Gen Chem 2 Midterm
1/165
Earn XP
Description and Tags
Liquids and Intermolecular Forces of Attraction, Solutions & Concentration Formulas, Solids, Colligative Properties, Thermodynamics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
166 Terms
1. All matter is made up of tiny particles.
2. These particles are in constant motion.
3. The speed of a particle is proportional to temperature.
Increased temperature means greater speed.
4. Solids, liquids, and gases differ in distances between
particles, in the freedom of motion of particles, and in
the extent to which the particles interact.
What are the Kinetic Molecular Theories of Liquids and Solids?
Solid and Liquid states are referred to as condensed phases because solid and liquid states of particles are closer together
What States of Matter are called condensed phases and why?
Assumes both volume and shape of its container
Expands to fill its container
Is compressible
Flows readily
Diffusion within a gas occurs rapidly
What are some characteristic properties of Gas?
Assumes the shape of the portion of the container it occupies
Does not expand to fill its container
Is virtually incompressible
Flows readily
Diffusion within a liquid occurs slowly
What are some characteristic properties of Liquid?
Retains own shape and volume
Does not expand to fill its container
Is virtually incompressible
Does not flow
Diffusion within a solid occurs extremely slowly
What are some characteristic properties of Solid?
The distance between particles
What is the fundamental difference between states of matter?
The kinetic energy of the particles
The strength of the attractions between the particles
The state a substance is in at a particular temperature and pressure depends on what 2 antagonistic entities?
molecules
strong
compounds
The attractions between _________ are not nearly as ______ as the intramolecular attractions that hold _________ together.
Intermolecular forces of attraction(IMFA)
intriguing interactions that occur between molecules, shaping the physical and chemical properties of substances.
forces that act between separate molecules.
These forces arise due to the varying distribution of electrons within molecules, creating temporary imbalances of charge that induce attraction or repulsion between
neighboring molecules.
van der Waals forces
What do you call intermolecular forces as a group?
Dipole-dipole interactions
Hydrogen bonding
London dispersion forces
What are the van der Waals forces?
A pair of equal and opposite electric charges or magnetic poles of opposite sign separated especially by a small distance
What are dipoles?
London dispersion forces or dispersion forces
forces present in all molecules, whether they are non-polar
Electrons are in constant motion and there will be instances wherein they collect in one
area of an atom, creating a partial negative side (Induced Dipole) which forces a partial
positive side on the neighboring atom (Instantaneous Dipole)
These fleeting attractions contribute to the condensation of gases into liquids and the solidification of liquids into solids.
When there is an attraction between an instantaneous dipole and an induced dipole.
When do London dispersion forces/dispersion forces occur?
Instantaneous dipole
the term given to a molecule when a dipole of uneven charges is created very quickly and randomly in a molecule
This causes an induced dipole
Induced dipole
a weak attraction that results when a polar molecule induces a dipole in an atom or in a nonpolar molecule by disturbing the arrangement of electrons in the nonpolar species.
Polarizability
The tendency of a molecule’s electron cloud to distort
the tendency of molecules to generate induced electric dipole moments when subjected to an electric field
As this increases, the dispersion forces also become stronger
The shape of the molecule or surface area
Factor affecting the strength of London Dispersion Forces:
Long, skinny molecules tend to have stronger
dispersion forces than short, fat ones due to the increased _______ ____
Linear Molecule
This shape of a molecule has a larger surface area that enhances intermolecular contact and increases dispersion force
Spherical Molecule
This shape of a molecule has a smaller surface area that diminishes intermolecular contact and decreases dispersion force
Molecular weight
Factor affecting the strength of London Dispersion Forces:
The strength of dispersion forces tends to increase with increased _________ ______.
Ex. Larger atoms have more electrons which means a stronger attractive force when they become dipoles
Dipole-Dipole Interactions
the attraction between molecules that have permanent dipoles
The positive end (δ+) of one is attracted to the negative end (δ−) of the other, and vice versa.
These forces are only important when the molecules are close to each other.
As the polarity of molecules increases, so does its dipole-dipole forces
What is the relationship of dipole-dipole forces and molecules’ polarity?
The higher the dipole-dipole force, the higher the boiling point is due to greater IMFA
What is the relationship of dipole-dipole forces and a molecule’s boiling point
Hydrogen Bonding
Dipole-dipole interactions that arise in part from the high electronegativity of nitrogen, oxygen, and fluorine.
Unusually strong
When ________ is bonded to one of
those very electronegative elements, the
________ nucleus is exposed.
Due to the difference in electronegativity, it becomes difficult to separate hydrogen from
NOF thus making water have a high boiling point.
The dipole–dipole interactions experienced when H is bonded to N, O, or F
What interactions causes hydrogen bonding
Ion-dipole interaction
the intermolecular force of attraction between a charged ion (cation or anion) and a molecule that has a dipole
The strength of these forces is what makes it possible for ionic substances to dissolve in polar solvents.
