Chapter 11 (Reaction Of Organic Compounds)
1/91
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
92 Terms
Alkane Combustion
Burn readily in the air - for an exothermic reaction (as such with hydrocarbons)
Substitution Reactions of alkanes
Atom or functional group is replaced by another atom or functional group
e.g. Creating halo alkanes (Replacing hydrogen with fluorine, nitrogen, CI)
Can only be initiated by UV light (the substitution reaction - cannot occur in darkness)
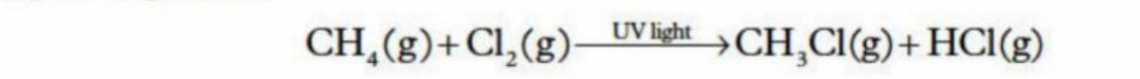
Explanation of example above
After single substitution, another substitution can also occur, until all hydrogens have been swapped out
Fractional Distillation
The process in which the different substituted products can be taken apart
Substitution reactions within haloalkanes
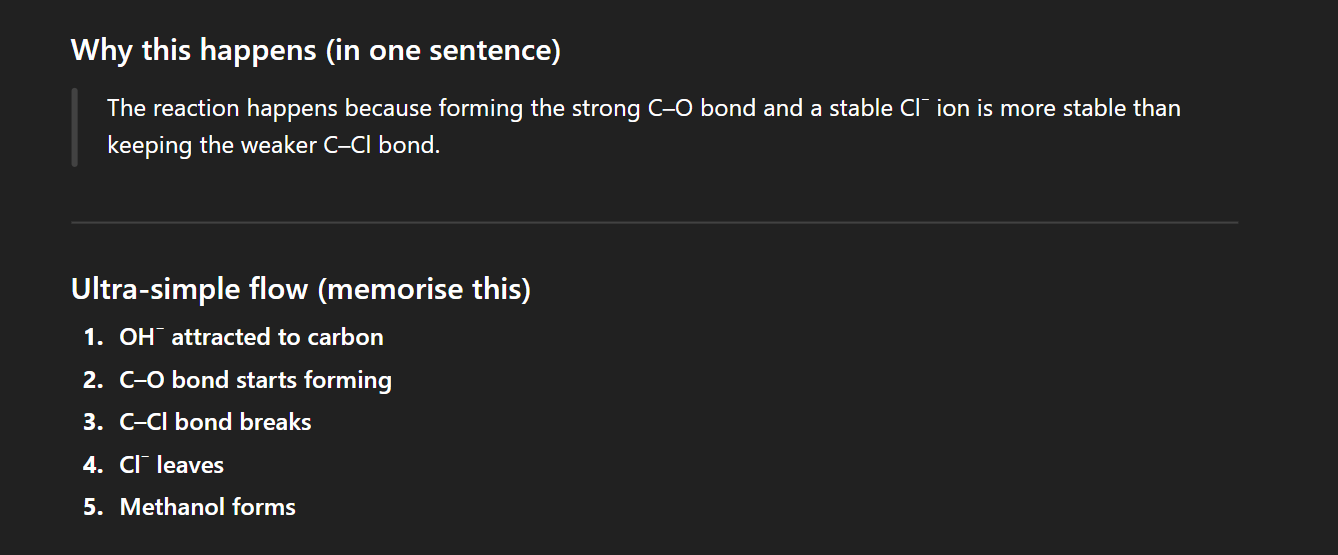
Substitution reactions within haloalkanes - Example
Susbtitutions with Ammonia
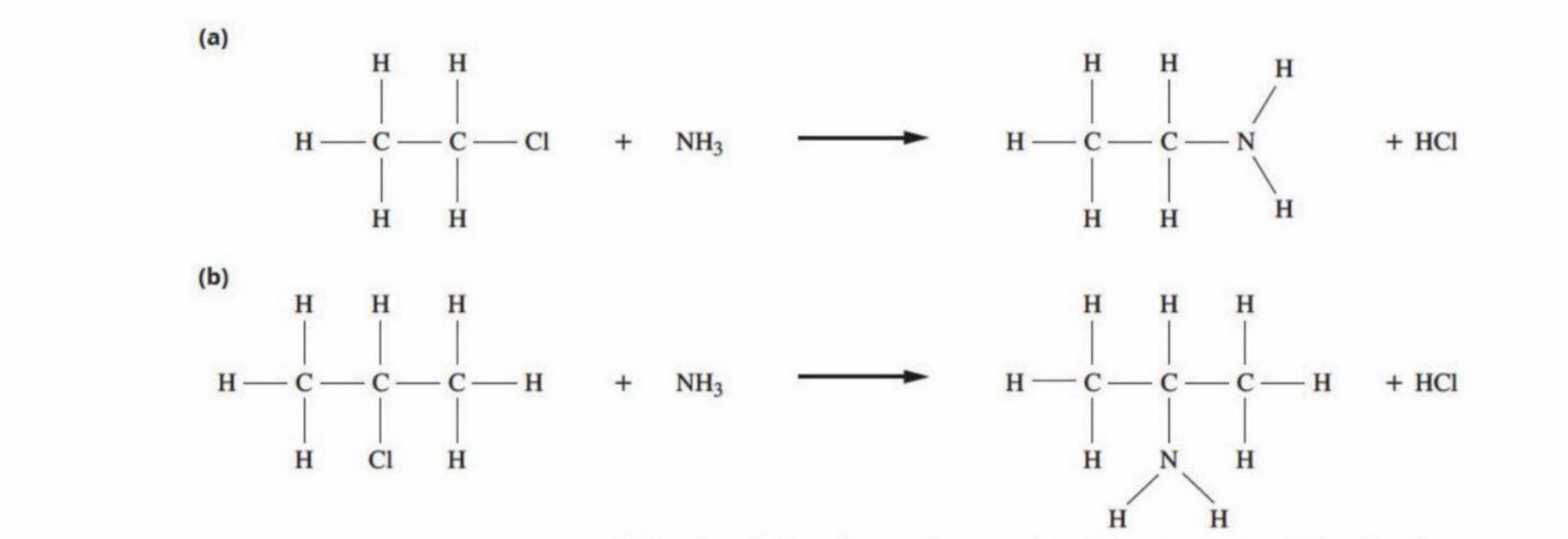
Trick (Unclear)
