Chapter 12: Liquids and Solids
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
Intermolecular Forces
Kinetic molecular theory states that the attractive forces between
molecules in a gas are negligible—gases have neither a definite
shape or volume.
• Liquids and solids have definite volumes, which means that there
are attractive forces holding the particles together.
• These temporary attractive forces are known as intermolecular
forces (they can apply to atoms and ions as well as molecules).
• While these intermolecular forces attractions, are much weaker
than covalent or ionic bonds.
Intermolecular vs. Intramolecular
• Within a molecule, atoms are held together by intramolecular
covalent bonds.
• Different molecules in a liquid or solid are held together by weak,
temporary intermolecular forces. (in between molecules)
• Intermolecular forces frequently break and reform between
different molecules in a liquid sample.
intra molecular is stronger
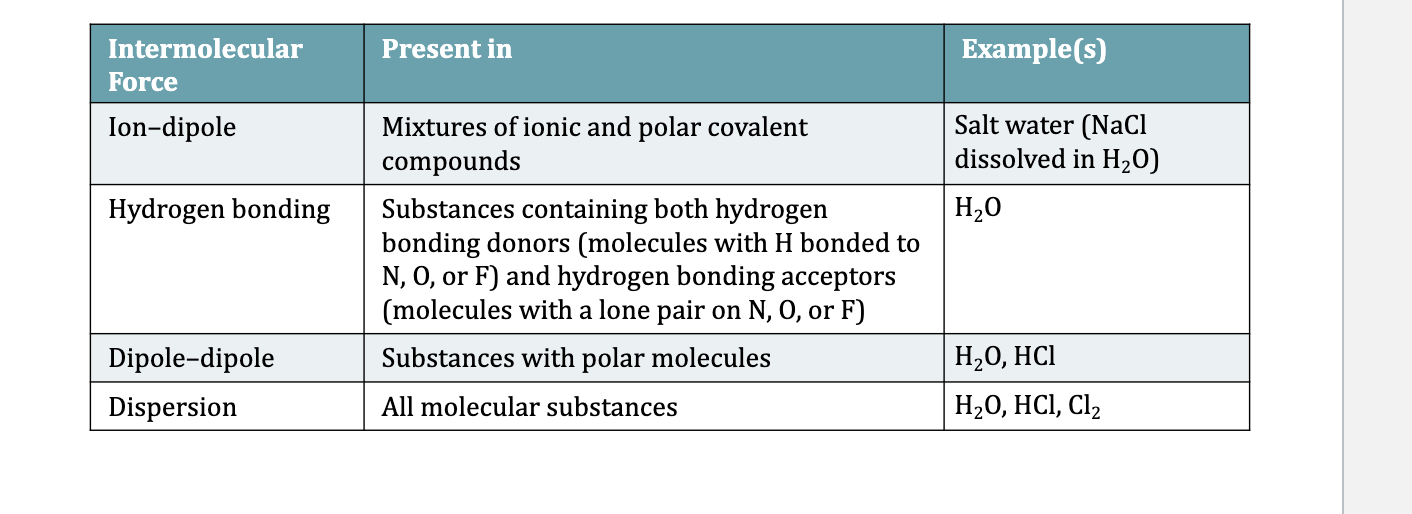
Types of intermolecular forces
Ion-dipole attractions form btwn ions and polar molecules
Dipole-dipole attractions form btwn 2 polar molecules
Hydrogen bonding is a particularly strong type of dipole-dipole attraction
Dispersion forces occur between all molecules but are most notable btwn nonpolar molecules
The strength and type of intermolecular forces can explain trends in MP and BP and solubilities and explains unique properties of water
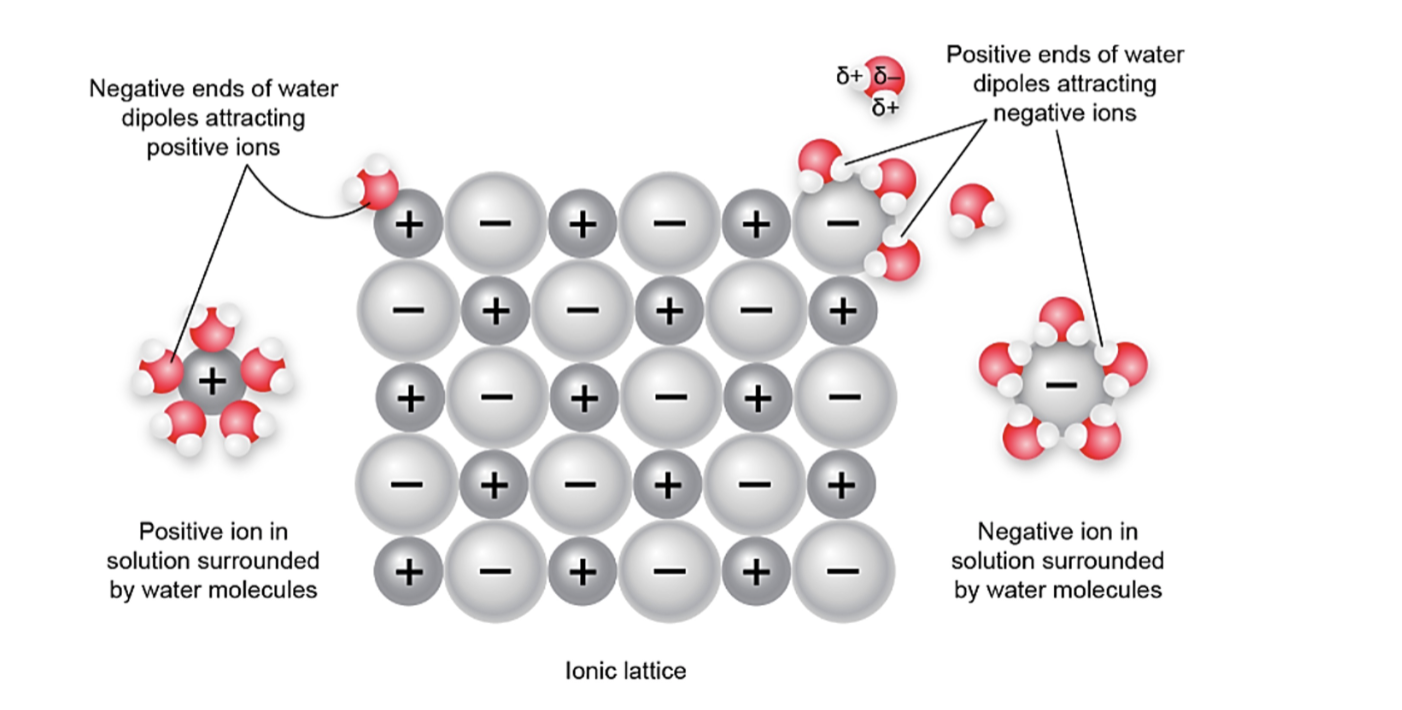
Dissolving Action of water on an ionic solid
Dipole-Dipole Attractions
• Found between polar molecules (same or different type).
• The negative pole of one polar molecule is attracted to the positive
pole of another polar molecule.
