(pt 1) exam #1 - immunohematology (cls 544)
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
90 Terms
history of blood banking (1400s-1700s)
Ancient beliefs: blood is the most important of the "four body humors"
1492: first recorded blood transfusion--Pope Innocent VIII
1628: discovery of the intravenous circulation by Dr. William Harvey
Late 1700s: bloodletting is common practice
“firsts” within transfusion medicine (1600s-1800s)
1667: 1st blood transfusion documented by Dr. Jean-Baptise Denis (France)
Sheep to human
1818: 1st successful human blood transfusion administered by Dr. James Blundell; patient experienced postpartum hemorrhage (human to human)
1869: 1st blood preservation research; Braxton hicks recommended sodium phosphate as a nontoxic anticoagulant
“firsts” within transfusion medicine (1900-1916)
1901: Karl Landsteiner discovered the ABO blood group system
1914: Dr. Albert Hustin reported use of sodium citrate as an anticoagulant for transfusions
1915: Richard Lewisohn determined the minimum amount of sodium citrate needed for anticoagulation
1916: First blood transfusion used blood stored and refrigerated by Oswald Robertson
“firsts” within transfusion medicine (1937-1953)
1937: The first US hospital blood bank by Bernard Fantus (Chicago, Cook county hospital)
1941: Dr. Charles Drew appointed as director of the first American Red Cross blood bank
1947: The American Association of Blood Banks founded (AABB)
1948: American Red Cross began operating their full-scale blood program
1953: Plastic blood bank bag invented by the Fenwal Company
components of blood (4)
Red blood cells (RBCs/PRBCs)
White blood cells (Granulocytes)
Platelets
Single donor vs random (pooled)
Plasma
Fresh frozen plasma, frozen plasma, cryoprecipitate AHF
red blood cells
Contains hemoglobin
Carries oxygen throughout the body and to the tissues
white blood cells
Help fight against infection
Neutrophil, eosinophil, basophil, monocyte, lymphocyte
platelets
Derived from the cytoplasm of the megakaryocyte
Plays important role in blood coagulation, hemostasis, and blood thrombus formation
Prevent massive blood loss
plasma
Liquid portion of whole blood
Contains water, electrolytes, glucose, fats, proteins, and gases throughout the body
Carries all clotting factors necessary for coagulation in inactive form
Upon occurrence of coagulation, the fluid converts into serum
shelf life of RBCs & platelets
RBC shelf life = up to 42 days
PLT shelf = up to 5 days
**Supply constantly replenished by blood collection facilities
blood statistics/facts
The average adult has 10.5 pints of blood in their body
No FDA approved substitute for blood products
Under normal circumstances, about every 2 seconds someone in the US will need blood
general steps to the blood donation process (5)
Pre-donation: hydration
Registration
Health history and mini-physical
e.g. hemoglobin, body temperature
Donation
Post-donation
Refreshments
collection method for whole blood
unit of whole blood is collected from a volunteer donor
After donation, the whole blood unit is separated into components
Process lasts ~1 hour
Can donate every 56 days, up to 6 times per year
eligibility requirements for whole blood donation
At least 16 years old
Weigh at least 110 lbs
Be in good "general" health
apheresis
automated process in which whole blood is removed from the body and passed through an apparatus that separates out one (or more) particular blood components
RBCs, WBCs (granulocytes), plasma and/or platelets
The remaining blood components are returned to the donor
(apheresis donation) platelet donation eligibility
1.5-2.5 hour process
Can donate up to 24 times a year
Shelf life = 5 days
Same general donation requirements as whole blood
Exception--no aspirin in the past 48 hrs
(apheresis donation) double RBCs donation eligibility
Process may last up to 1.5 hours
Can donate every 112 days up to 3 times per year
Shelf life = 42 days
Age, weight, and height requirement (ARC)
Males: 17 yrs, 130 lbs or more, 5'1" or taller
Females: 19 yrs, 150 lbs or more, 5'3" or taller
Be in good health
where does blood go after a blood donation?