If it is an alkane —> haloalkane (then is a substitution reaction)
Reactivity of alkenes
More reactive than alkanes (due to unsaturation)
The bonding that alkenes undergo
Addition reactions
(like how a monomer takes on additional bonds)
Makes no byproduct (everything is contained within the molecule) - no inorganic molecule formed
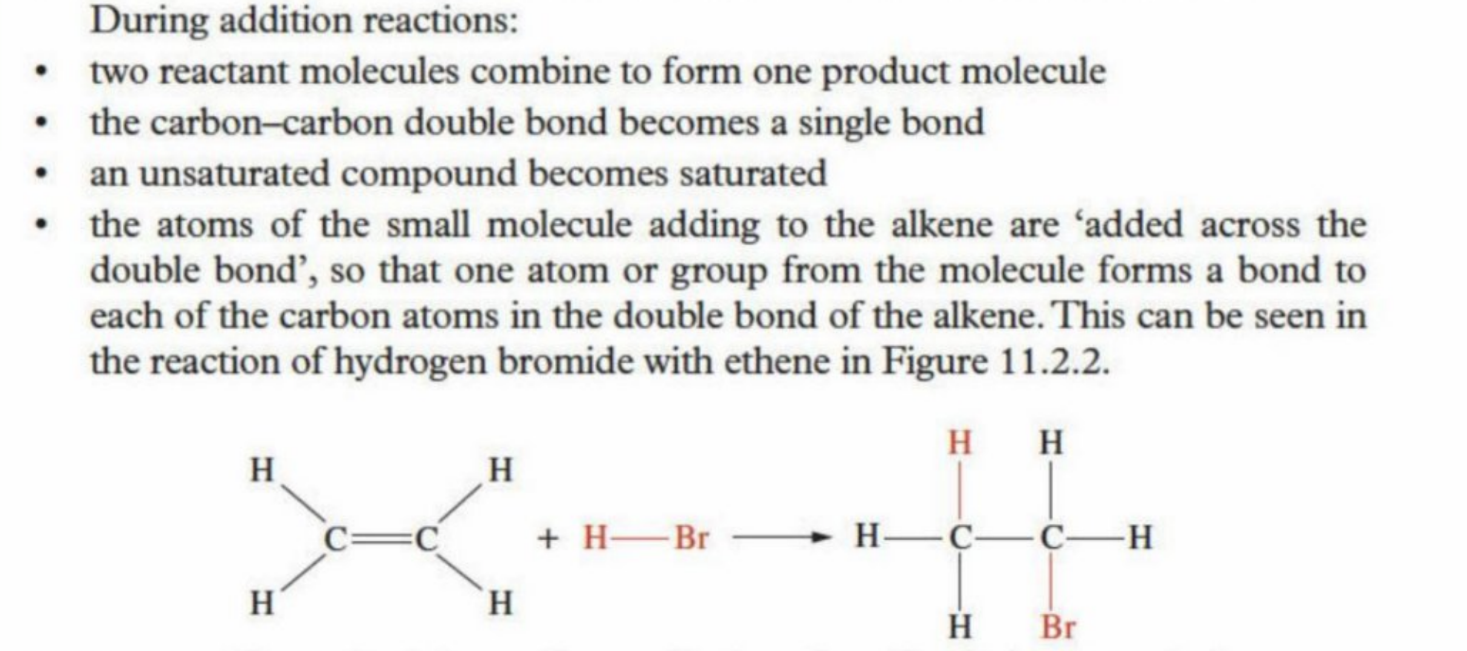
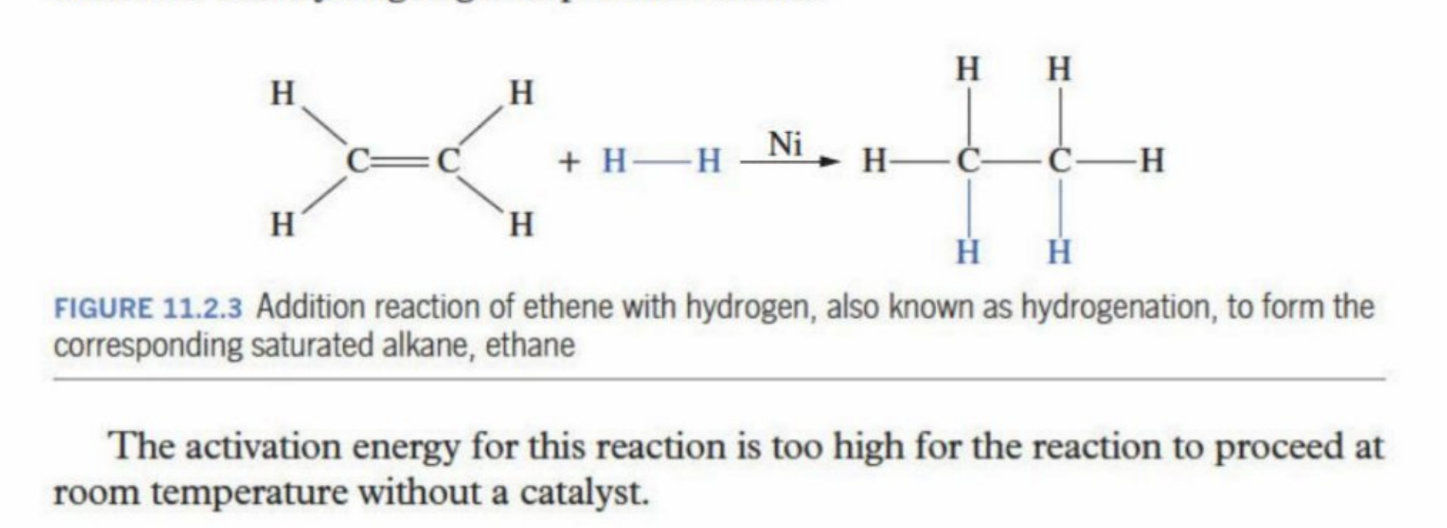
Reaction for alkenes with hydrogen (hydrogenation reaction)
Simply the addition of hydrogen atoms into the unsaturated alkene
Is done with a catalyst as the activation is too high for the reaction to proceed without a catalyst
Addition reactions with halogens
Alkene reacts with halogens to become saturated
Reaction with Bromine (halogen that is orange in color) can be used to distinguish alkane vs alkene - used to test for a C=C bond
Alkene - adsorbs bromine and the orange color, as it becomes fully saturated - solution becomes clear in color
Alkane - No change, as it is already saturated
Addition reactions with hydrogen halides
