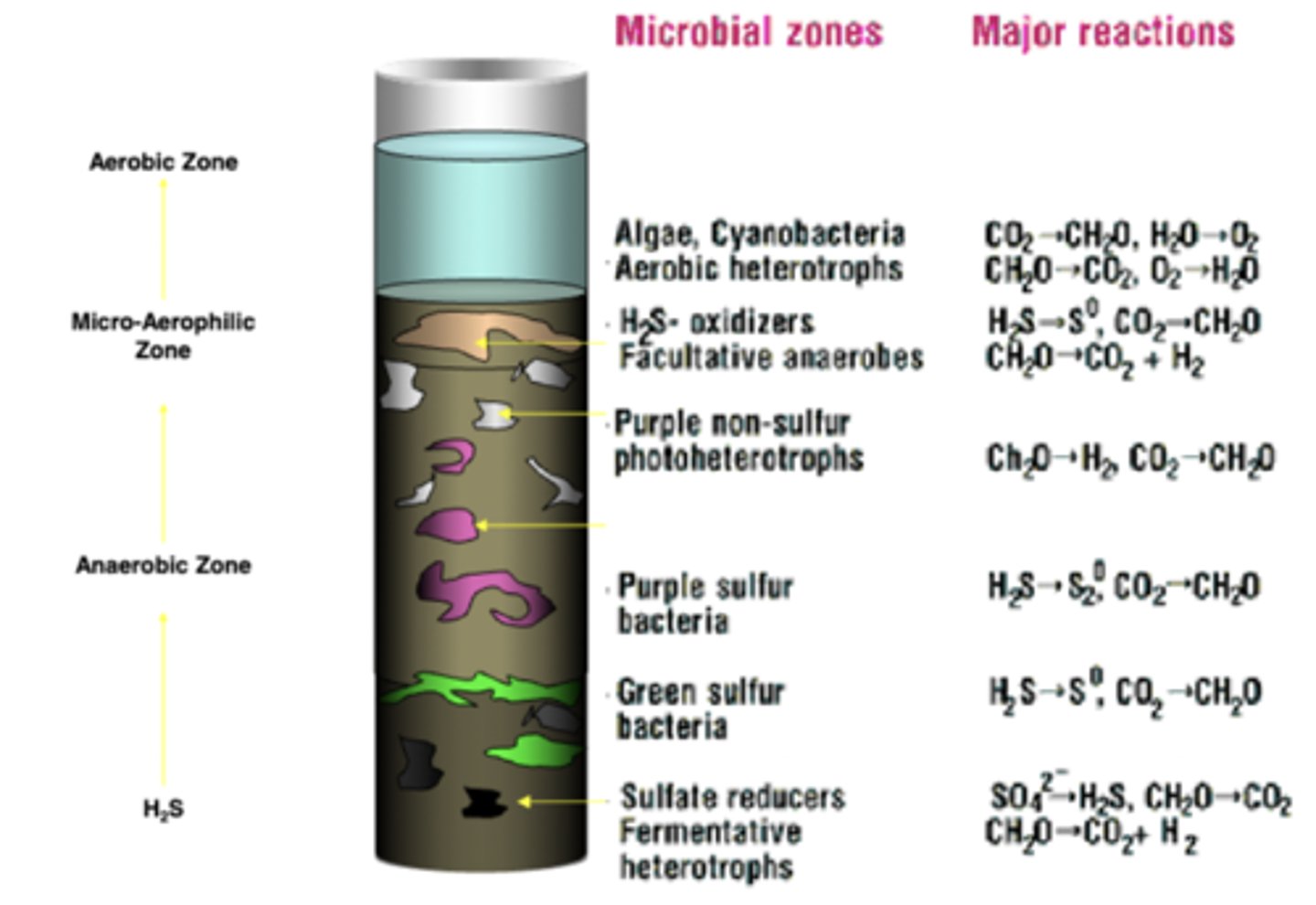
Top layer of Winogradsky Column
-Aerobic & light
-common microbes: algae, cyanobacteria
-- aerobic heterotrophs
Upper middle layer of Winogradsky Column
-microaerophilic
-common microbes: purple non-sulfur bacteria
-- photoheterotrophs
Lower middle layer of Winogradsky Column
-anaerobic & light
-common microbes: green sulfur bacteria, purple sulfur bacteria
bottom layer of Winogradsky Column
-anaerobic & dark
-common microbes: sulfate reducers
-- fermentative heterotrophs
pure cultures
one bacterium gives rises to one colony
enumeration of bacterial cells
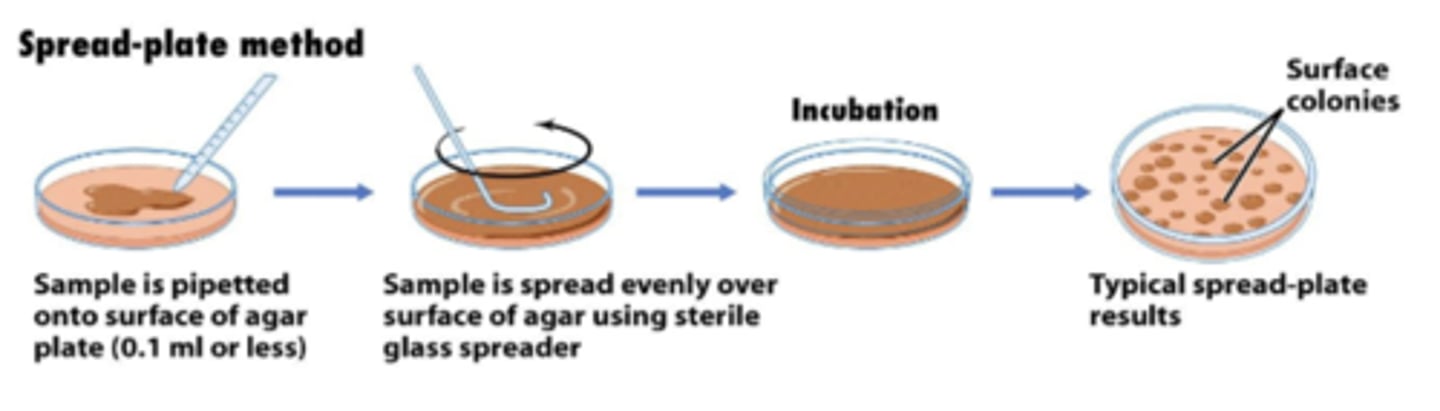
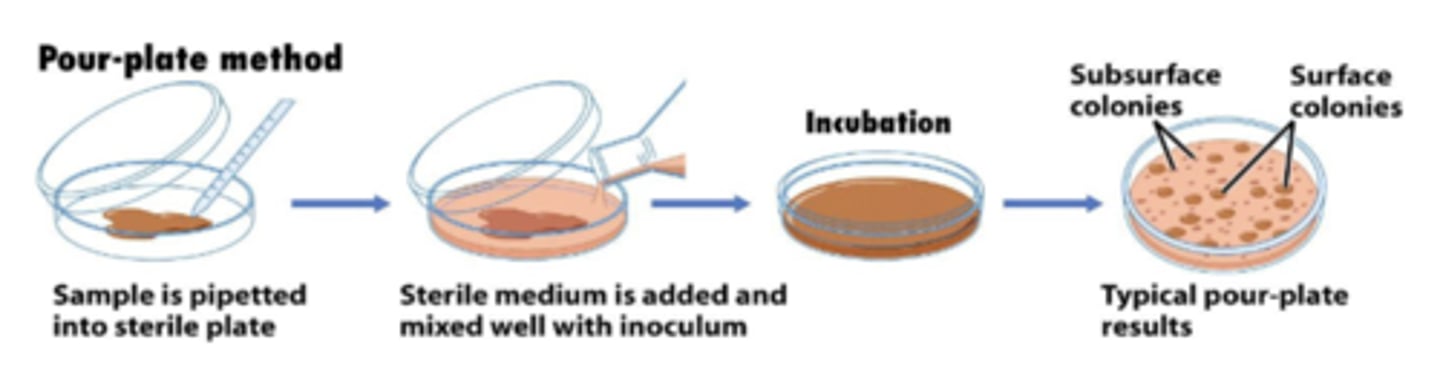