• These attractions are a type of intermolecular force known
as dipole–dipole attractions
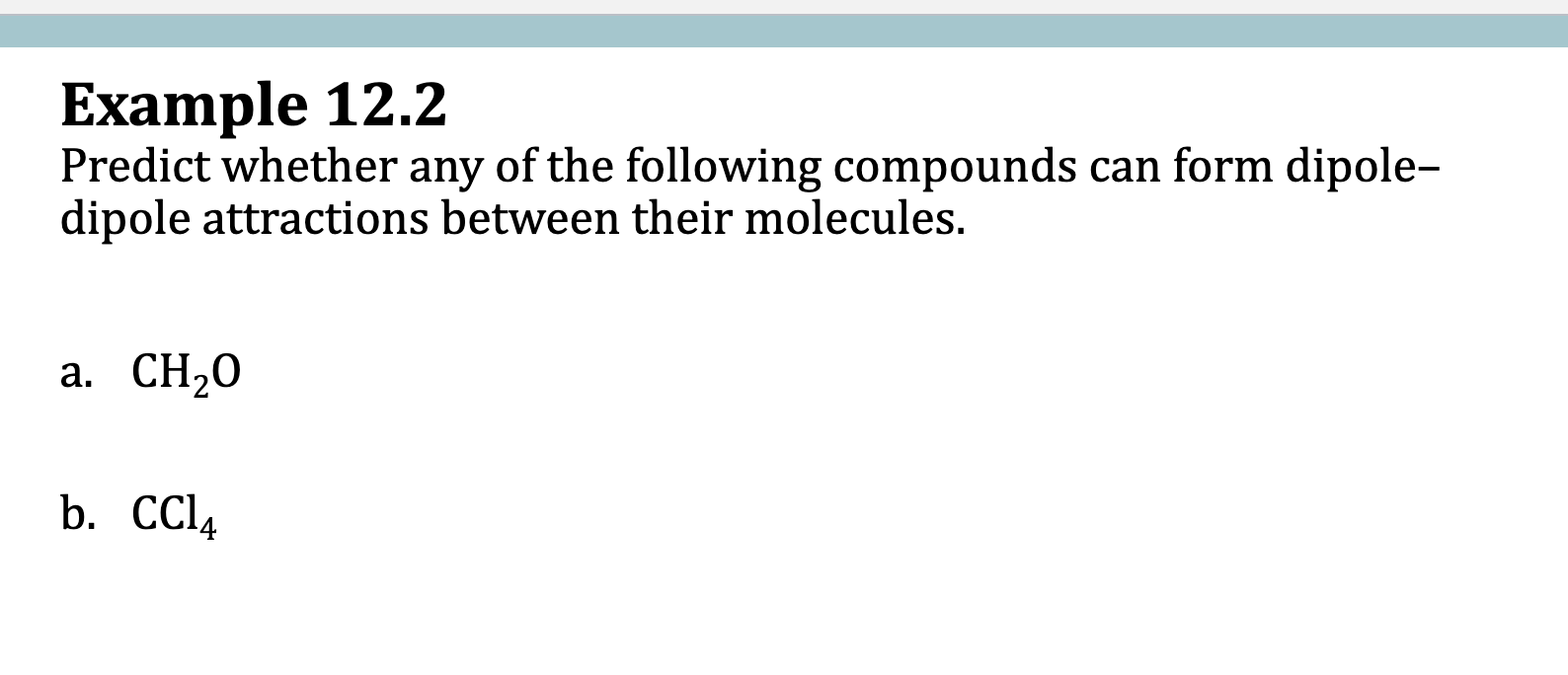
Example 12.2
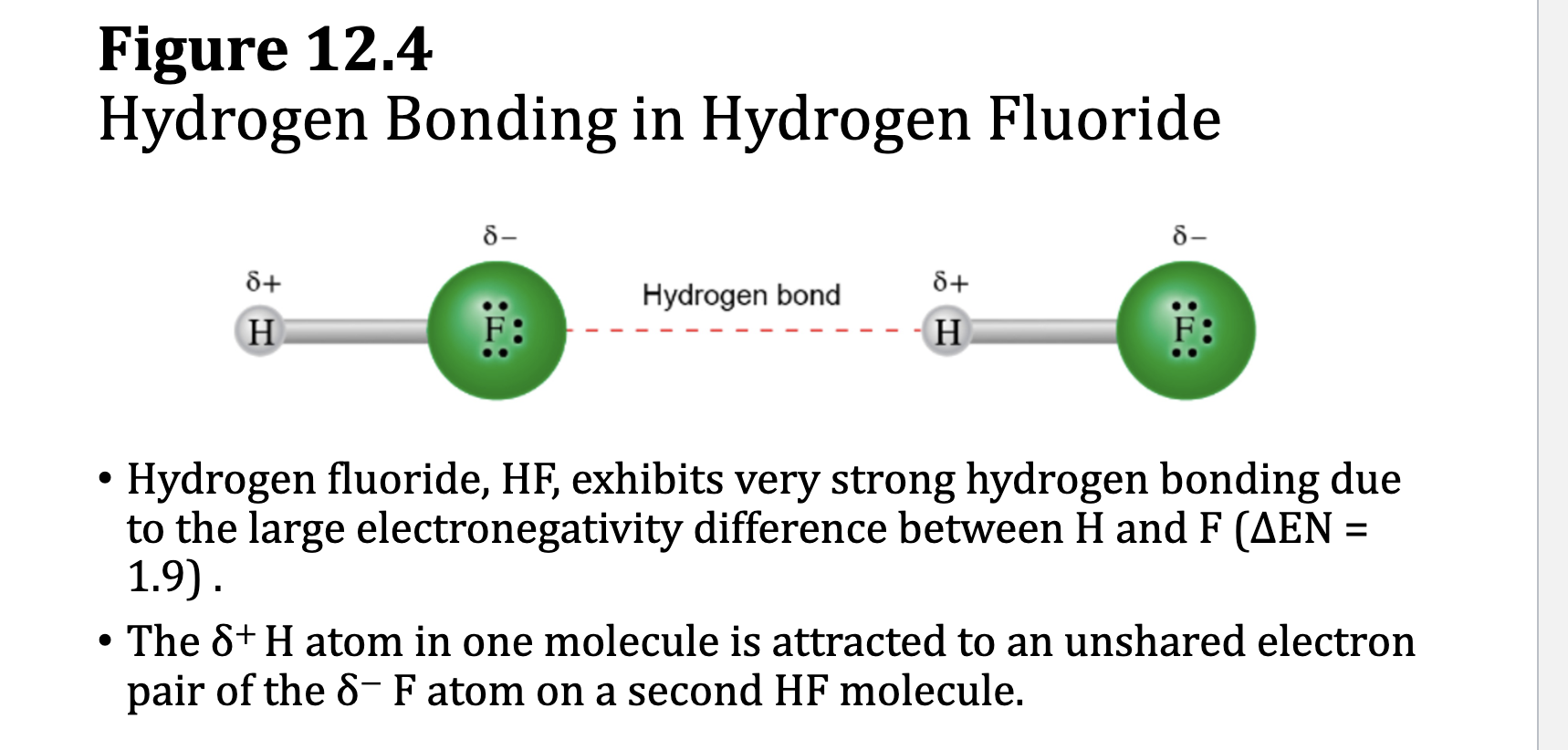
Hydrogen Bonds
• A hydrogen bond is a special case of dipole–dipole attractions and is
stronger than other dipole–dipole attractions.
• Hydrogen bonding requires two very specific components:
• A hydrogen bond donor molecule containing a partially positive H atom
bonded to F, O or N
• A hydrogen bond acceptor molecule containing a partially negative N, O
or F with lone pair electrons
• A hydrogen bond is the attraction between the δ + H in the donor molecule
and a lone pair of electrons on the δ − O, N, or F of the acceptor molecule.
Dispersion Forces or London Dispersion Forces
• Default type of intermolecular forces, found in between all type of
atoms and molecules
• Weakest IMF, so important only when no other type of IMF are
present
• Caused by temporary asymmetric distribution of electrons,
forming an instantaneous dipole.
• Also known as dipole induced dipole attraction.
• All atoms and molecules exhibit dispersion forces.
• These forces are especially important for understanding the
physical properties of nonpolar substances.
strongest in larger, heavier molecules; greater surface area; linear molecules have stronger dispersion than compact ones
Factors Affecting the strength of dispersion forces: atomic size
• The ability of an electron cloud to become asymmetric, its
polarizability
• Smaller atoms do not polarize readily.
• Larger atoms polarize more readily.
• Larger atoms or molecules have stronger LDF compared to smaller
atoms or molecules.
Atomic Size and Polarizability
• Consider the diatomic halogens: F2, Cl 2, Br 2 , and I 2.
• Fluorine and chlorine, the smaller halogens, are gases at room
temperature.
• Bromine, which is larger, is a liquid.
• Iodine, which is still larger, is a solid.