? Increasing interaction strenght
a. London-dispersion forces
b. Dipole-dipole forces
c. Hydrogen bonding
d. Ion-dipole bonding
e. Ion bonding
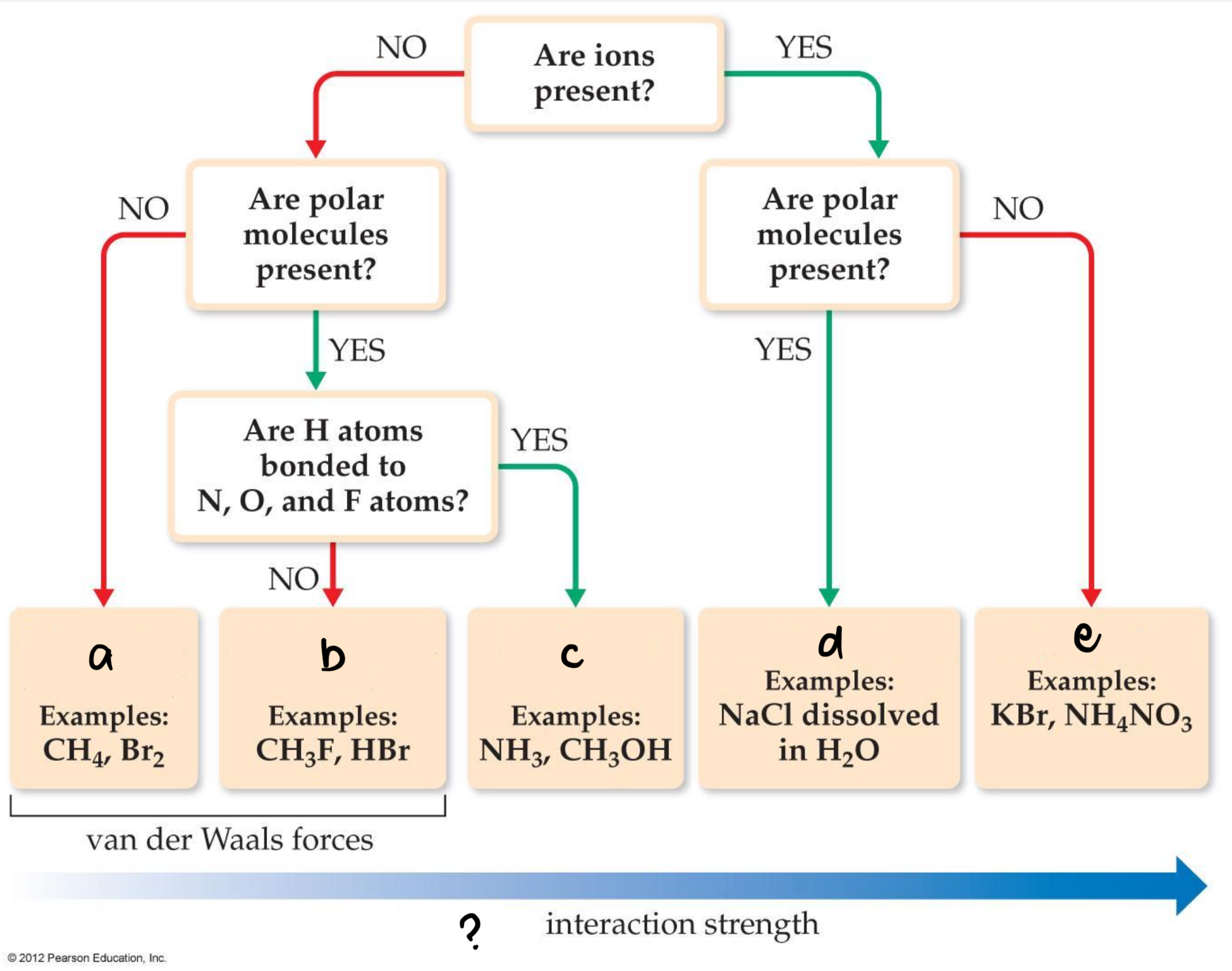
Boiling Point and Melting Point
Viscosity
Surface Tension
Capillary Action
List the Liquid Properties Affected by Intermolecular Forces
Boiling Point
the temperature at which liquid boils
Melting Point
the temperature at which a substance changes state from solid to liquid
The stronger the IMFA, the higher the boiling and melting point of a substance
What is the relationship of a substance’s Intermolecular force of attraction(IMFA) to its boiling & melting point?
Viscosity
Resistance of a liquid to flow
related to the ease with which molecules can move past each other
Longer Carbon Chain = Higher _________
Centipoise
What is the unit of measurement of Viscosity?
Viscosity increases with stronger IMFA
Viscosity decreases with higher temperature
What is the relationship of a substance’s viscosity to its Intermolecular force of attraction(IMFA) and temperature?
Surface tension
The property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of its molecules.
Water acts as if it has a “skin” on it due to extra inward forces on its surface.
The stronger the IMFA, the greater the surface tension of a substance
What is the relationship of a substance’s Intermolecular force of attraction(IMFA) to its surface tension?
Cohesive Forces
Intermolecular forces that bind similar molecules to one another(Mercury = Convex)
Adhesive Forces
Intermolecular forces that bind a substance to a surface(Water = Concave)
Capillary Action
The rise of liquids up narrow tubes
Ex. Water has stronger adhesive forces with glass; mercury has stronger cohesive forces with itself.
Adhesive forces attract the liquid to the wall of the tube.
Cohesive forces attract the liquid to itself.
How are cohesive and adhesive forces important for capillary action?
Vapor pressure
Pressure exerted by vapor onto a liquid in a container
At any temperature, some liquid
molecules have enough energy to escape the surface and become a gas.
The higher the vapor pressure, the weaker the intermolecular forces
How do intermolecular forces of attraction affect the vapor pressure of a liquid?
As the temperature of a substance increases its vapor pressure also increases
As the temperature rises, the fraction of
molecules that have enough energy to
break free increases.