Blood center/manufacturing center
Blood processed into separate components and stored based on product type
Laboratory
Donor samples sent for infectious disease testing to ensure safety of blood for transfusion
Blood center
Blood components labeled and distributed to hospitals as needed
Hospital
Healthcare providers determine patient needs for transfusion
Lab responsible for compatibility testing
Blood components are transfused to the patient
US Food and Drug Administration (FDA)
Regulates the donor screening process
Code of Federal Regulations (CFR)
Blood treated as both a biologic and a drug
Ensure compliance in all aspects of transfusion medicine
Provides licenses for:
Collection and processing facilities
Blood products and derivatives
Reagents used in the processing and testing of those products
Center for Biologics Evaluation and Research (CBER)
Regulates the collection of blood and blood components
Used for transfusion
Manufactured pharmaceuticals derived from blood
Enforces quality standards
Inspects blood establishments
Monitors errors, accidents, & adverse clinical events
Any fatality or adverse events need to be documented to this organization
Centers for Medicare and Medicaid Services (CMS)
part of the Department of Health and Human Services (HHS)
provides health coverage through Medicare, Medicaid, and the Children's Health Insurance Program (CHIP), while also overseeing the Health Insurance Marketplace
Clinical Laboratory Improvement Amendments of 1988 (CLIA ‘88)
Regulate laboratory testing and require clinical laboratories to be certificated by their state (and CMS) before they can accept human samples for diagnostic testing
Three federal agencies are responsible for CLIA
FDA, CMS, and CDC
American Association of Blood Banks (AABB)
International association of blood centers, transfusion and transplantation services and transfusion medicine
Provides the highest standard of care for transfusion medicine
Voluntary inspection and accreditation program for member institutions
Approved by CMS and CLIA requirements
Resources for donor screening procedures
AABB standards for blood banks and transfusion services
AABB technical manual
the College of American Pathologists (CAP)
Provides voluntary inspection and accreditation program for member institutions
Blood bank is usually included in CAP inspections along with general lab
Approved by CMS and meet the CLIA requirements
blood bank history (antigen/antibody discoveries)
1901: Landsteiner discovers the ABO blood group system
1927-1947: M, N, PI antigens were discovered
1945: the antihuman globulin (AHG) testing technique was developed by Coombs, Mourant, and Race
Prior to the AHG test, only IgM antibodies were detected
First used for Rh antibody detection
Later, used for antibody detection in other blood groups
1946: Kell blood group system discovered
antihuman globulin (AHG)
derived from serum in rabbits or other animals (e.g. mice) that were previously immunized with purified human globulin to produce antibodies against human globulin
AHG is used in the direct and indirect antiglobulin tests
AHG binds to human globulins such as IgG or complement
In blood bank, the primary focus is on IgG and IgM blood group antibodies
immunoglobulins
Two functions:
Bind w antigens
Mediate various biological effects by binding to host tissues, various cells of the immune system, phagocytic cells, and the first component of the classical complement system
The ability to recognize antigens is the product of the adaptive immune system
Immunoglobulins are proteins composted of two heavy chains and two light chains
Five isotypes: IgA, IgD, IgE, IgG, IgM
IgG
Predominant class of antibody produced in the secondary immune response (non-agglutinating antibodies)
Comprises 75% of immunoglobulins in plasma = most abundant
Monomer → bivalent antibody molecule → capable of binding to two antigen sites that are very close together
due to its smaller size, IgG are not as detectable in "non-enhanced" testing environments
subclasses of IgG
IgG1 and IgG3 = activate complement, readily recognized and bound by macrophages
IgG2 = weakly activates complement and not easily recognized
IgG4 = does not activate complement and not recognized at all (in most cases)
Ability to cross the placenta (IgG1 is best at doing this, then 4, 3, & 2))
IgM
First immunoglobulin class produced in a primary immune response
Comprises 6% of immunoglobulins in the plasma
Pentamer with a valency of 10 (10 potential antigen-binding sites)
Considered a direct agglutinate (or complete antibody)
Can bind to multiple RBCs and agglutinate in the absence of AHG reagent
Very efficient at complement activation
Does NOT cross the placenta
(general) direct antiglobulin test (DAT)
One-stage procedure that detects in vivo RBCs that have been sensitized by antibodies
Detects if your cells are coated by antibodies from autoimmune reactions or other processes that may cause agglutination when other RBCs are introduced
**antihuman globulin (AHG) reagent is added to form a "bridge" when antigen-antibody complexes present—allows for visualization of the Ag/Ab reaction (e.g. agglutination)
(general) indirect antiglobulin test (IAT)
Two stage procedure that demonstrates in vitro reactions between RBCs and corresponding IgG antibodies
Detects if you have antibodies that can coat other cells and cause agglutination
**antihuman globulin (AHG) reagent is added to form a "bridge" when antigen-antibody complexes present—allows for visualization of the Ag/Ab reaction (e.g. agglutination)
AHG (Coombs) tests overview
Used to detect RBCs sensitized with IgG alloantibodies, IgG autoantibodies, and complement (e.g. C3d)
AHG reagents
Polyspecific AHG
Specificities: contains antibodies to human IgG + the C3d human complement component
Monospecific AHG--one specificity
Anti-IgG
Anti-complement (anti-C3b, C3d)
how are AHG reagents made?