Halide - a halogen with a charge
Hydrogen atom + halogen - Both attach onto alkene, making it saturated
Can make isomers
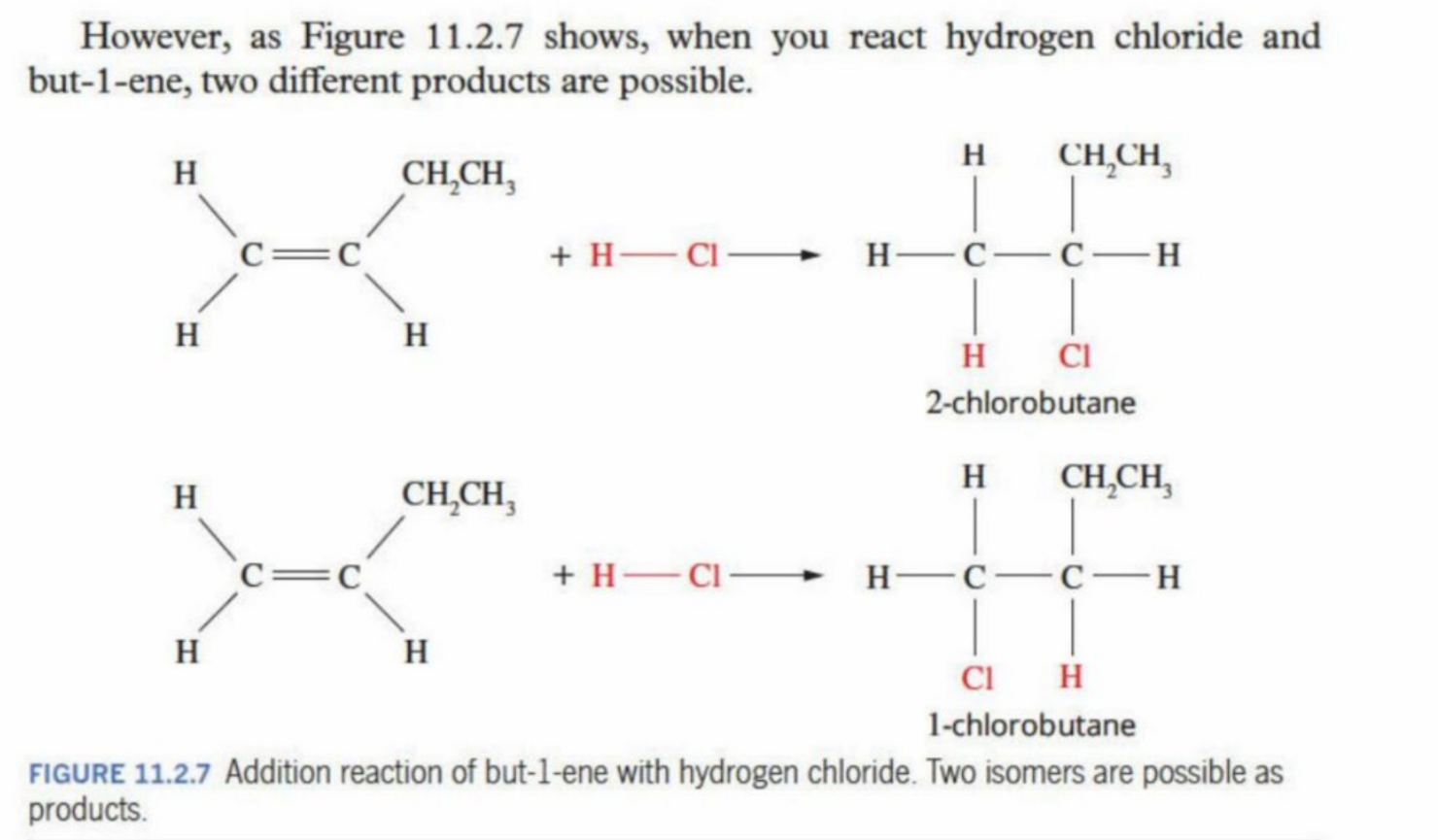
Isomers made upon asymmetry of molecules
When an asymmetrical alkene bonded with an aysmmetrical reactant - isomers are produced
e.g. but-1-ene (C=C is is between C1 and C2 + HCI (is asymmetrical as the CI draws more electrons away from the H creating an imbalance)
Alkenes with Water
Used to make an alcohol
Is used with an increase of heat and catalyst to speed up reaction
Is termed as hydration reaction
The making of ethanol using reactions with water
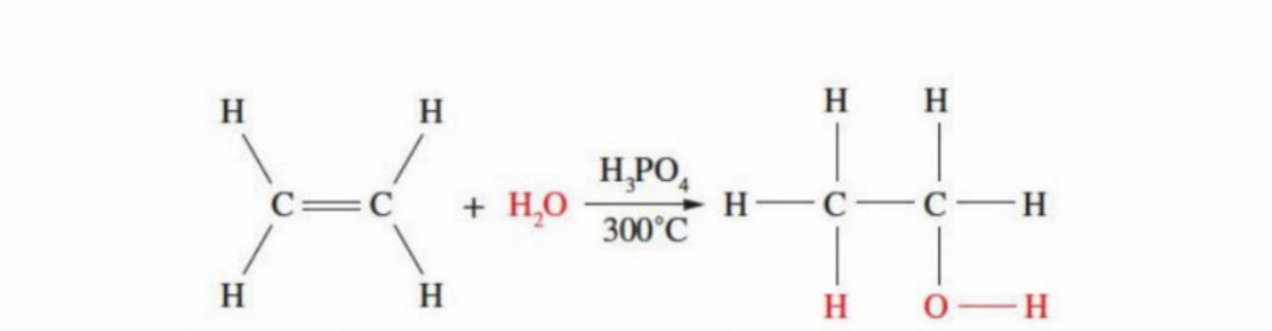
Explanation
Occurs at high temp (300oC) - reactants are passed over “bed” of the catalyst and gaseous ethanol is formed
Uses H3PO4 (Sulfuric Acid) as the catalyst
Commercial method of making ethanol - uses less energy (apart from initial heating), and is a one-step process