What is the relationship of a substance’s temperature to its vapor pressure?
When liquid molecules evaporate and vapor molecules condense at the same rate.
rate of vaporization = rate of condensation
When do liquid and vapor pressure reach a state of dynamic equilibrium?
The boiling point of a liquid is the temperature at which its vapor pressure equals atmospheric pressure.
Boiling point of a liquid is when vapor pressure = atmospheric pressure
Define the boiling point of a liquid in relation to temperature, vapor pressure and atmospheric pressure
The temperature at which its vapor pressure is 760 torr
What temperature is considered the normal boiling point of a liquid?
Volatility
describes how easily a substance will vaporize (turn into a gas or vapor)
a substance that evaporates readily at normal temperatures
one that has a measurable vapor pressure
The more volatile, the weaker the intermolecular forces.
How do intermolecular forces of attraction affect volatility?
Solutions
are homogeneous mixtures of two or more pure substances.
the solute is dispersed uniformly throughout the solvent.
Solute
the component of a solution that is lesser in quantity; the substance being dissolved.
Solvent
the component of a solution that is greater in amount; the dissolving substance of a solution.
The natural tendency of substances to mix and spread into larger volumes when not restrained in some way
The types of intermolecular interactions involved in the solution process
The ability of substances to form solutions depends on 2 factors
Mixing of gases is a spontaneous process.
Each gas acts as if it is alone to fill the container.
Mixing causes more randomness in the position of the molecules, increasing a thermodynamic quantity called entropy.
The formation of solutions is favored by the increase in entropy that accompanies mixing.
Give the Natural Tendency towards Mixing
When molecules of different types are brought together, mixing occurs spontaneously unless the molecules are restrained wither by sufficiently strong intermolecular forces
How does IMFA affect the ability of substances to form solutions?
Solute-solute interactions
Intermolecular Interaction Involved in Solution Formation:
interactions between solute particles must be overcome to disperse the solute particles through the solvent
Solvent-solvent interactions
Intermolecular Interaction Involved in Solution Formation:
interactions between solvent particles must be overcome to make room for the solute particles in the solvent
Solvent-solute interactions
Intermolecular Interaction Involved in Solution Formation:
interactions between solvent and solute particles occur as the particles mix
Solubility
is the maximum amount of solute that can dissolve in a given amount of solvent at a given temperature.
Saturated solutions
solutions that have the maximum amount of solute dissolved.
Unsaturated solutions
solutions that have any amount of solute less than the maximum amount dissolved in solution.
Supersaturated solution
solutions where the solvent holds more solute than is normally possible at that temperature.
These solutions are unstable; crystallization can usually be stimulated by adding a “seed crystal” or scratching the side of the flask.
These are uncommon solutions.
Solute-solvent interactions
Pressure
Temperature
List the factors that affect solubility
The stronger the solute-solvent interaction, the greater the solubility of a solute in that solvent
The larger the gas(molar mass), the more soluble it will be in water.
Polar organic molecules dissolve in water better than nonpolar organic molecules
hydrogen bonding increases solubility
What is the relationship between solute-solvent interactions and how it affects a solute’s solubility?
Miscible
Liquids that mix in all proportions
Immiscible
Liquids that do not mix in one another
A solution of Hexane and water are a good example becuase hexane is nonpolar and water is polar
External pressure has very little effect on the solubility of liquids and solids.
In contrast, the solubility of gases increases as the partial pressure of the gas above a solution increases.
How does pressure affect the solubility of solids, liquids and gases?
If the pressure of a gas over liquid increases, the amount of gas dissolved in the liquid will increase proportionally.
How does Henry’s law explain the relationship of the pressure of gas and the solubility of solutes?
an increase in temperature produces an increase in solubility for solids[most of the time].
The addition of more heat facilitates the dissolving reaction by providing energy to break bonds in the solid.
How does temperature affect the solubility of a solid in a liquid?
the solubility (concentration) increases with an increase in temperature.
An increase in temperature puts a stress on the equilibrium condition and causes it to shift to the right. The stress is relieved because the dissolving process consumes some of the heat.
How does temperature affect the solubility of a liquid in a liquid?
as the temperature increases, solubility decreases accordingly.
How does temperature affect the solubility of a gas in a liquid?