injecting animals with specific human protein and harvesting antibody that animal produces in responses
polyspecific AHG
contains both anti-IgG and anti-C3d
Reagent of choice for DAT on adults
It is common practice to perform initial DAT workup with polyspecific AHG reagent
If positive, repeat testing of patient sample using monospecific AHG reagent
monospecific AHG
contains either anti-IgG or anti-C3d
Anti-IgG reagent preferred over polyspecific AHG for antibody detection to avoid detecting clinically insignificant cold-reactive antibodies that bind complement
Anti-IgG mixture primarily comprised of IgG1 and IgG3 subclasses
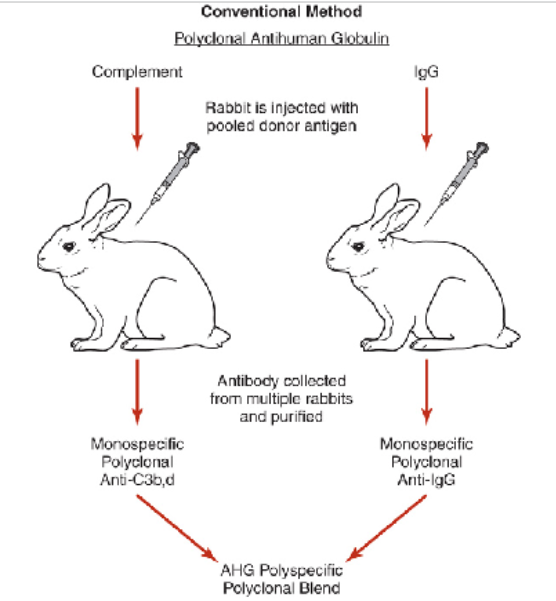
polyclonal AHG production
prepared by injecting human globulins into rabbits
Advantages:
Detects many different IgG antibodies
Disadvantages
Manufacturing--if excess antibody present (IgG), then prozoning may occur resulting in potentially false-negative results
Unable to determine potency of anti-C3d, C3b individually
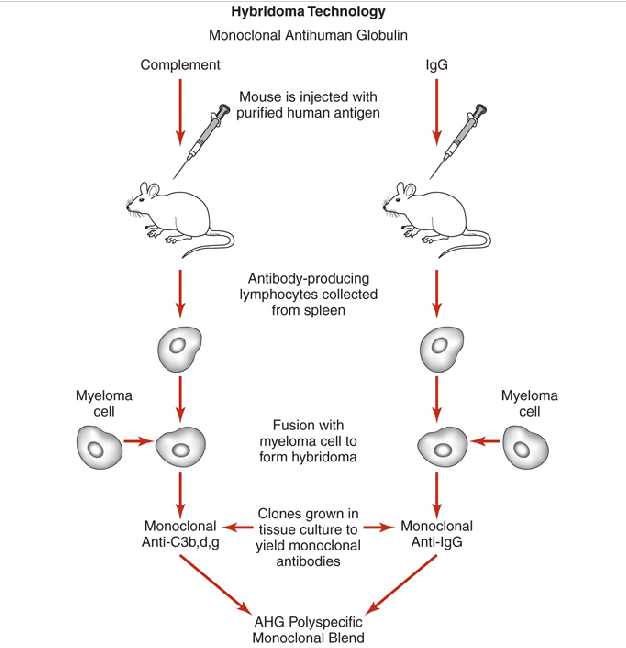
monoclonal AHG production
prepared by injecting human globulins into mice (e.g. murine reagents)
Advantages:
Produces higher antibody titers with well-defined specificities to IgG and fragments of C3
Clonal line produces a single antibody: no need to remove heterospecific antibodies
Disadvantages:
Detects single epitopes: IgG antigens are composed of multiple epitopes
clinical applications of the IAT
Used for the detection of in vitro sensitization of RBCs
Use patient plasma/serum
Clinical applications
Antibody screen
Antibody identification
Crossmatch (or compatibility) testing
RBC phenotype (antigen typing)
Antibody titration
polyspecific vs monospecific AHG in the IAT
Most clinically significant antibodies are IgG class
Use of enhancement media
Increase rate and sensitivity of antibody attachment to RBC antigens
Reduce incubation period
Bovine serum albumin (BSA)
Low ionic strength solution (LISS)
Polyethylene glycol (PeG)
Some clinically significant antibodies are detected with the anticomplement component of AHG but not with anti-IgG
e.g. Anti-Jka may be detected with polyspecific AHG & LISS but not with albumin
clinical applications of the DAT
Used for detection of in vivo sensitization of RBCs with IgG antibodies and/or complement
Use patient blood
Clinical applications
Hemolytic disease of the fetus and newborn (HDFN)--maternal antibody