• However, both bromine and iodine vaporize readily, indicating the
weakness of the dispersion forces
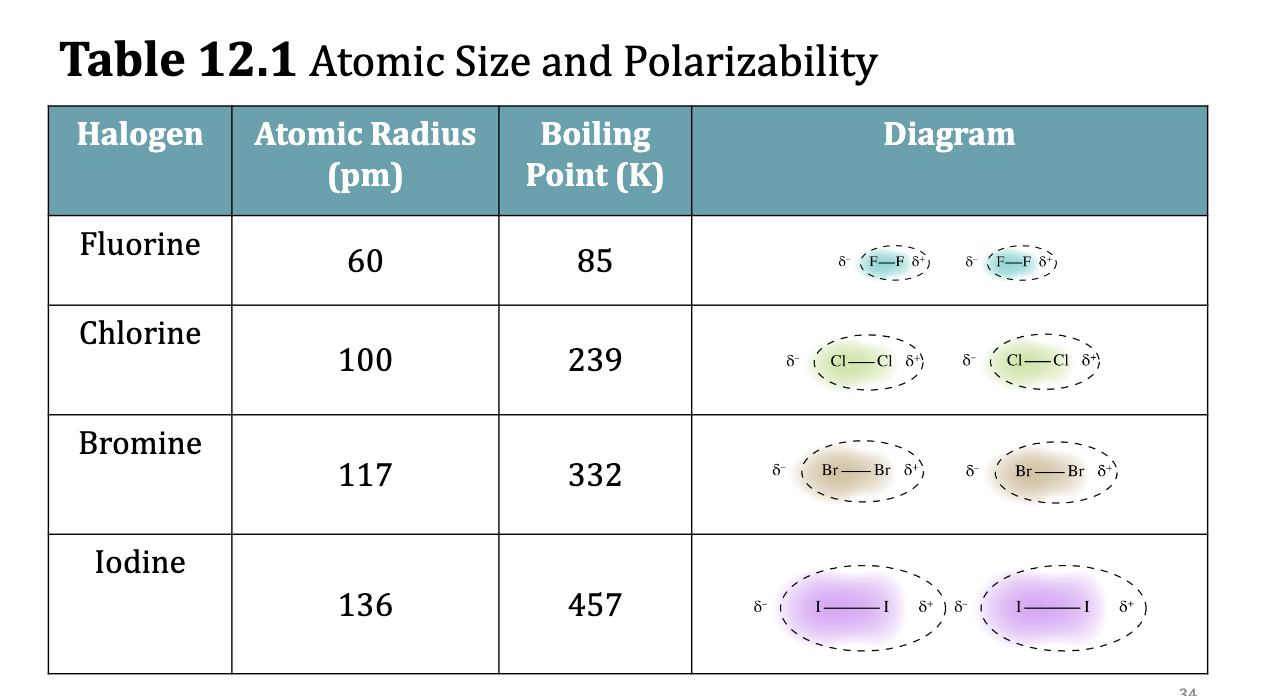
Table 12.2 Typs of Intermolecular Forces strongest to weakest
Properties of Liquids
• The particles of a liquid are in contact with one another but not as
closely as in the solid state.
• The particles of a liquid are in constant motion and interact via
intermolecular forces, which are weak and temporary.
• These weak and temporary forces give liquids their fluid
properties of viscosity, surface tension, and capillary action.
Viscosity
• The resistance to flow, viscosity, is determined by the strength of
the intermolecular attractions and temperature.
• Stronger IMF = High Viscosity
• High temperature = low viscosity
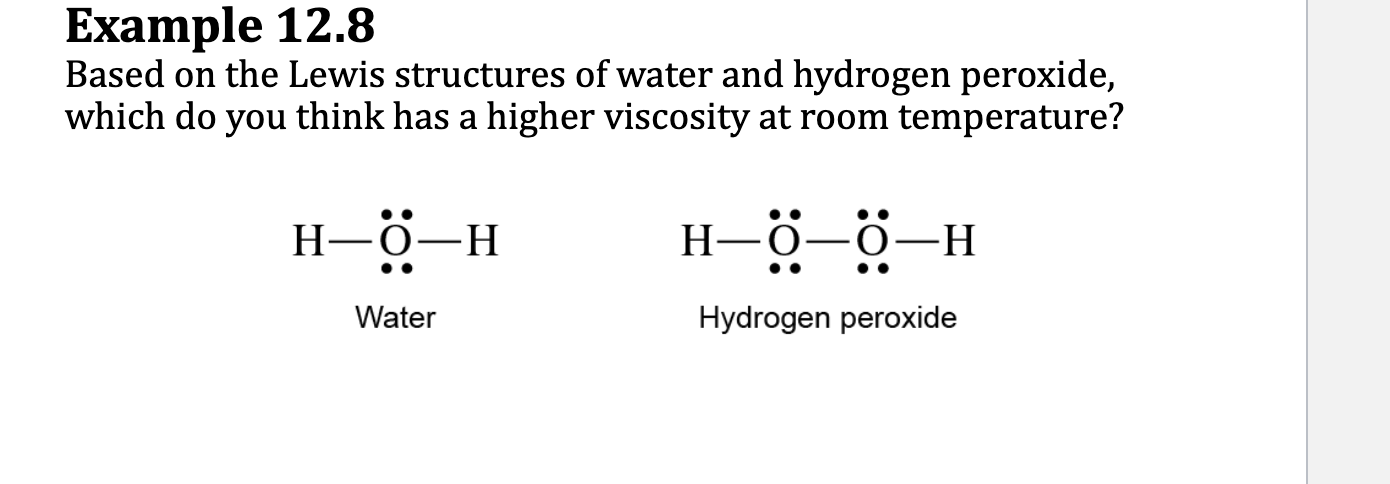
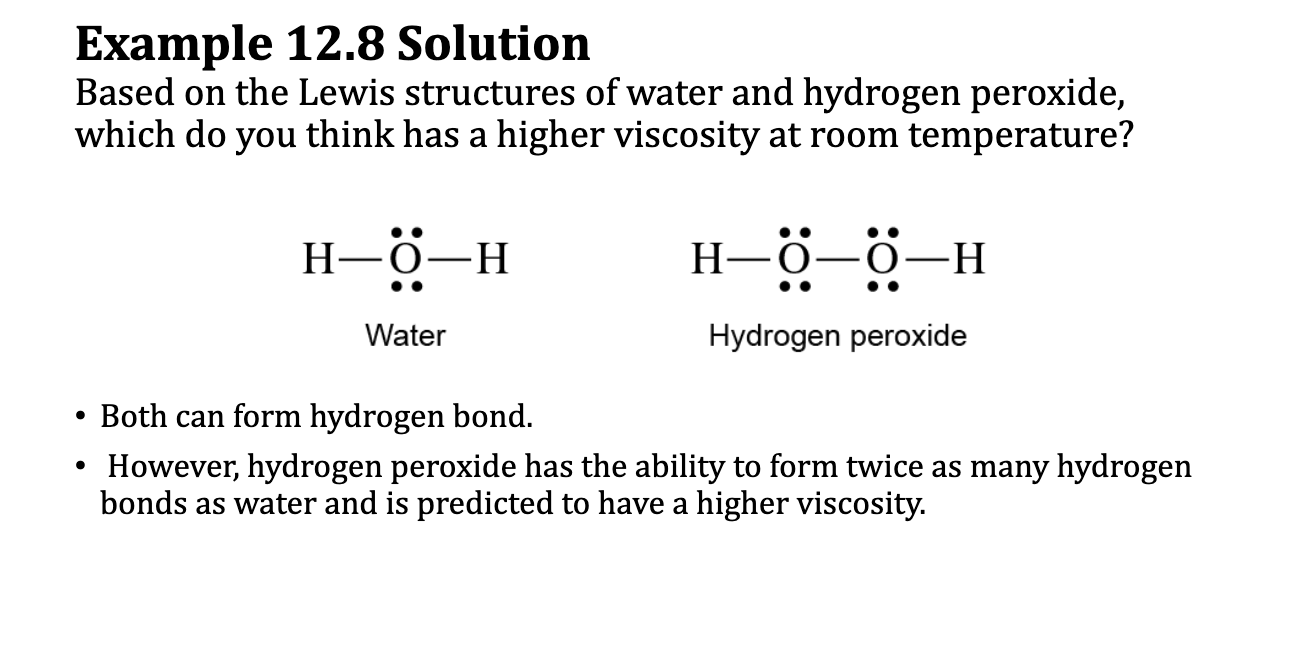
E.x. 12.8
Surface Tension
the tendency of a liquid to minimize its surface area
molecules along hte surface of a liquid behave differently than those in the bulk liquid
cohesive forces attract the molecules of the liquid to one another
strong ST = strong IMF
Capillary Action
The ability of a liquid to flow against gravity up a narrow tube, or
the rise of liquids up narrow tubes is called capillary action
The lead molecules of a liquid attach, via intermolecular
forces to the inner surface of the tube, and then rest of the
molecules follow the attachment.