Mass percentage
Parts per million (ppm)
Mole fraction
Molarity
Molality
List the Units of Solution Concentration
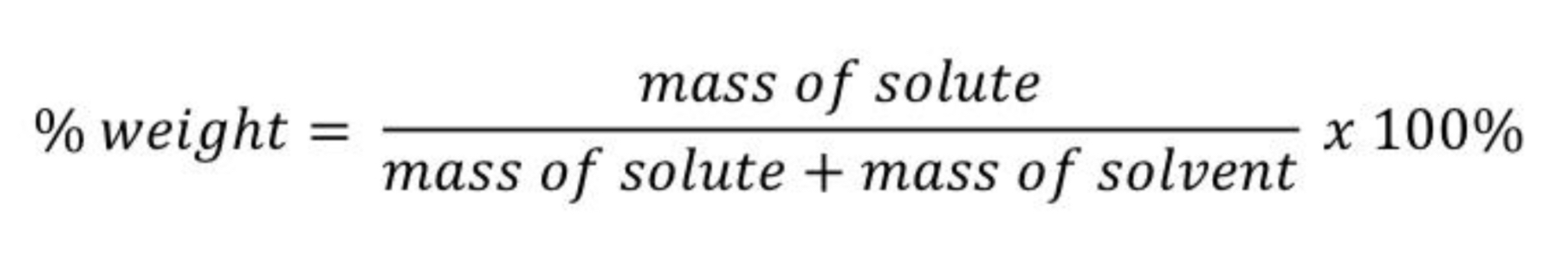
Give the % by weight(w/w) formula
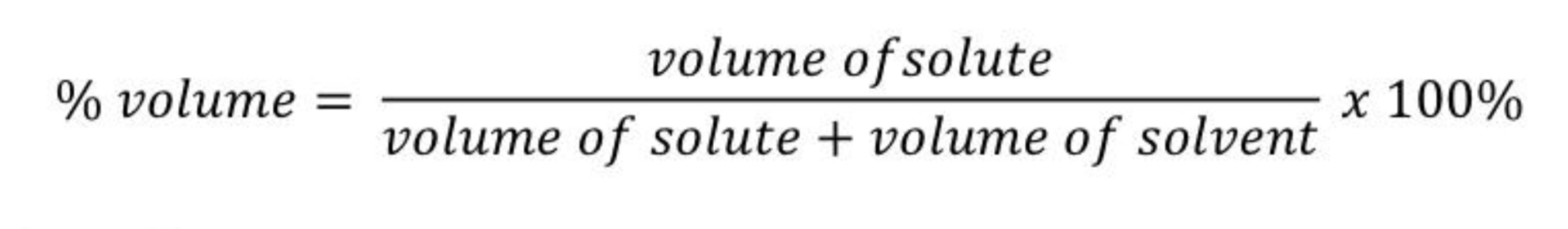
Give the % by volume(v/v) formula
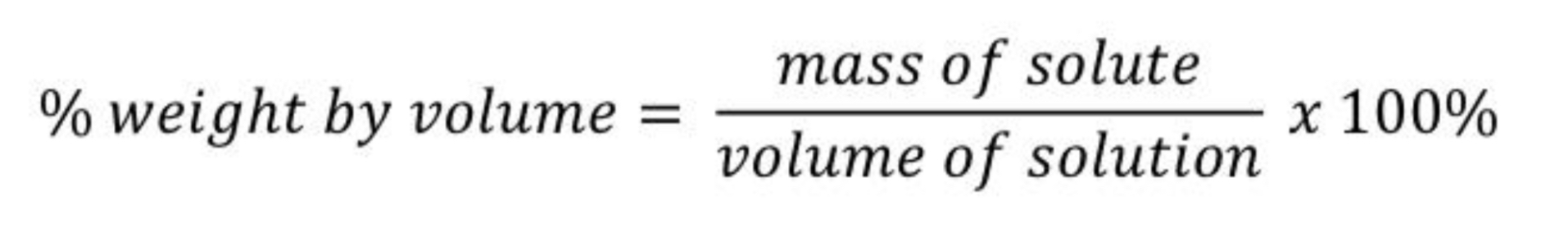
Give the % by weight by volume(w/v) formula
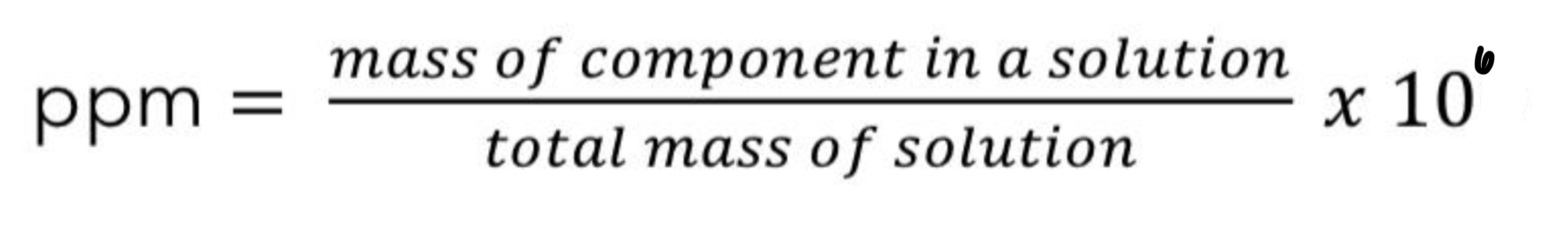
Give the Parts per million formula(ppm) still relating mass of a solute to the total mass of the solution
Note: Since percent is out of 100, we multiplied by 100.

Give the formula to find the moles of a component
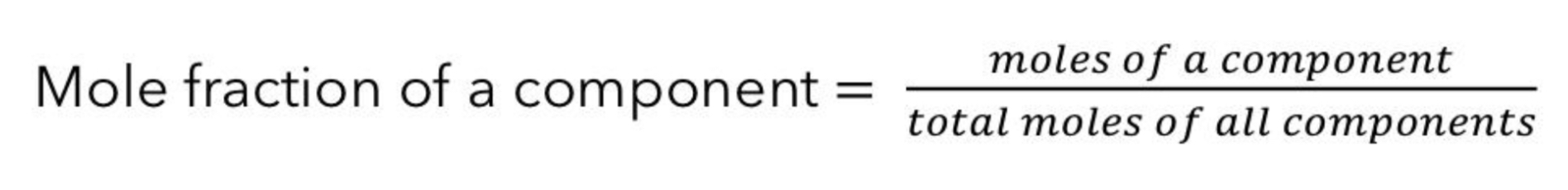
Give the mole fraction formula
Mole fraction is the ratio of moles of a
substance to the total number of moles in a
solution.