spread-plate method and pour-plate method
spread-plate method
pour-plate method
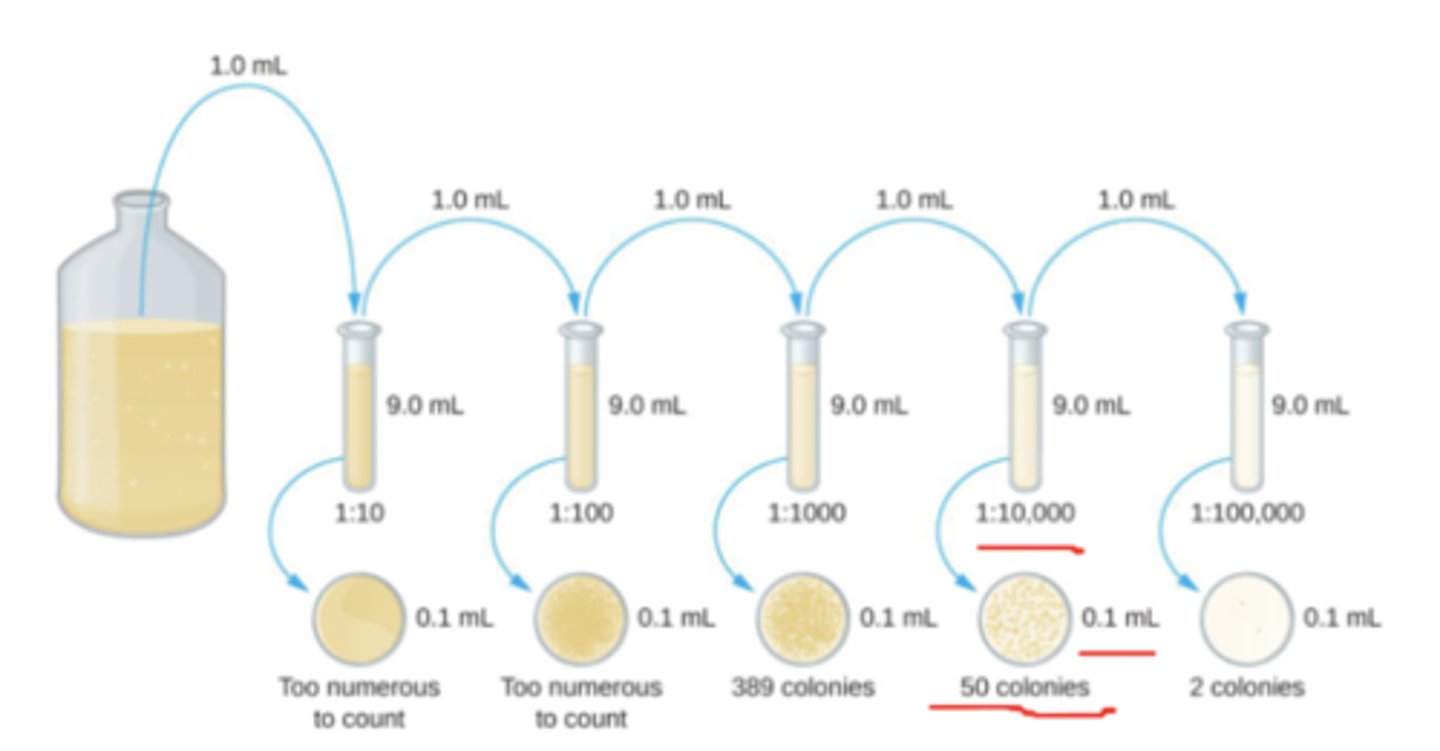
Serial dilutions
CFU/mL= (number of colonies per volume plated in μL) (1000μL/volume plated) (1/dilution factor)
example of serial dilutions
-CFU/mL= (389)(1000μL/100μL)(1/(10^-3)= 3891010^3= 3.89*10^6
-CFU/mL= (2)(1000/100)(1/10^-5)= 21010^5=2000000= 2*10^6
-CFU/mL= (50)(1000/100)(10^4)= 5*10^5
-the average CFU/mL is the average of the 3 plates
-between 30 to 300 colonies is good
colony
1 colony is a population of bacterial cells from the same mother cell, thus they are genetically identical
total cell count
-accounts for all cells, regardless of viability and culturability