Solid-gas (heterogenous) nature of reaction - easy to remove product (ethanol as a gas) from reaction mixture, leaving catalyst (liquid)
Why having an hydroxyl group makes the hydrocarbon more reactive
OH bond - is highly polar and is very vulnerable to substitutions (with other atoms) and the lone pair on oxygen can also allow such additional reaction pathways
Alkene bond is non-polar - need higher activation energy
Combustion Of Alcohols

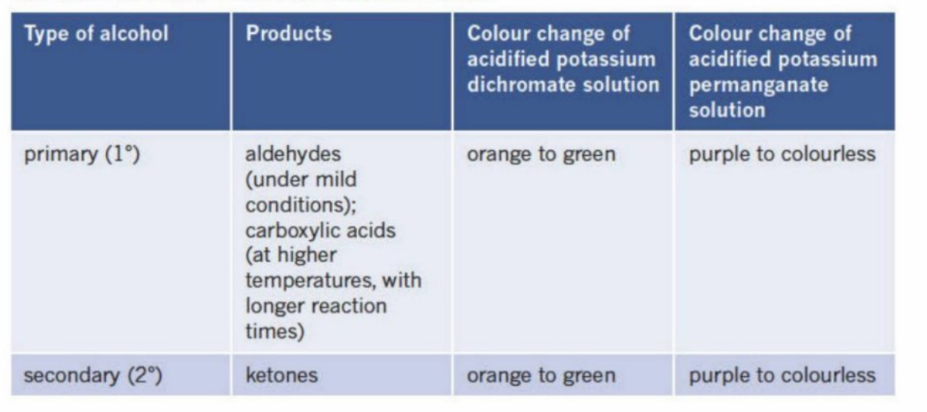
Primary Alcohols
The OH group is bonded to a carbon that is only bonded to one other carbon
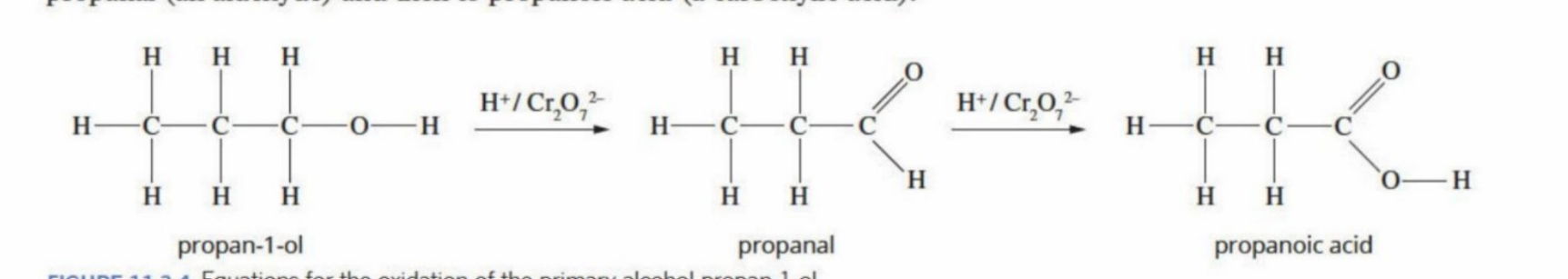
They oxidize to become aldehydes before oxidizing into carboxylic acids (is bonded to two hydrogens - can undergo oxidation twice)
Oxidation of primary alcohols
Is first oxidised into aldehyde, and then into a carboxylic acid (group) - the carbon chain stays intact
An oxidising agent is used
Why an additional catalyst is used
Alcohols are not very reactive on their own
The C-OH bond is polar but strong, so the alcohol doesn’t easily give up hydrogens.