It does not matter if it is for a solute or for a
solvent.
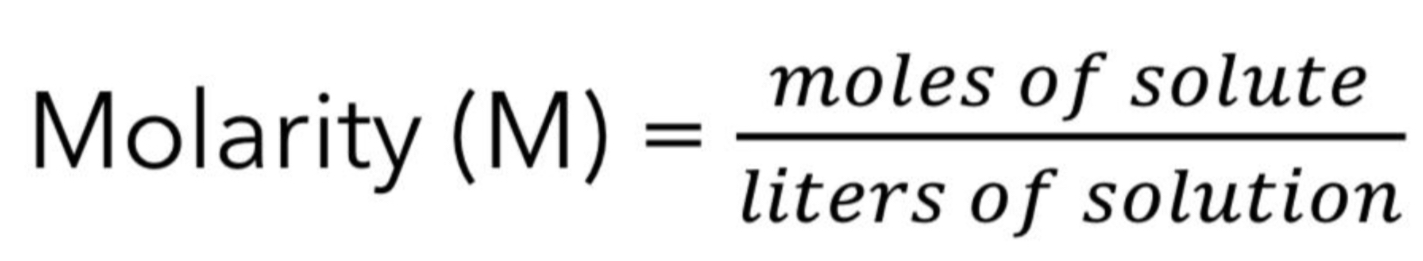
Give the formula for Molarity(M)
Molarity varies with temperature (volume
changes).
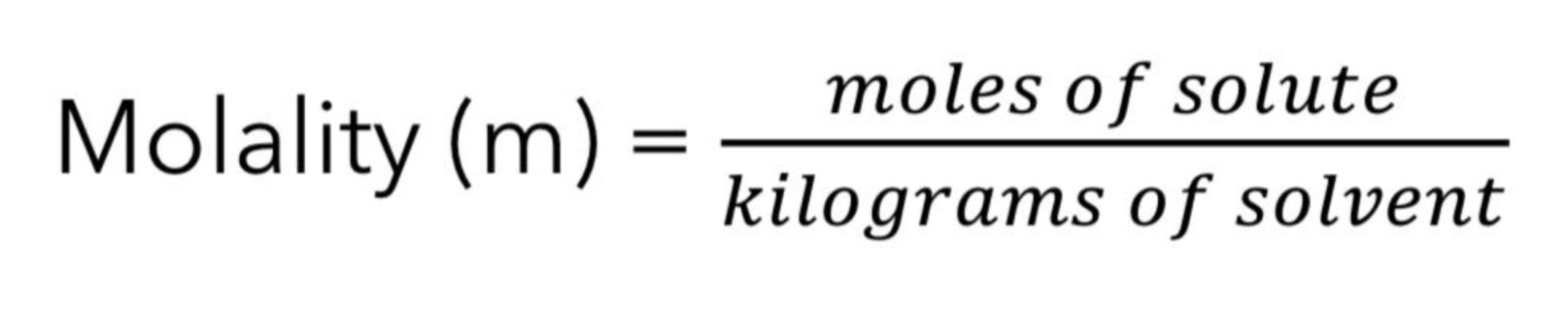
Give the formula for Molality(m)
Molality does not vary with temperature
(mass does not change).
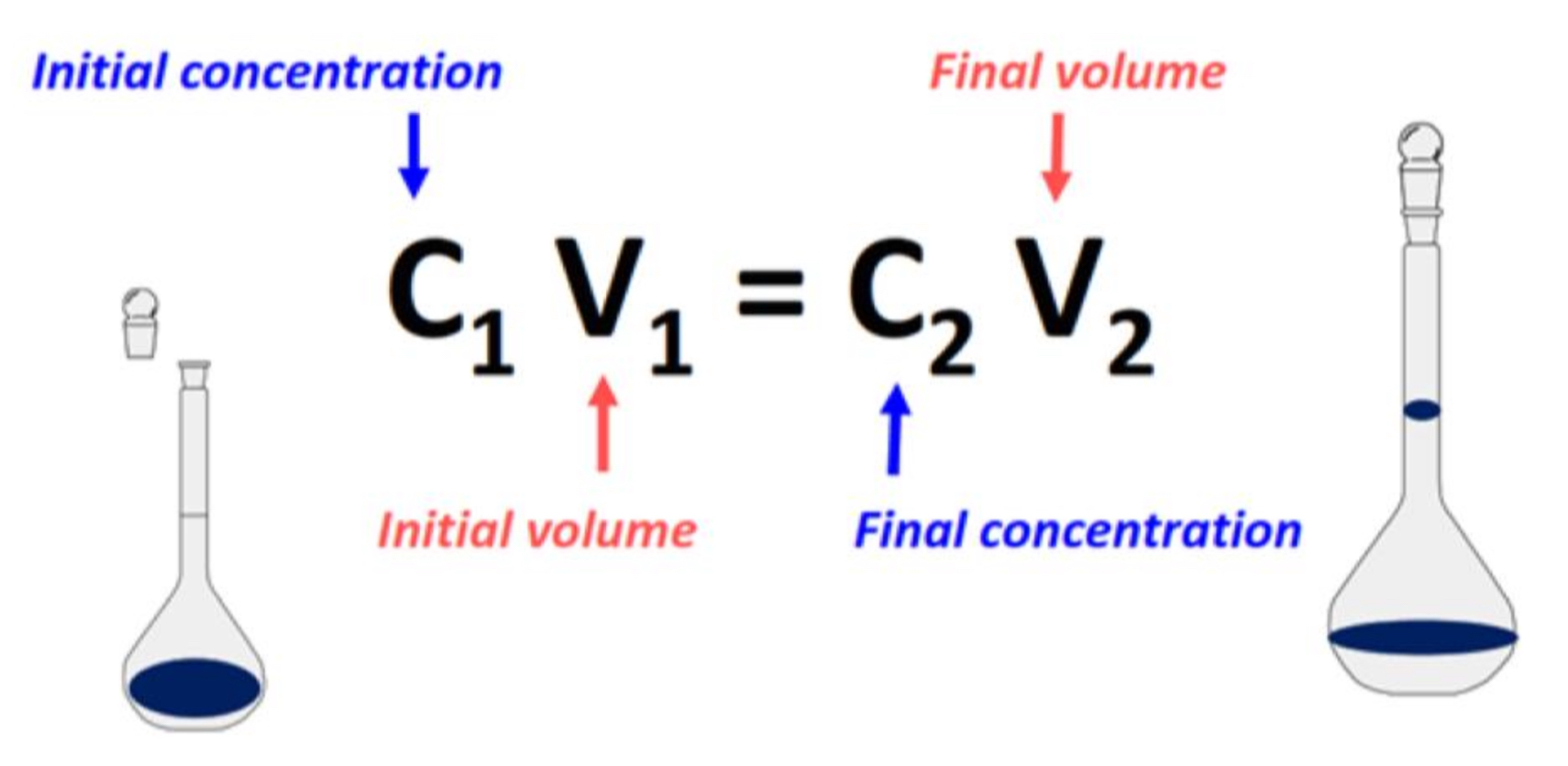
Give the Dilution Formula
Metallic solids
Type of Solid and how it they bond:
share a network of highly delocalized electrons meaning they have free movement around the solid
Generally created through metallic bonding
Due to the delocalized nature of the electrons, it is a good heat and electricity conductor.
It is also shiny due to the photoelectric effect. (Basically, the free electrons when taken in
light release excess energy absorbed thus making the metal look reflective.)
Has a high melting point due to the strong IMFA
Because it is covalently bonded, when distorted it does not break easily.
Properties of Metallic Solids
Ionic Solids
Type of Solid and how it they bond:
are sets of cations and anions mutually attracted to one another.
Are held together by ionic bonding (difference in charges).
Usually have structures that minimize the distance between oppositely charged and maximize the distance between like-charged
Really hard and have high melting points due to strong IMFA
Poor conductor when solid due to the rigid nature of the lattice
Good conductor when liquid to the electronegative ions being mobile.
Is brittle. This is due to the alternating structure of the solid, a slight change can cause
like-charged particles to interact thus destroying the structure of the lattice.
Properties of Ionic Solids
Covalent-network solids
Type of Solid and how it they bond:
are joined by an extensive network of covalent bonds.
All atoms in the lattice are covalently bonded thus giving it a really robust structure
Diamonds are an example of this.
Is hard, has a high melting point, and is a poor conductor.
Though it is brittle, powdered forms are rarely seen due to most covalent-network solids
being inherently small.
Properties of Covalent-Networks Solids
Molecular solids
Type of Solid and how it they bond:
are discrete molecules that are linked to one another only by van der Waals forces.
Graphite is the only _________ ______ to be able to conduct electricity due to the large gap in
between the sheets allowing for easy passage of particles.
They are soft(weak attractive forces),
Have low melting points(weak attractive forces),
Poor conductors(particles cannot move easily),
Brittle(deformation cause attractive forces to be broken).
Properties of Molecular Solids
Crystalline solids
type of solids that has atoms arranged in a very regular pattern
Amorphous solids
type of solids characterized by a distinct lack of order in the arrangement of atoms.
Crystal Lattice
the symmetrical three-dimensional structural arrangements of atoms, ions or molecules (constituent particle) inside a crystalline solid as points.
It can be defined as the geometrical arrangement of the atoms, ions or molecules of the crystalline solid as points in space.
Lattice point
represents each atom, molecule or ions (constituent particle) is in crystal lattices
joined together by a straight line in a crystal lattice.
Unit Cell
the smallest part (portion) of a crystal lattice. It is the simplest repeating unit in a crystal structure.
The entire lattice is generated by the repetition of this in different directions.