Hemolytic transfusion reaction (HTR)--recipient antibody
Autoimmune hemolytic anemia (AIHA)--patient autoantibody
Drug-induced hemolytic anemia--drug/anti-drug complex
testing/sample criteria for DAT
Patient sample must be collected in an anticoagulant (e.g. EDTA--preferred, ACD, CPD)
Positive DAT result is not definitive--need further investigation
e.g. Patient's diagnosis, blood transfusion history, list of medications
check cells (Coomb’s control cells)
IgG-sensitized or complement-coated RBCs (should produce agglutination when tested)
D positive cells coated with anti-D (IgG)
Cells coated with anti-complement
positive reaction means that reagent has been added and ensures test works
Lack of reaction due to improper washing
antibody screen test
determines if patient has unexpected antibodies present in plasma
Utilizes indirect antiglobulin test (IAT)
Immediate spin (IS) phase
Incubation/37 deg C phase
Antihuman globulin (AHG) phase
levels of detection for DAT/IAT
overall goal is to detect all clinically significant antibodies for both DAT and IAT and none of the clinically insignificant antibodies
e.g. warm and cold reacting autoantibodies
DAT level of detection
100 to 500 IgG molecules per RBC
400 to 1100 molecules of C3d per RBC
IAT level of detection
100 to 200 IgG or C3d molecules on the RBC to obtain a positive reaction—more specific
factors affecting the AHG phase of the antibody screen
plasma:cell ratio ; ionic strength ; pH
Enhancement media:
Albumin, LISS, PeG
temperature ; incubation time
Washing of RBCs & saline used
Addition (or lack thereof) of AHG reagent
Centrifuge setting for reading purposes
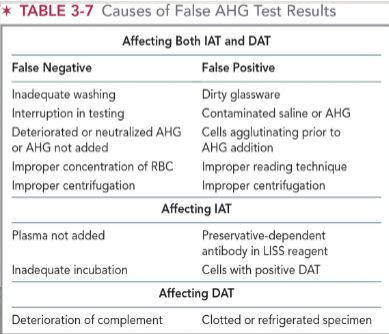
sources of error for AHG test (DAT/IAT)
most encountered sources of error:
Inadequate washing of RBCs
Non-reactive AHG reagent used in the procedure
Failure to add AHG reagent to the tubes
False negative reactions: seen most in tube testing and inadequate washing of RBCs
Note: all negative AHG test reactions must be checked by the addition of check cells!
AHG testing methods (3)
Tube Test: the "gold standard" for detection of Ag/Ab reactions
Gel: the sensitivity is equivalent to the polyethylene glycol (PeG) test tube method
Solid Phase: RBC antigens coat the bottom of microtiter plate
contributions to immunology by edward jenner; robert koch; pasteur; bordet; and kaus?