Catalyst provides the right environment
For example, in H+/Cr2O7-2
The acid protonates the alcohol (-OH → -OH₂⁺), making the oxygen a better leaving group.
Cr2O7-2 pulls electrosn from carbon as it is very “electronb-hungry” - disrupts C-H bond on alcohol carbon
This allows the oxidizing agent to accept electrons more easily.
Speeds up the reaction without being consumed
The catalyst lowers the activation energy, so the reaction happens faster and under milder conditions.
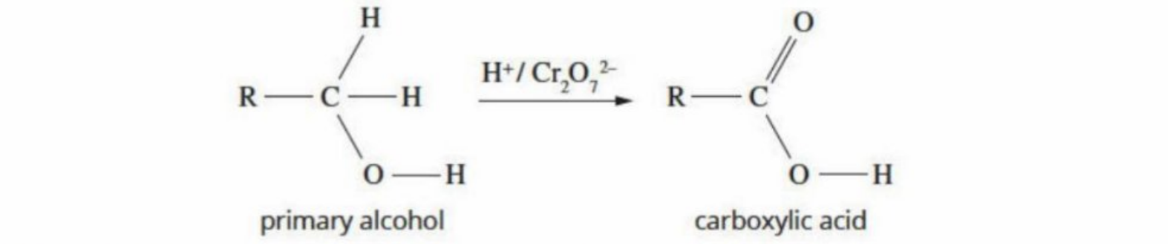
Primary Alcohols Oxidation (Visual)
General Primary Alcohols Oxidation Equation
Secondary Alcohol
OH bonded Carbon is bonded to two other Carbon atoms
Produces a ketone in the presence of a strong oxidizing agent (reduces itself)
Secondary Alcohol Oxidation
Creates a ketone by the oxidation of the secondary alcohol
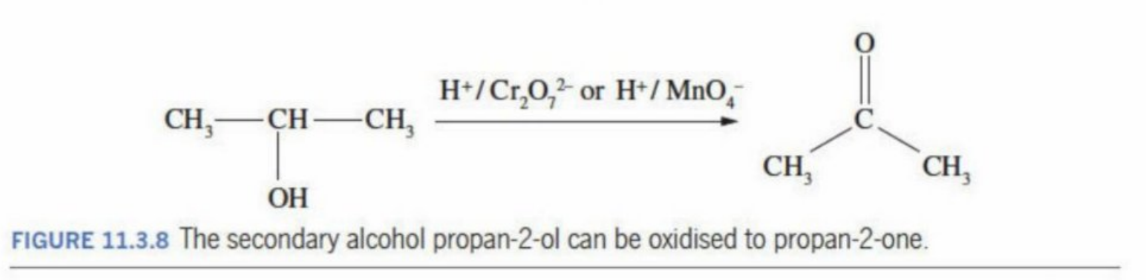
Secondary Alcohol General Reaction
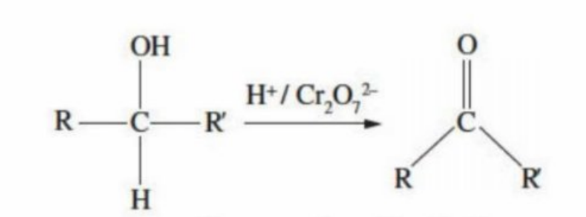
Tertiary Alcohol Oxidation
As there are no hydrogens bonded to the carbon that is connected to the OH group, it cannot undergo oxidation (must loose a Hydrogen)
Tertiary alcohols are resistant to oxidation, no matter how strong the oxidation agent
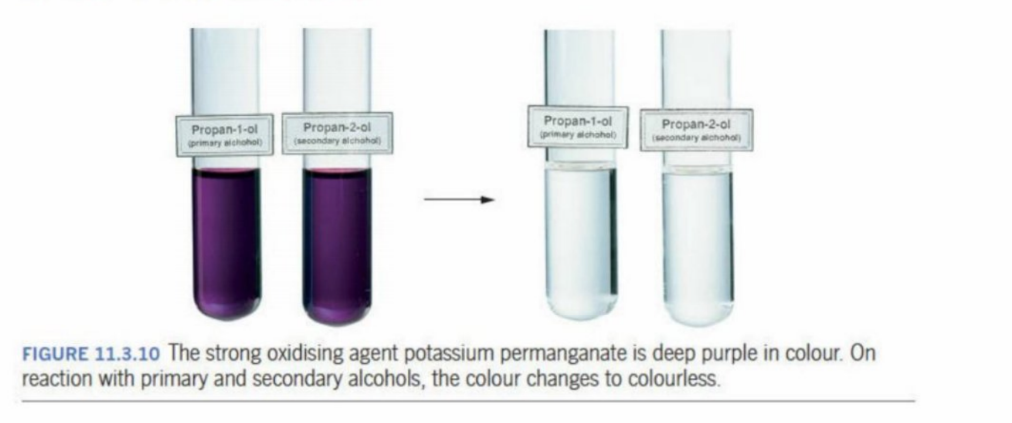
Using dichromate and potassium permanganate to indicate oxidation
When a primary or secondary alcohol is placed into these oxidising agents, they turn in colour, siganlling that oxidation has occured
The change in colour - shows gain in electrons
Dichromate
Cr —→ reduced to Cr+3 (its own charge - oxidation number)
If tertiary alcohol - no colour change is observed

Potassium permanganate
Changes from purple (MnO4-) to colorless (reduced to Mn+2), when secondary or primary alcohols are used.