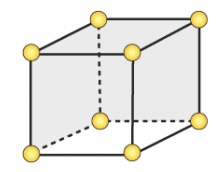
Primitive/Simple Cubic Unit Cell
Type of Unit Cells
points only at the corners
Coordination Number = 6
One above, 4 surrounding the center, and one below
Has only 1 atom (1⁄8 atom for 8 corners)
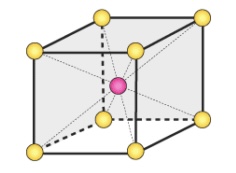
Body Centered Unit Cell(BCC)
Type of Unit Cells
points at corners and one at the center
Coordination Number = 8
4 above and 4 below
Has 2 atoms (1 atom at center + 1⁄8 atom for 8 corners)
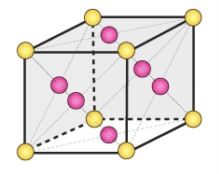
Face-Centered Unit Cell(BCC)
Type of Unit Cells
points at corners and at faces
Coordination Number = 12
4 above, 4 surrounding center, 4 below
Has 4 atoms (1⁄2 atoms for 6 faces + 1⁄8 atom for 8 corners)
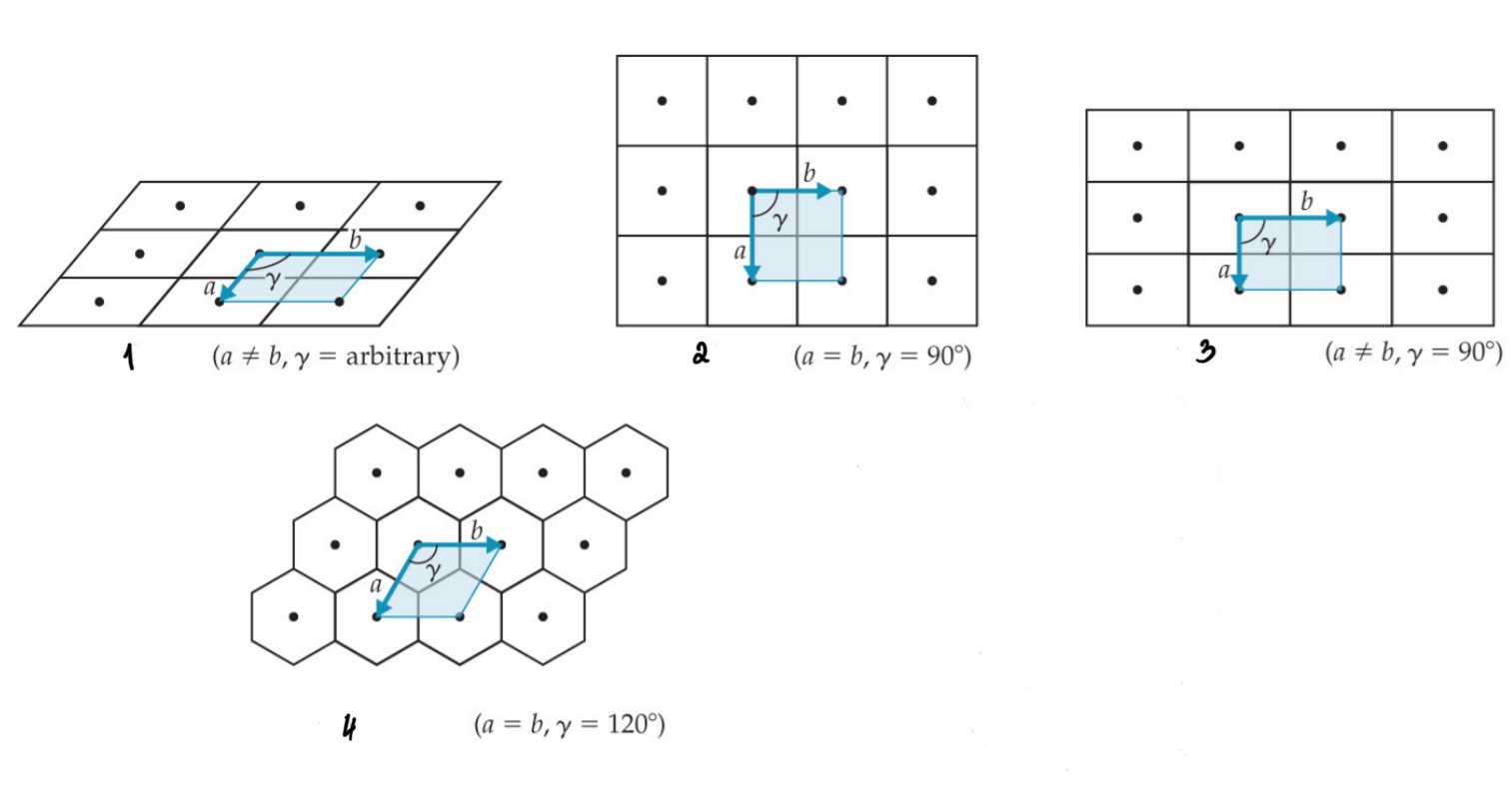
Oblique
Square
Rectangular
Hexagonal