Edward Jenner: discovered vaccination (used cowpox to protect against smallpox)
Robert Koch: proved infections were caused by microorganisms
Louis Pasteur: developed rabies vaccine
Bordet: discovered complement
Robert Kaus: discovered precipitins
antigen-antibody reaction that produces a visible precipitate
who developed the idea of the “lock and key” binding for antigens and antibodies?
Paul Ehrlich
postulation that specialized cells (e.g. plasma cells) carried antibodies and the molecular structure had receptor sites for the antigens that stimulated their formation
general characteristics of the immune response
occurs when the human body is exposed to foreign substances, organisms, and environmental toxins
equipped with remarkable immune defense mechanisms
innate/natural immunity
primary line of defense ; acts quickly, nonspecific
Physical: skin, mucosal linings
Biochemical: chemical secretions, (e.g. tears, saliva)
Immune cells: phagocytic cells & NK cells remove invading orgs
Humoral: cyto/chemokines initiate inflammation process
acquired/adaptive immunity
“learned” immunity; needs time, is specific, and capable of memory
Cellular: B and T lymphocytes
Humoral: antibodies
ability to respond to infinite variety of antigens upon reintroduction
primary vs secondary immune responses
Primary antibody response: accomplished by B cells with help of antigen-specific T cells; IgM antibodies
Secondary (anamnestic) antibody response: occurs upon re-exposure to an antigen
Involvement of memory B cells
Primarily IgG antibodies
antigen
a foreign substance that can trigger an immune response or damaged host cells
Chemically complex
Foreign or non-self
Usually, high molecular weight
antigens associated with blood bank testing are proteins that sit on the surface of blood cells
antigen vs immunogen
Antigen: recognized and bound by antibodies
Immunogen: initiates and immune response
All immunogens are antigens; not all antigens are immunogens
immunogenicity
chemical composition and molecular complexity of the antigen
in blood bank: ABO > D > K > c > E > k > e
major histocompatibility complex (MHC)
molecules for presentation to lymphocytes
Consists of a group of genes located on chromosome 6
MHC Class I antigens: found on nucleated cells in the body
MHC Class II antigens: found on antigen presenting cells
functions of antibody molecules (3)
Neutralization—antibodies bind to pathogens or toxins/prevent entry into cells
Opsonization—antibodies bind to the bacteria coating them, allow phagocytes to recognize Fc portion of the antibody molecule and eliminate bacteria
Activation of complement—enhances the bactericidal actions of phagocytes
summary of the five classes of immunoglobulins
IgA - mucosal secretions, 13%
IgD - bound to surface of naive B lymphocytes with surface IgM, 1%
IgE - factor in serum that causes allergies; activates mast cells, <1%
IgG - principal isotype in blood and extracellular fluid, 80%
IgM - first antibody produced in primary immune response; found in blood and lymph, 6%
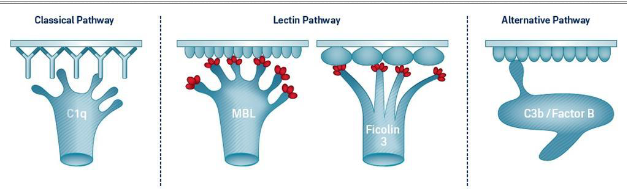
activation pathways of complement (3)
all three lead to formation of membrane attack complex (MAC)
Classical – presence of antigen-antibody complex activate complement
Alternative – activated by high molecular weight molecules with repeating units on the surfaces of target cells
Lectin – activated by the attachment of plasma mannose-binding lectin (MBL) to microbes
**regulated by C1 inhibitor that inhibits formation of C3 convertase
how does complement relate to blood bank?