Two types of forces are important in capillary action: Cohesion and ADhesion
Shape of the meniscus could be convex or concave, and
depends upon the strength of Cohesive forces and Adhesive
forces.
cohesive forces ˃ adhesive forces =Convex
meniscus (like Hg)
adhesive forces ˃ cohesive forces = Concave
meniscus (like water)
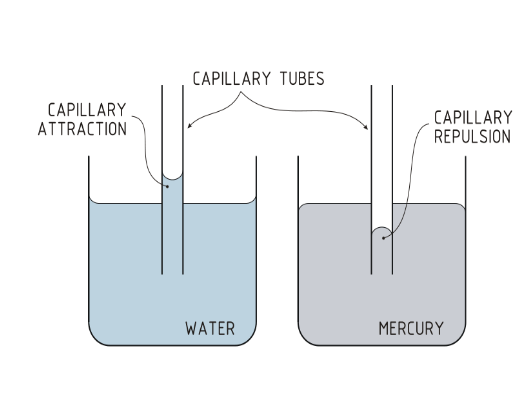
Cohesion and Adhhesion
• Two types of forces are important in capillary action
• Intermolecular forces that bind similar molecules (such as
water and water) to one another are called cohesive forces.
• Intermolecular forces that bind a substance to a surface (such as
water and glass) are called adhesive forces.