Give the names of the 2D Unit Cells
a ≠ b, y is arbitrary
a = b, y = 90°
a ≠ b, y = 90°
a = b, y = 120°
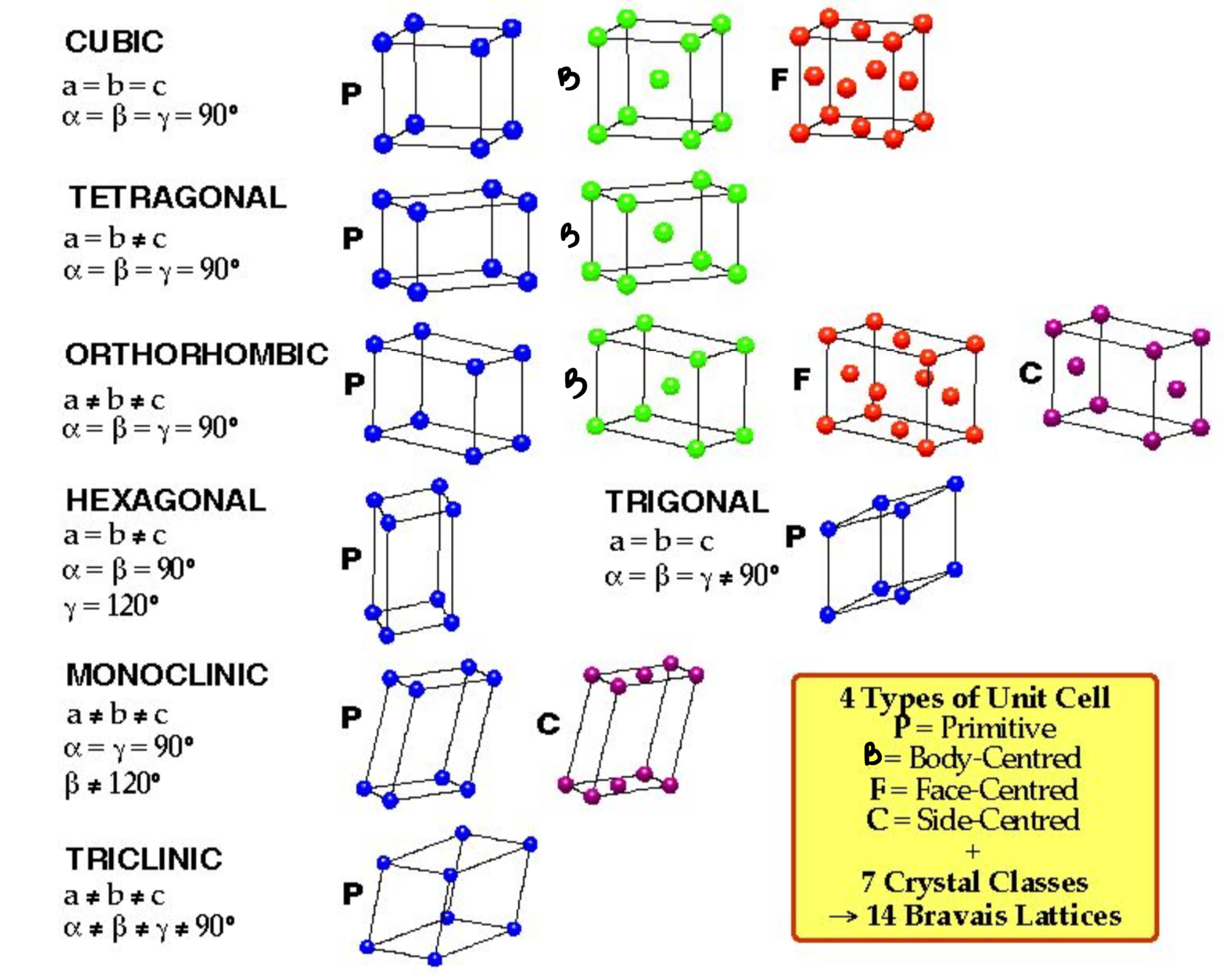
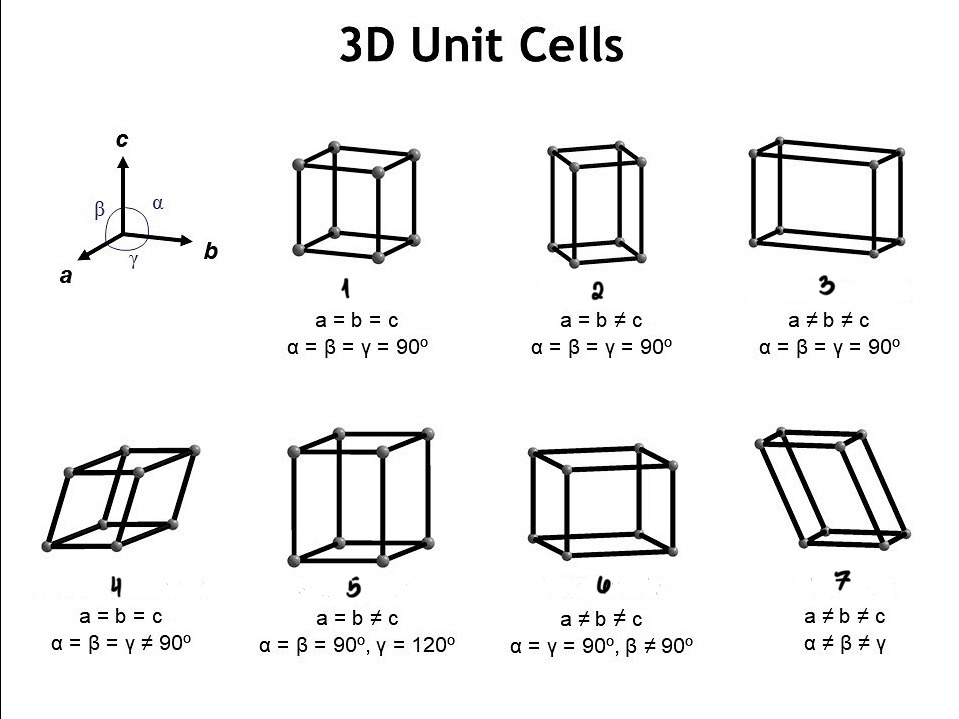
Cubic(PBF)
Tetragonal(PB)
Othrorhombic(PBFC)
Rhombohedral(P)
Hexagonal(P)
Monoclinic(PC)
Triclinic(P)
Give the names of the 3D Unit Cells
All axes are the same and are all perpendicular
Two axes are the same length and are all perpendicular
Different lengths and are all perpendicular
Equal lengths but no perpendicular axes
Partial part of a full hexagon has two axes of the same length
No axes have the same length and two are perpendicular
No axes are the same length nor are they perpendicular
Close Packing
The atoms in a crystal pack as close together as they can based on the respective sizes of the atoms.