Complement system plays role in blood group testing
Some antigen-antibody complexes activate complement; hemolysis or RBC lysis
Complement components related to blood group serology
Chido/Rodgers blood group (epitopes on C4 protein)
Fixation tests
May be used to detect the amount of antigen or antibody present
Fixed via the classical pathway
effects of storage/anticoagulants on complement
C1 and C2 components inactivated by heating serum to 56 deg C for 30 minutes
Degrades at room temp and refrigerated storage
Activity inhibited by EDTA (chelation of calcium)
membrane attack complex
Final step of complement activation
Consists of C5b and C6-C9
Classical pathway regulated by two mechanisms in fluid phase and complement control proteins
IgM is very efficient in activating complement, causes intravascular hemolysis
IgG is less efficient but may cause extravascular hemolysis
primary factor in the formation of an immune complex?
antigen's chemical composition plays the biggest role in the formation of an immune complex
Determines type of noncovalent bonds
Exothermic: released energy appears as heat
Enhanced at lower temperature
Endothermic: need energy from the environment
Enhanced at warmer temperatures
affinity
strength of the interaction between the antigen and the antibody's binding site at ONE individual site
avidity
SUM of affinities between antigens and antibodies
Increased avidity can make up for decreased affinity
influence of attraction on AGN-ABY complexes
fit between antigen and antibody must be high enough to allow for the formation of multiple noncovalent bonds
complementary nature needs to exist between antigen and antibody (e.g., size, shape, charge), lock and key
basis of all blood bank testing is centered around the reaction between antigen and antibody/antibodies
law of mass action
Antigen and antibody bonds are reversible
Low repulsion and high attraction = binding is likely to occur
High repulsion and low attraction = binding is less likely to occur
agglutination
Visible endpoint of antigen-antibody reactions for blood bank testing
Particulate matter caused by combination with specific antibody
reversible reaction between antigen and antibody
stage 1 of agglutination
Sensitization
Antigen and antibody come together to form an immune complex
Binding only occurs if antigen and antibody are complementary in nature
Factors affecting stage 1
Temperature
Incubation time
pH ; Ionic strength
Antigen-antibody concentration
stage 2 of agglutination
Lattice formation
Antibody cross links form between RBCs forming a lattice that allows visualization of the antigen and antibody reactions
Dependent upon the strength of test system, pH, and temperature
Overcoming forces that keep red blood cells apart (zeta potential)
Repulsion may occur in physiologic saline
factors influencing agglutination reactions
Centrifugation—enhances agglutination
Antigen-Antibody (serum-to-cell) Ratio
Equivalent amount of antigen-antibody bind
pH
Test system, 6.5 -7.5
Saline
Temperature—IgM vs. IgG antibodies
Enhancement Media
22% Albumin - adjusts for zeta potential between RBCs
LISS - reduces zeta potential, RBCs take up antibody more rapidly
PeG - increases test sensitivity
Aggregates RBCs causing closer proximity of RBCs to each other/enhances antibody cross-linking
genetics
study of inheritance of transmissible characteristics or traits, including red blood cell antigens
genetic material that determines each trait is found in nucleus of cells
Chromosome--linear thread of DNA
mitosis
somatic or nonsexual cell division
Diploid: human cells, except for sexual cells, contain two sets of chromosomes
DNA replication: chromosomes within nucleus duplicated before cell division
meiosis
sex cell division
Daughter cells contain half of number of chromosomes in parent cell
Mutations--increase genetic variability; potential to create new phenotypes
Crossover--when two genes are near each other on the chromosome
DNA structure
Usually exists in coiled double strand
Made up of nucleotides: deoxyribose sugar, phosphate group, one of four bases
Four bases: cytosine, guanine, adenine, thymine