Why is heat used
1. Overcoming Activation Energy (Ea)
Even though the reaction between an alcohol and potassium dichromate is "energetically favorable" (exothermic), it doesn't happen instantly at room temperature.
The Barrier: The molecules need a specific amount of kinetic energy to collide with enough force to break existing bonds (like the C-H and O-H bonds) and form new ones (C=O).
The Solution: Heat provides this "spark," increasing the speed of the molecules so that more collisions result in a successful reaction.
2. Controlling the "Oxidation Ladder"
Heat is the "throttle" that determines how far the reaction goes. This is especially true for primary alcohols, which can be oxidized in two stages:1
Alcohol —→Aldehyde
Aldehyde ——> Carboxylic Acid
Distillation (Gentle Heat): If you want to stop at the aldehyde, you heat the mixture gently and use a distillation setup.6 Because aldehydes have lower boiling points than alcohols, they evaporate and leave the reaction flask as soon as they are formed, preventing them from being oxidized further.7
Reflux (Strong Heat): If you want the carboxylic acid, you use a reflux condenser.8 This allows you to boil the mixture vigorously for a long time. Any aldehyde that evaporates hits the cold condenser, turns back into a liquid, and falls back into the flask to be "hit" by the oxidizing agent again until the reaction is complete.9
3. Increasing Reaction Rate
Organic reactions are notoriously slow compared to the "instant" reactions you see in inorganic chemistry (like acid-base titrations). Without heat, you might have to wait days to see the orange-to-green color change. By heating the mixture, you compress that time into a few minutes, making it a practical laboratory test.
Summary Of Reactions with primary and secondary alochols
Carboxylic Acids Reactions With Alcohols (Esterification - Forming Of Esters) - Why Catalysts are added
Are gently stirred with heat and with catalyst (e.g. sulfuric acid H2SO4 - adds hydrogen to carbonyl oxygen)
The H⁺ attaches to the oxygen of the carbonyl.
Oxygen now has more positive charge, so it pulls even more electron density away from the carbon.
Effect: The carbonyl carbon becomes more positively charged (even more δ⁺).
3. Why this matters
Alcohol attacks carbon by donating a pair of electrons (nucleophile).
Normally: the carbon isn’t positive enough → attack is slow.
After protonation: the carbon is more electrophilic → alcohol can attack much more easily.
Esterification (Visual)