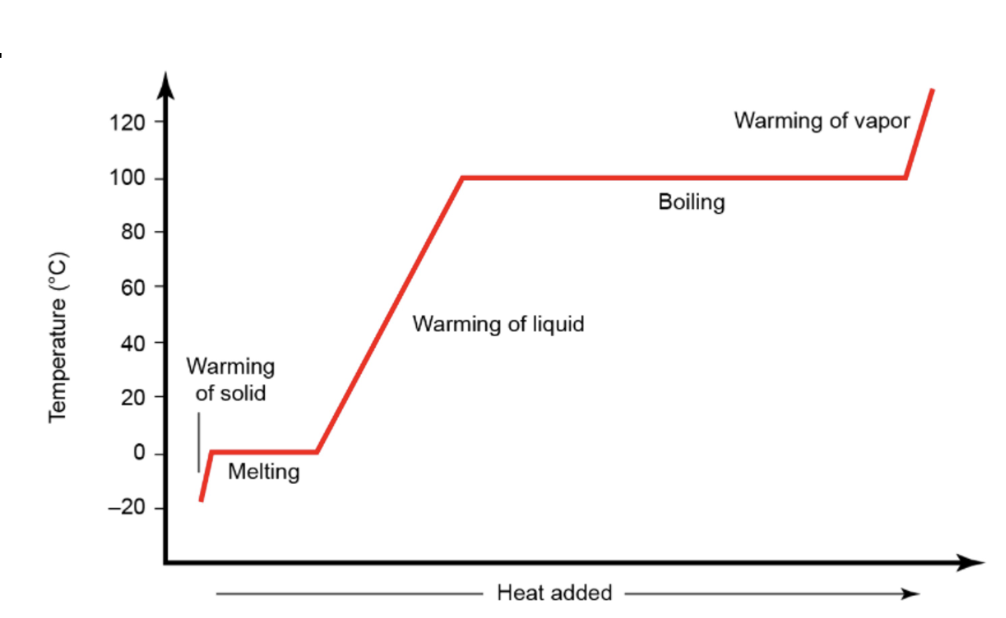
Heating Curve for Water
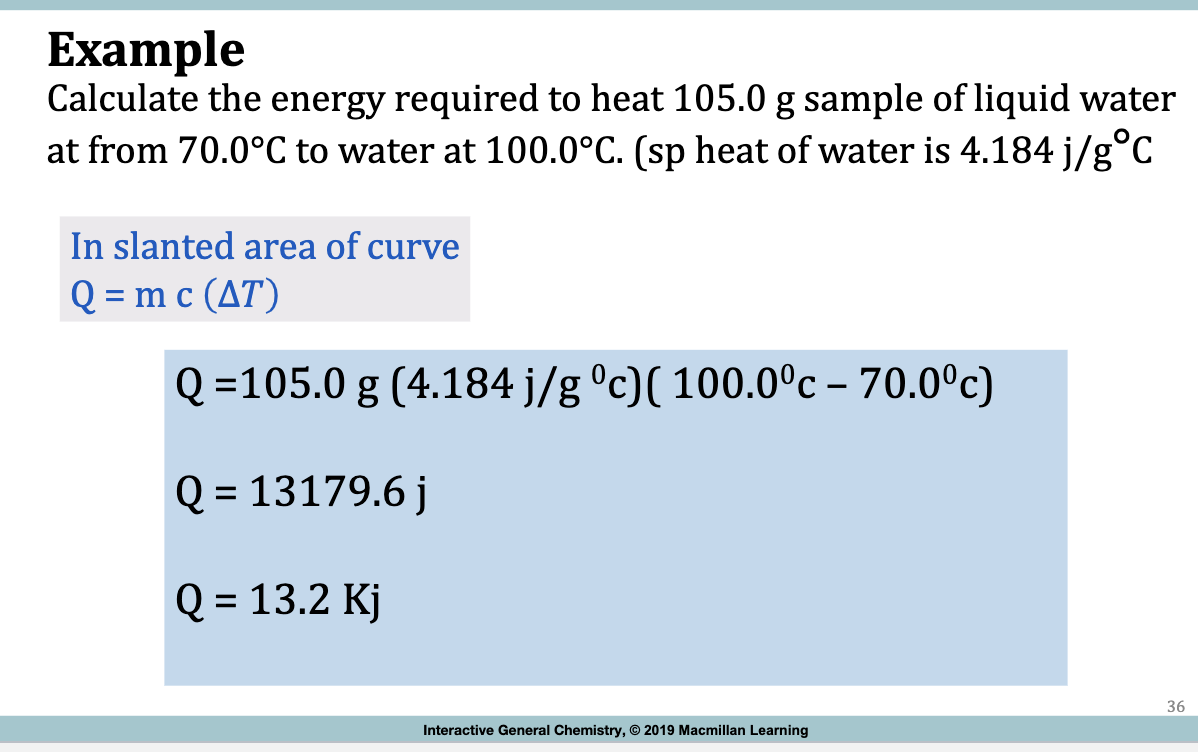
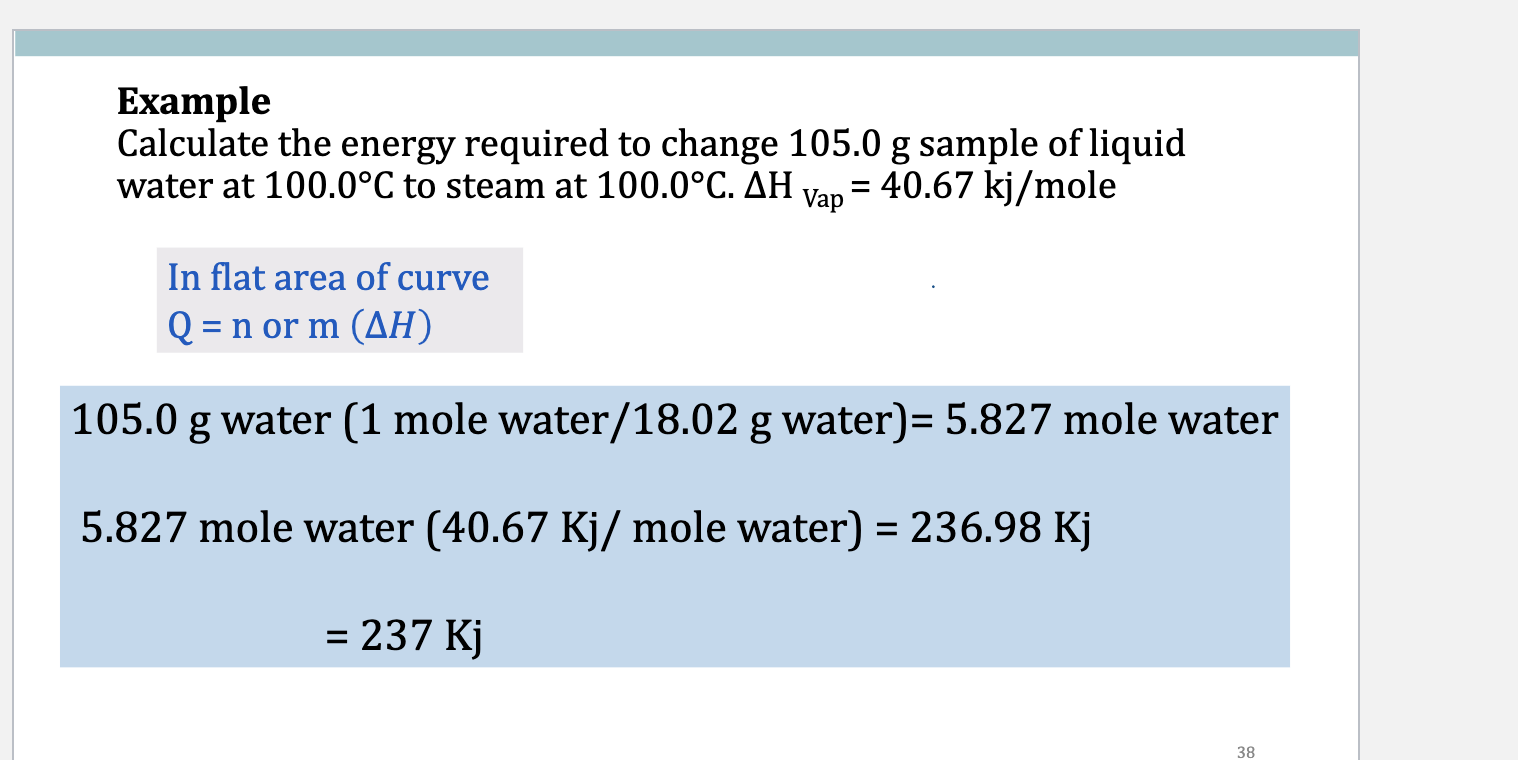
Example
Calculate the energy required to heat 105.0 g sample of liquid water
at from 70.0°C to water at 100.0°C
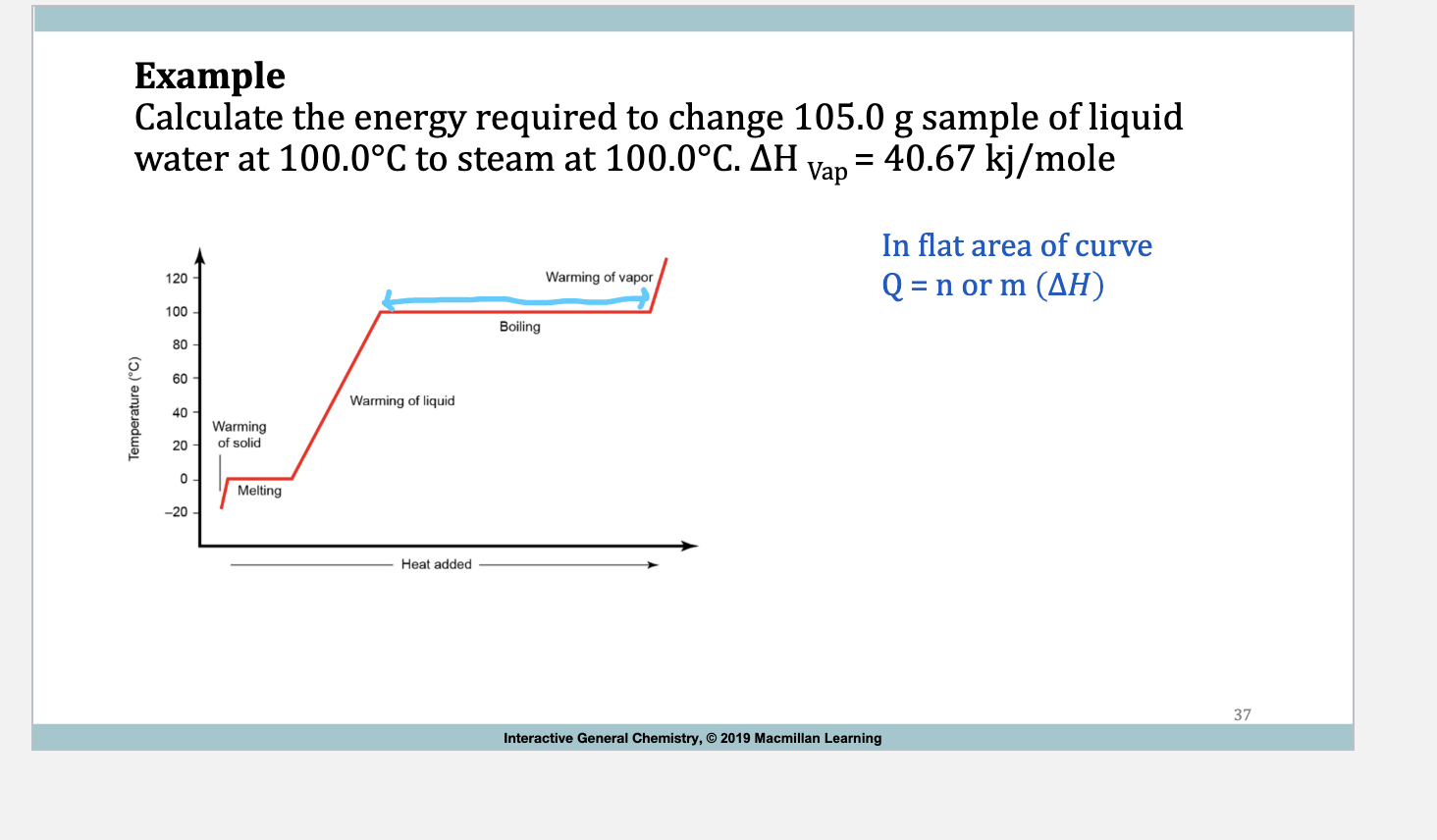
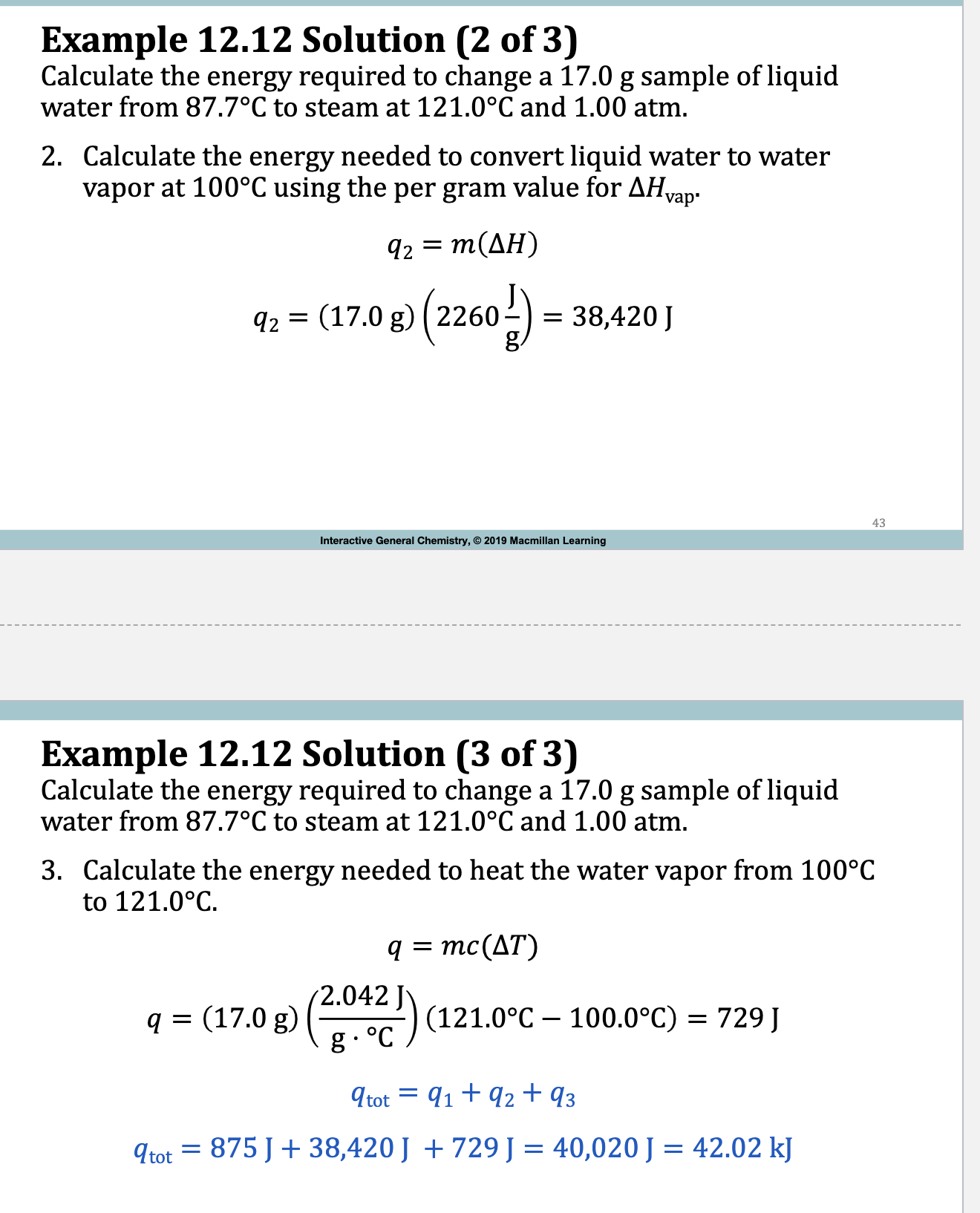
Example:
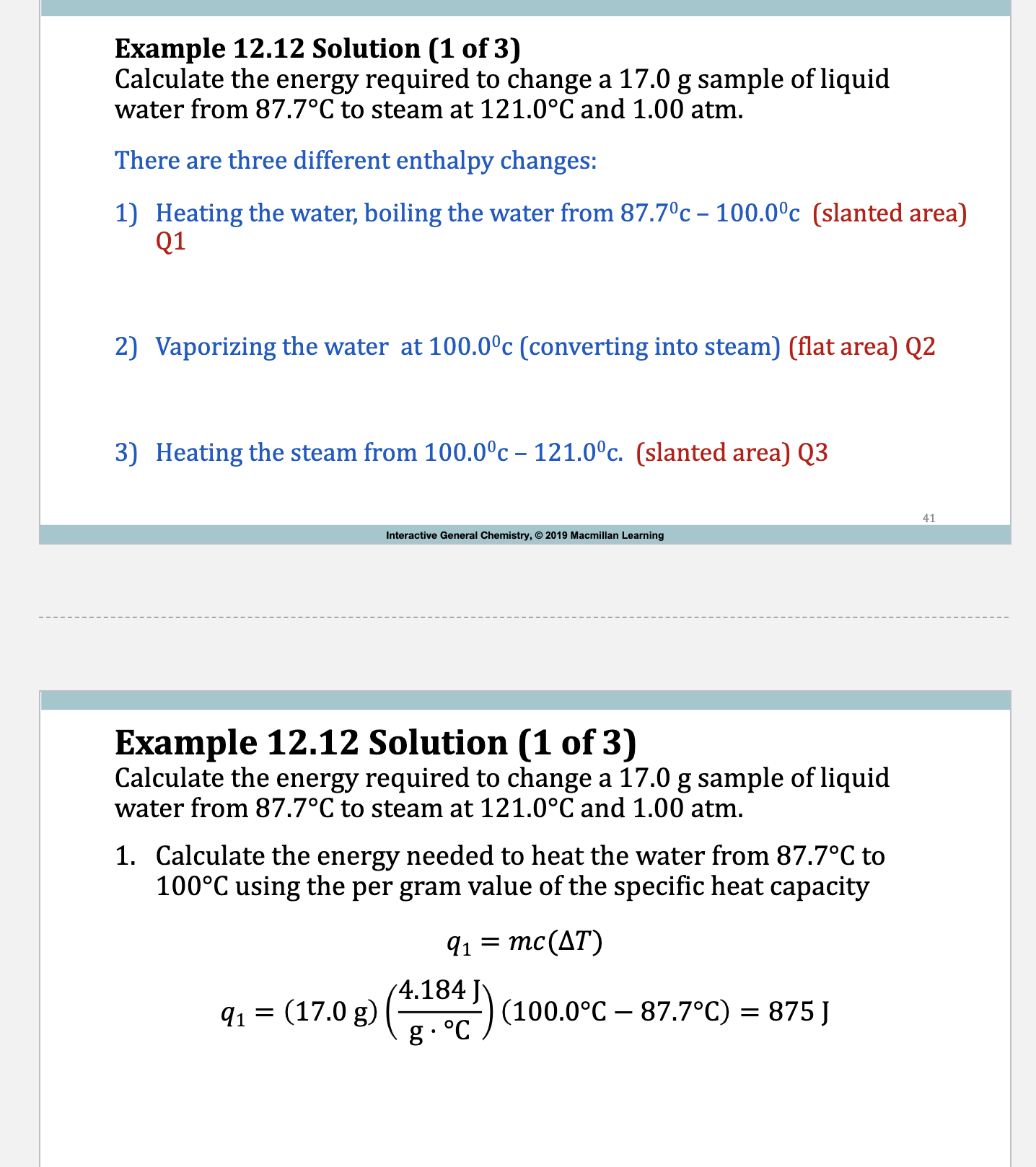
Example 12.12
Vapor Pressure
• Substances that vaporize easily are said to be volatile, and substances that do
not easily vaporize are nonvolatile.
• Volatile substances have more gas phase molecules above the surface of the
liquid, creating vapor pressure.