-may not accurately represent potential for infection or food spoilage
total cell count importance
-useful for monitoring overall microbial load in a sample
viable cell count
-accounts only for cells capable of reproducing and forming colonies
viable cell count importance
-crucial for assessing food safety, water safety and pathogenicity
-used to evaluate the effectiveness of sterilization and antimicrobial treatments
counting chambers
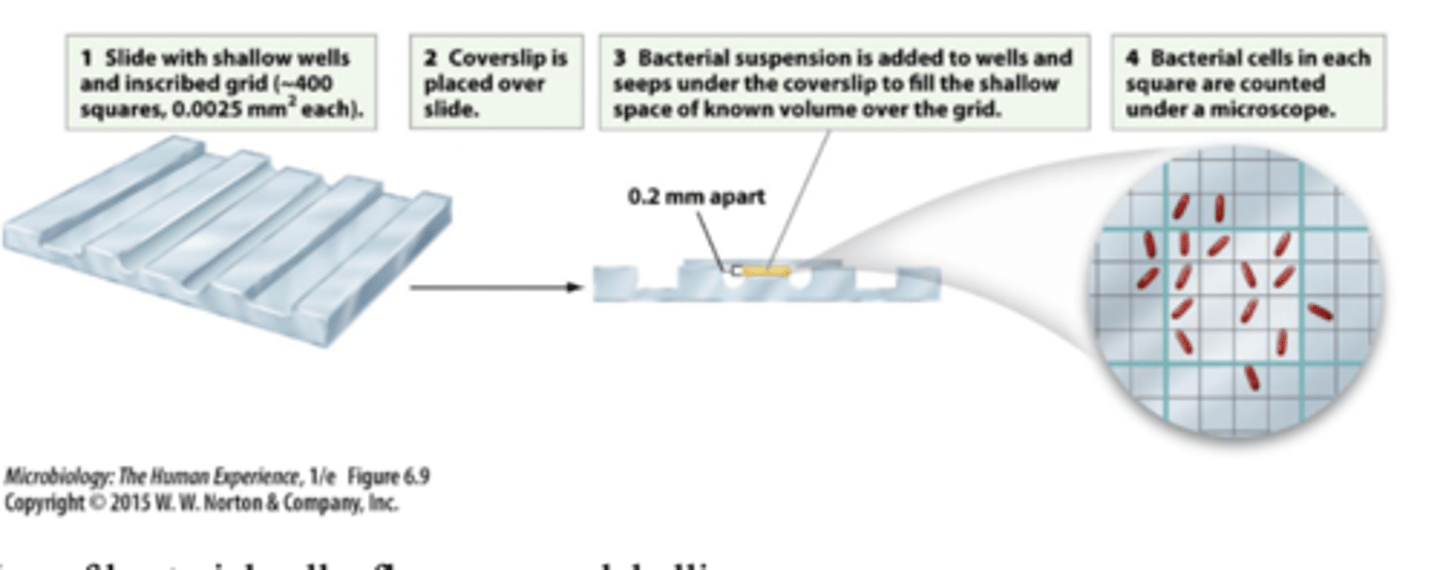
fluorescence labelling
-live/dead stain
-propidium iodide
-syto 9 green
propidium iodide
is unable to cross the cell membrane of intact/live cells. But can stain the nucleic acid of lysed cells red
Syto 9 green
is membrane permeable and will bind both live and dead cells
-Live cells are stained green, dead cells are stained red
Enumeration of bacterial cells
direct counting without a microscope
Fluorescence-activated cell sorter (FACS)
enumeration can be accomplished with an electronic instrument called FACS, which can count and separate bacterial cells with different properties
-used for viable cell count
turbidity
-Population can be calculated by measuring the turbidity of a cell culture. The degree to which the liquid medium has become cloudy because of microbial growth
-can be measured in real time using a spectrophotometer, which passes a beam of light through a sample of the culture