factors affecting DNA replication
Errors in the primary nucleotide sequence
Chemical and environmental factors
Ionizing radiation and strong oxidants
Ultraviolet (UV) radiation
Medications
mutations in DNA
any changes in the structure or sequence of DNA (physical of biochemical)
various chemicals and conditions that can cause mutations are referred to as mutagens
original form of the DNA sequence and the organism in which it occurs is called the wild type
common blood group antigens result from SNPs
(general) DNA translation
Genetic information carried by DNA transformed into an organism
In cell nucleus, DNA unwound, and complementary strand of messenger ribonucleic acid (mRNA) produced
mRNA transported from cells’ nucleus to cells’ cytoplasm
Set of three base pairs (codon) translate into a particular amino acid or stop codon
define gene, locus, allele, & antithetical
genes - made of DNA = basic unit of heredity
locus - site of a gene on a chromosome
allele - one or more different forms of a gene at specific locus on a chromosome
antithetical - antigens that represent different forms of a gene product from same locus
define homozygous, heterozygous, hemizygous, and the dosage effect
homozygous—two identical alleles at a given locus on both chromosomes
heterozygous—alleles are non-identical at a given locus (two different alleles)
hemizygous—refers to the condition when one chromosome has a copy of the gene and the other chromosome has that gene deleted or absent
dosage effect—serologic difference encountered with heterozygous versus homozygous antigen
mendel’s first law of inheritance
aka law of separation ; shows that alleles of genes have no permanent effect on one another when present in the same plant but segregate unchanged by passing into different gametes
unlike the flower color of many types of plants, most blood group genes are inherited in a codominant manner
in codominance, both alleles are expressed, and their gene products are seen at the phenotypic level
ex: In the MNSs blood group system, a heterozygous MN individual would type as both M and N antigen positive
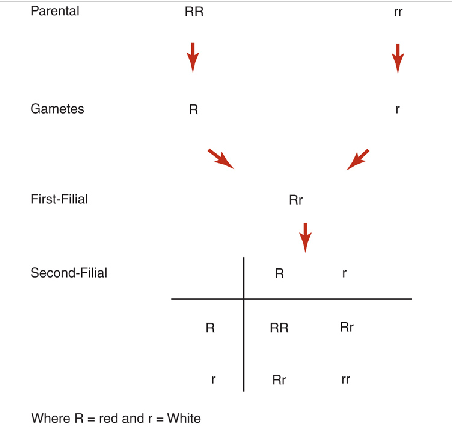
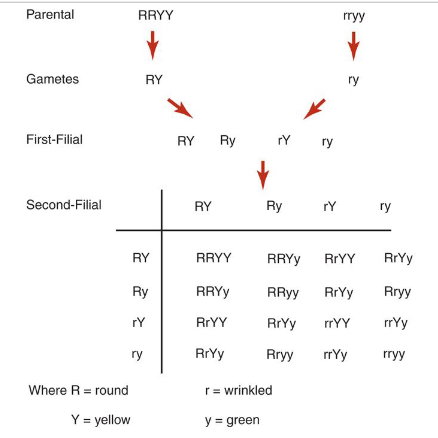
mendel’s second law of inheritance
aka law of independent assortment ; genes for different traits are inherited separately from each other
allows for all possible combinations of genes to occur in the offspring
Mendel's laws apply to all sexually reproducing diploid organisms
hardy-weinberg principle
allows the study of Mendelian inheritance in detail
formula states if there are only alleles for a trait in a population, then p + q = 1
p = gene frequency of the dominant allele
q = frequency of the recessive allele
This can be stated as:
p2 + 2pq + q2 = 1
(p + q)2 = 1
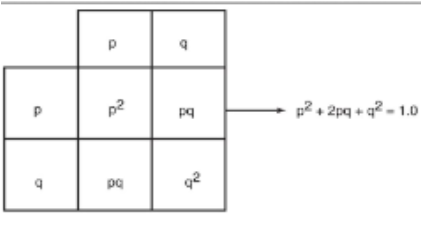
five assumptions under the hardy-weinberg principle
Large population size
Random mating
No natural selection
No mutations in parents or offspring
No migration, no new alleles introduced or lost
problems with the five assumptions under the hardy-weinberg principle
Collecting sample data from a significantly large enough segment of a population is not always feasible
Mating is not always random
Mutations happen
Mixing of populations on a global scale leads to “gene flow” on a constant basis
examples of various inheritance patterns
Autosomal dominant: ABO blood system
Autosomal codominant: S/s phenotype
Autosomal recessive: OO phenotype
X-linked dominant: Xga blood group system
X-linked recessive inheritance: Hemophilia A
Linkage: tendency for genes close together on same chromosome to be inherited as unit (haplotypes)
what can we use for genotype predictions?
Punnett squares
Shows different ways that genes can separate and combine
Pedigree chart
Records a family tree in which a trait is mapped through several generations
practical applications for genetics in blood bank
Paternity testing ; transfusion
High- and low-prevalence antigens
High-prevalence red blood cell antigens present in 98-99% of population
Genetic testing methods
PCR
DNA microarrays
Restriction fragment length polymorphism analysis (RFLP)