• Vapor pressure is affected by two factors, the strength of the intermolecular
forces and the temperature.
• stronger attractive forces = have lower vapor pressures
• high temperatures = high vapor pressure.
inversely proportional to IMF

Example 12.14

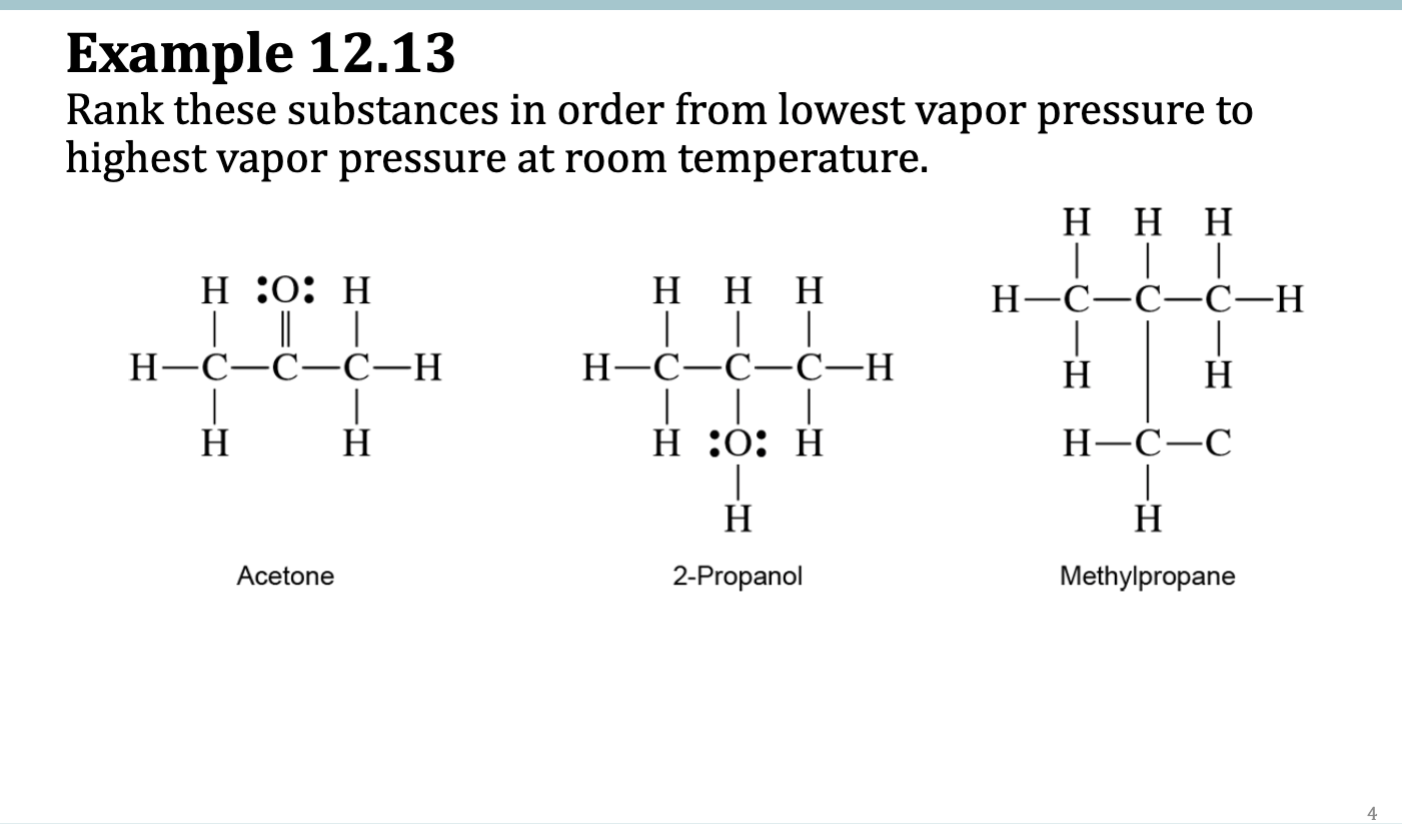
Example 12.13
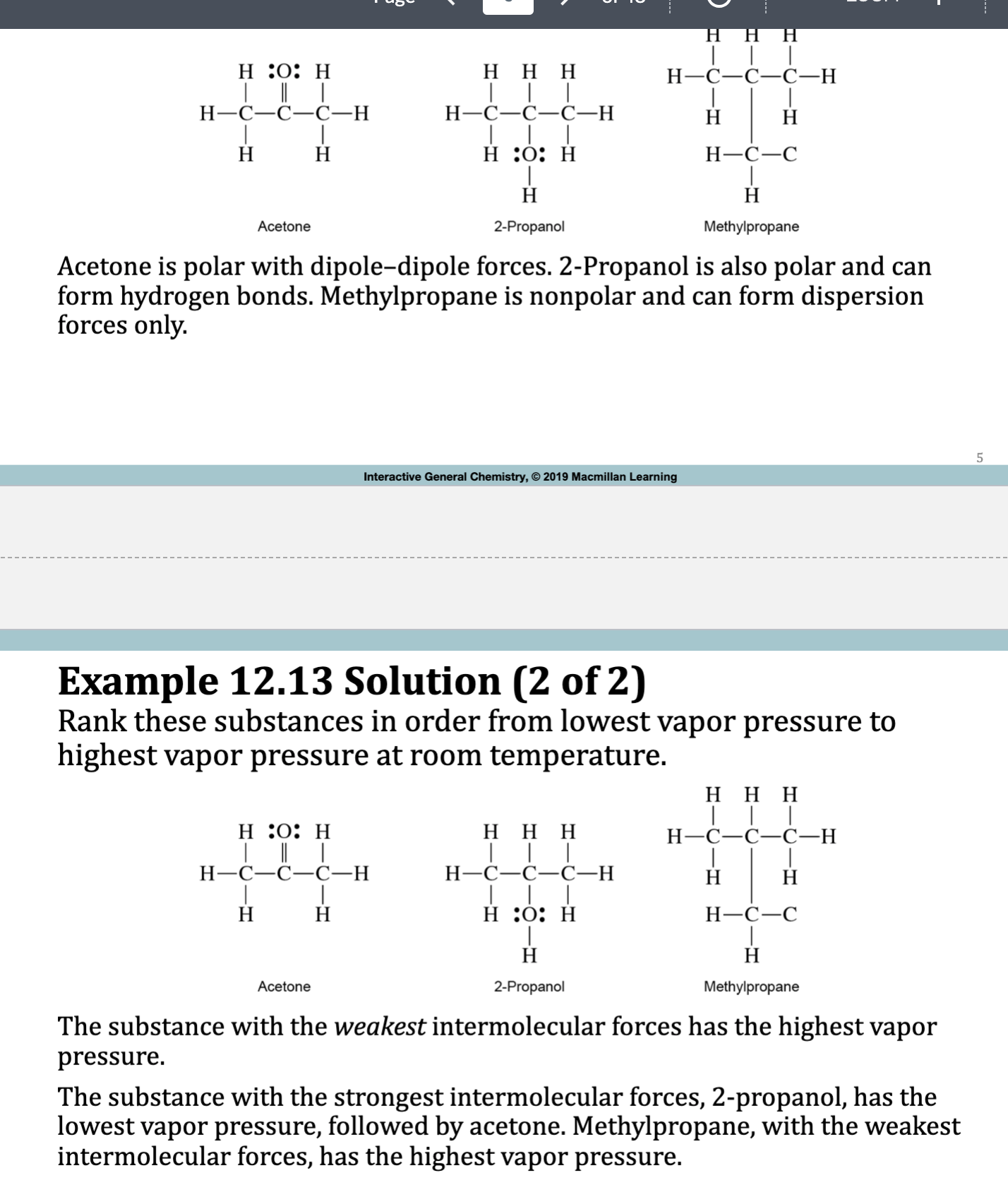
Clausius Clapeyron Equation
• Used for calculating enthalpy of vaporization of a liquid from its
vapor pressure at different temperatures
• Pvap is the vapor pressure in atmospheres, R is the gas law
constant (8.3145 J/mol · K), T is the temperature in kelvins,
and β is a constant specific for each liquid.
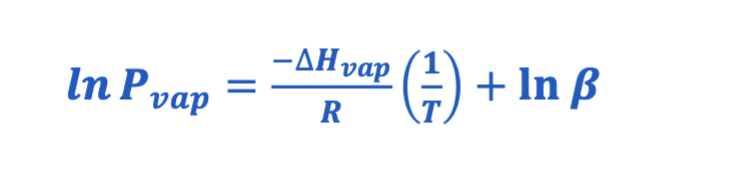
Figure 12.20: Clausius-Clapeyron Plot
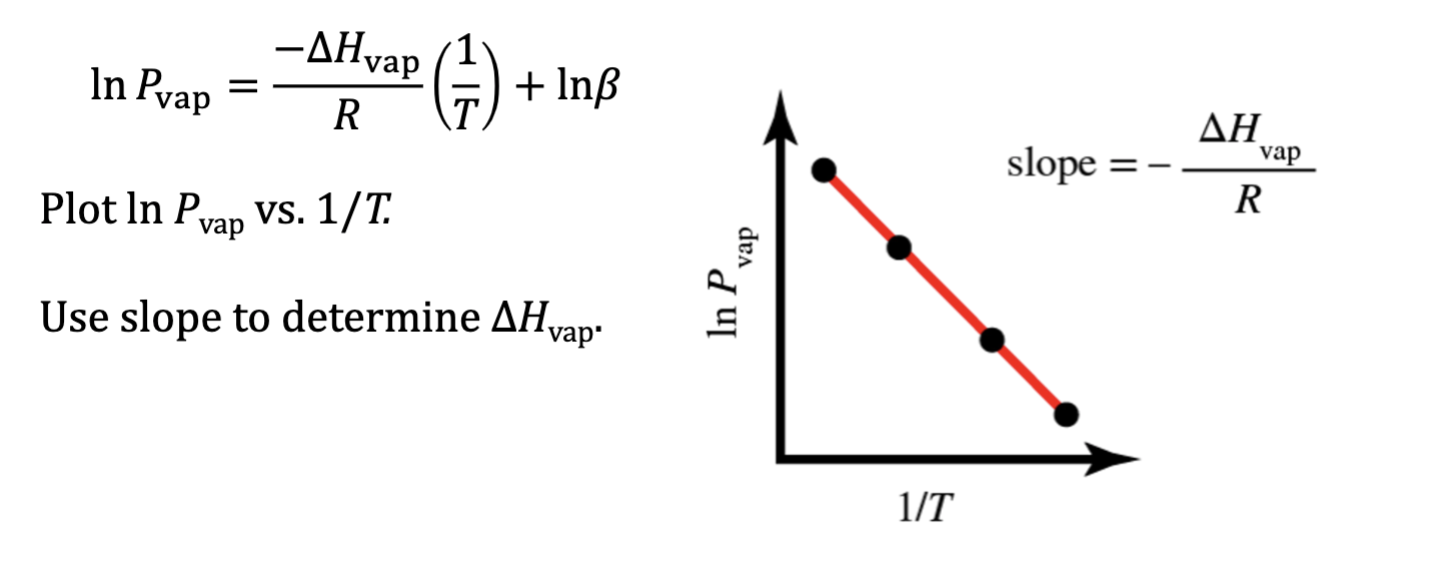
Two-Point Version of the Clausius-Clapeyron Equation
This equation allows you to calculate the ΔH vap by measuring vapor
pressure at two different temperatures or the vapor pressure of a
liquid at a certain temperature if you already know the ΔH vap
Boiling Point and Normal Boiling Point
• Boiling occurs when the vapor pressure of the liquid equals the
pressure of the surroundings.
Boiling Point: VPvap = Patm
• The normal boiling point is the boiling point of a liquid at a
pressure of 1.00 atm.
Normal Boiling Point: VPvap = 1 atm.
Factors Affecting Boiling Point
IMF: As the strength of IMFs increase, BP increases
External Pressure: Lower externa; pressure = lower BP
Liquid will boil at lower temperature
Higher external pressure = high BP & Liquid will boil at higher temperature
Vapor Pressure: High vapor pressure = low BP
Increases with size / molar mass = more electrons = more IMF/dispersion forces
Volatility is inversely related to BP
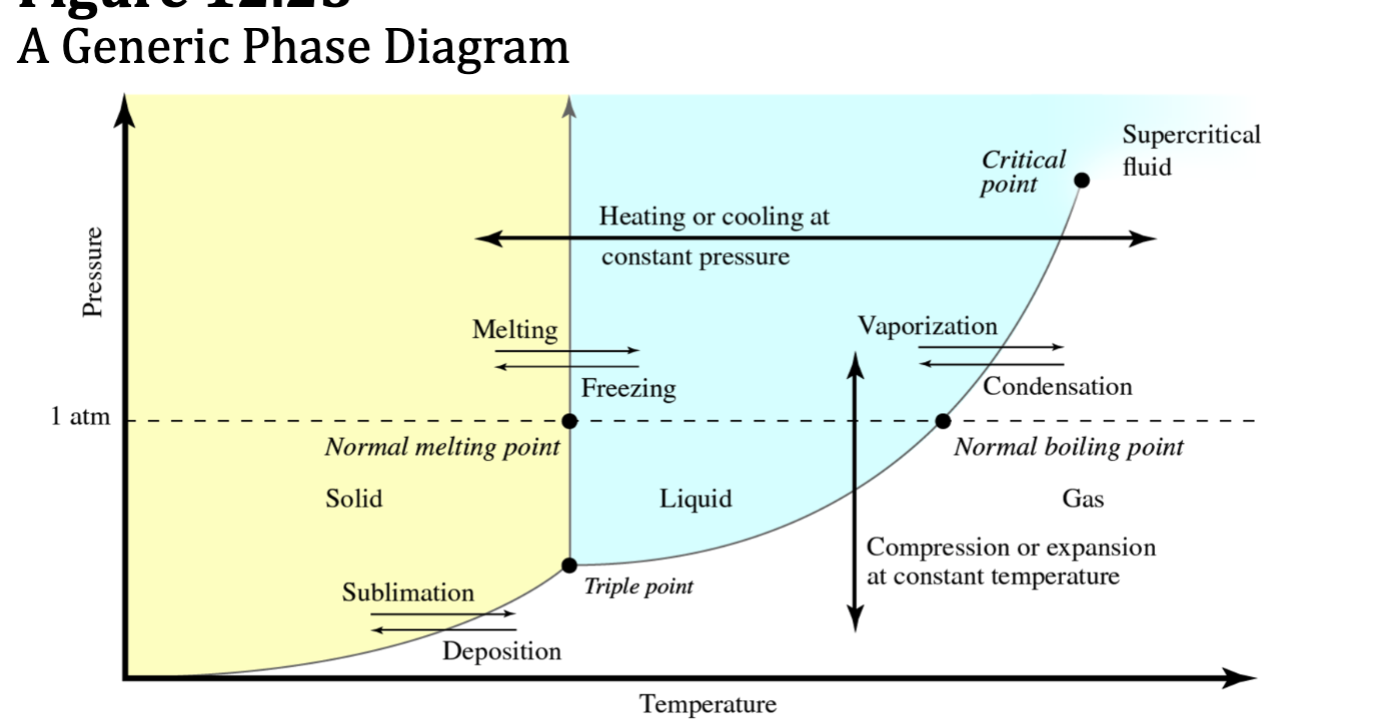
Phase Diagrams
shows the phase of a specific substance under all possible. pressure-temperature combinations
understand how to label
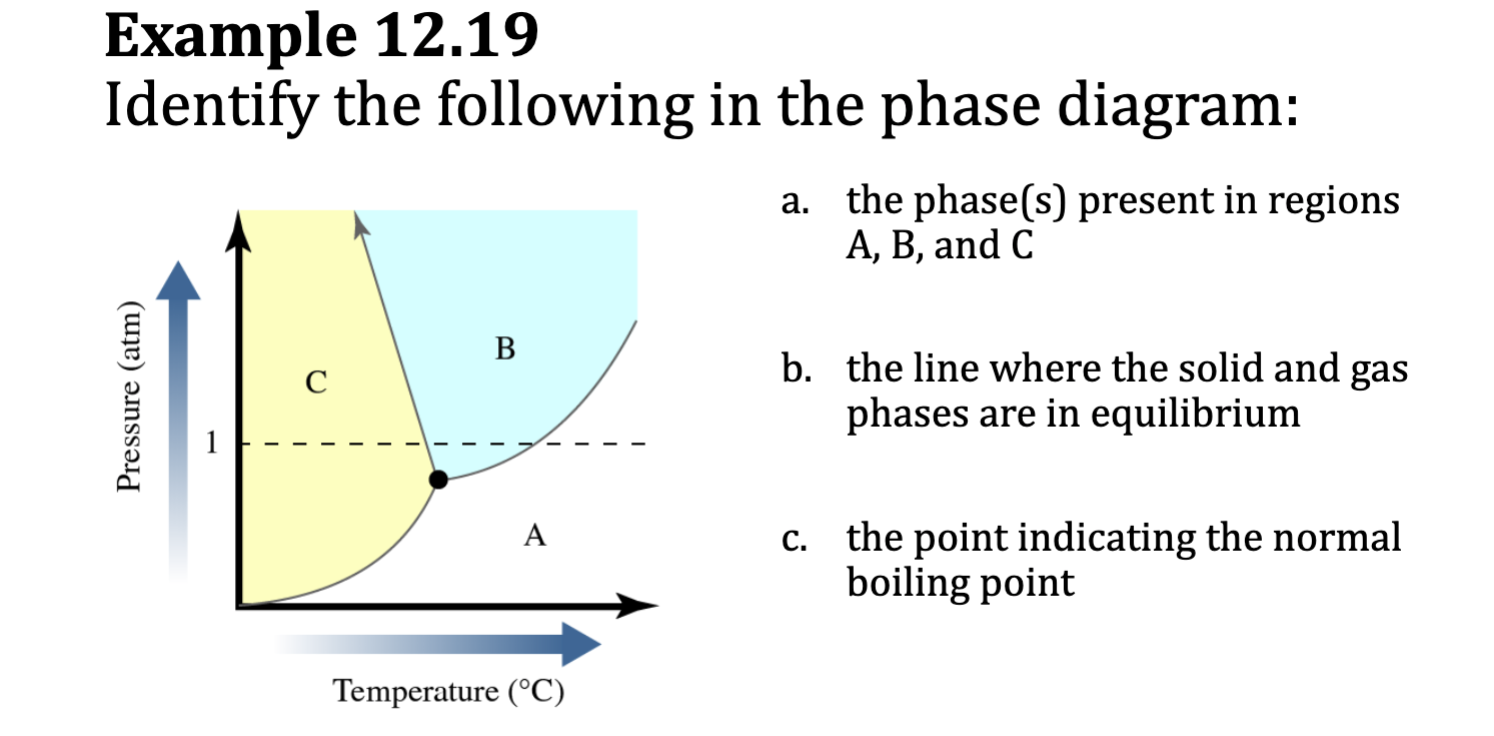
Example 12.19
a. the phase(s) present in regions
A, B, and C
Region A has lowest pressures and
highest temperatures: gas phase.
Region B has moderate pressures
and moderate temperatures: liquid
phase.
Region C has highest pressures and
lowest temperatures: solid phase.
b. the line where the solid and gas
phases are in equilibrium
The boundary line between the
solid region (C) and gas (A) region
represents all the T and P
conditions under which the solid
and gas phases are in
c. The horizontal dashed line
represents 1 atm. This dashed line
crosses the borderline between the
liquid (B) and gas (A) regions at
the normal boiling point. equilibrium.
Crystalline solids
have definite melting points, abruptly forming a liquid once the melting point is reached.
Amorphous solids
get softer as their temperature is raised,
gradually forming a liquid
Molecular Solids
ontain molecules held to each other by
intermolecular forces and have relatively low melting points.
Ionic Solids
are composed of ions held together by ionic
bonds and have quite high melting points
Covalent-Network Solids
are composed of atoms connected by
covalent bonds throughout the solid and have extremely high melting
points
Metallic Solids
are composed of metal ions loosely held together by
their valence electrons and have a broad range of melting points.
• Metal atoms in a metallic solid neither transfer nor share electrons.
• The valence electrons in a metallic solid surround the metal cations, forming
a sea of electrons.
• In the electron-sea model, each metal atom contributes it valence electrons
and forms a cation with the valence electrons free to move throughout the
structure.
Properties of Metals
• Metals are malleable, which means they can be hammered or
bent into different shapes without breaking.
• Metals are also ductile, which means they can be drawn into long,
thin wires.
• Metals are conductors of heat and electricity.
• These properties are possible because metallic bonds do not have
a specific direction.
• The electrons can flow within whatever shape the metal takes
Like Dissolves Like
Homogeneous solutions form best when intermolecular forces are similar between solute and solvent.