Splitting apart an ester (hydrolysis)
Goes back to carboxylic acid and alcohol by adding water (what was taken away during condensation)
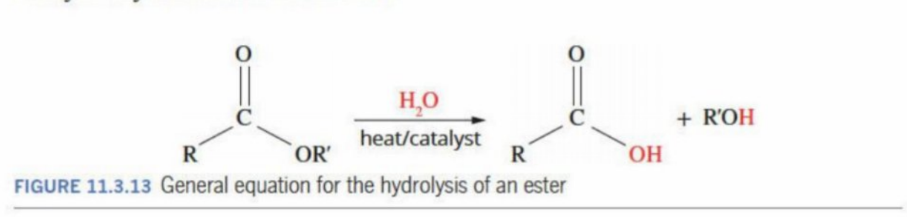
Example
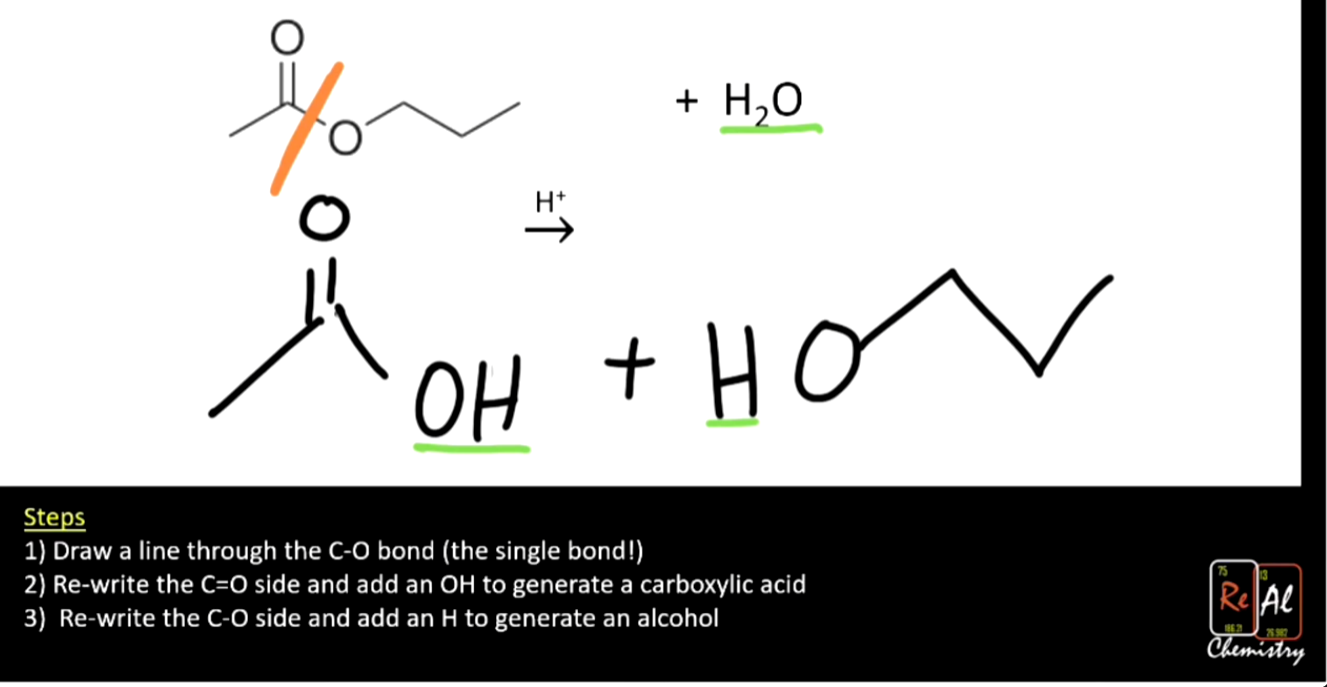
Example #2
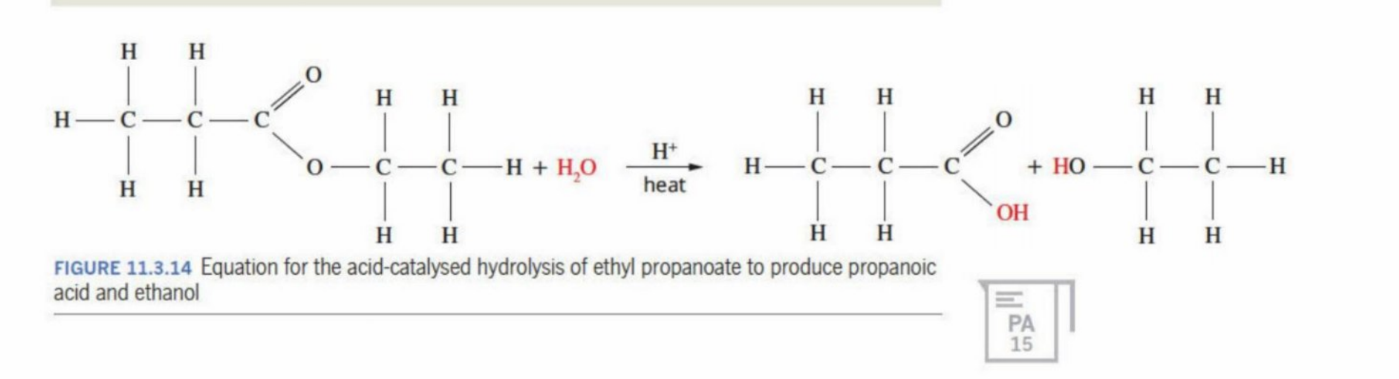
When a catalyst like an alkali (e.g. NaOH) is used (unclear)
Salt is produced of the carboxylic acid, but can be turned into a solution through adding dilute acid solution

When using catalyst (what happnes)
The ion of the catalyst is added onto the molecule, preventing it becoming a carboxylic acid
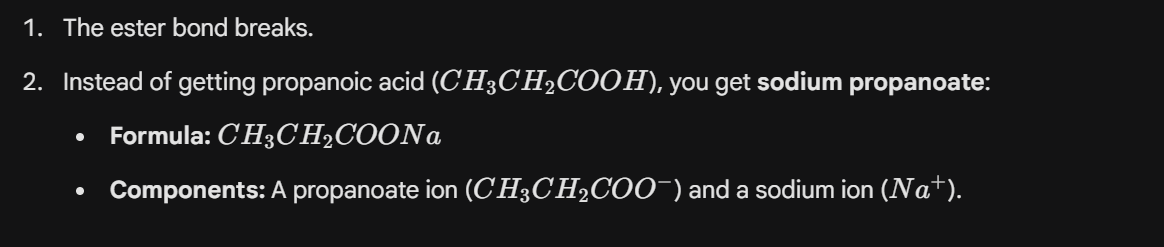
How to resolve this problem
HCI is used and creates the acid

Reaction pathway
A series of one or more steps or reactions that are used to convert a reactant into a desired product with different functional groups
Can be done so in many different ways
Simple Reaction Pathways
With alkanes and alkenes, they generally produce the same products using the same inorganic reactants and reaction conditions
Because they share similar properties with one another
Example of reaction pathway (alkane)
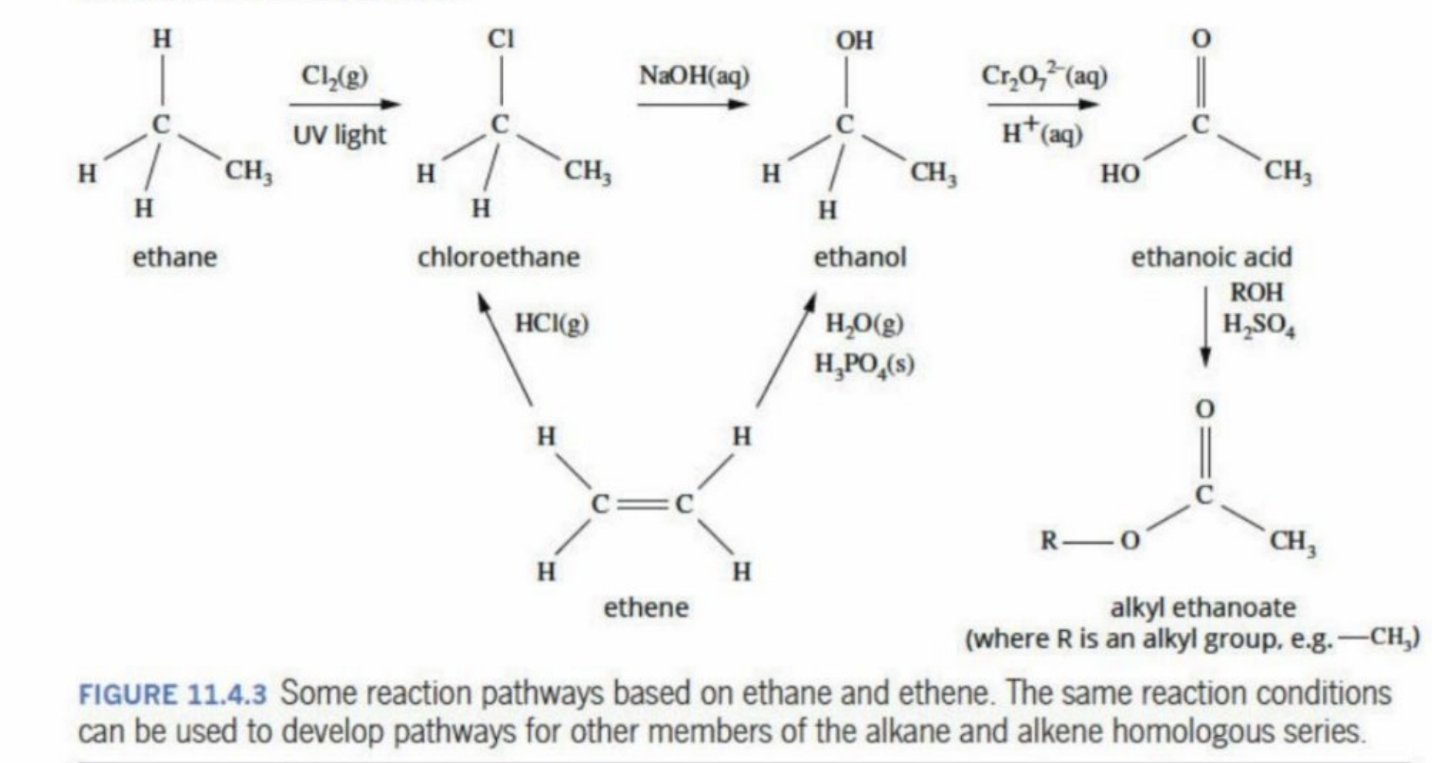
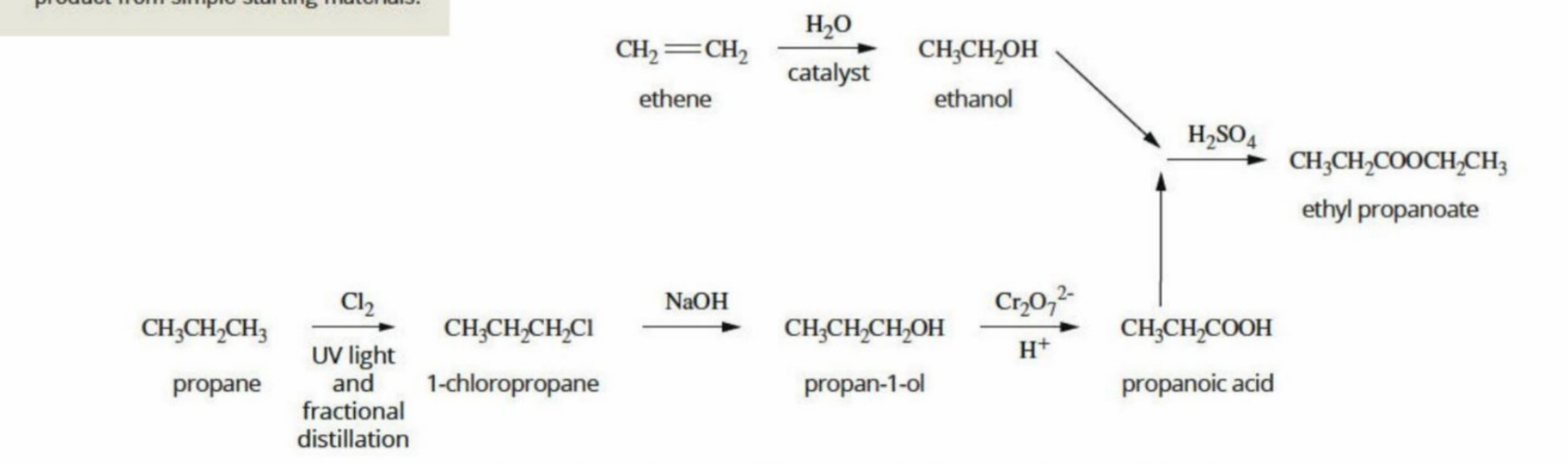
Making ethanol (complex reaction pathway) - in order to make an ester
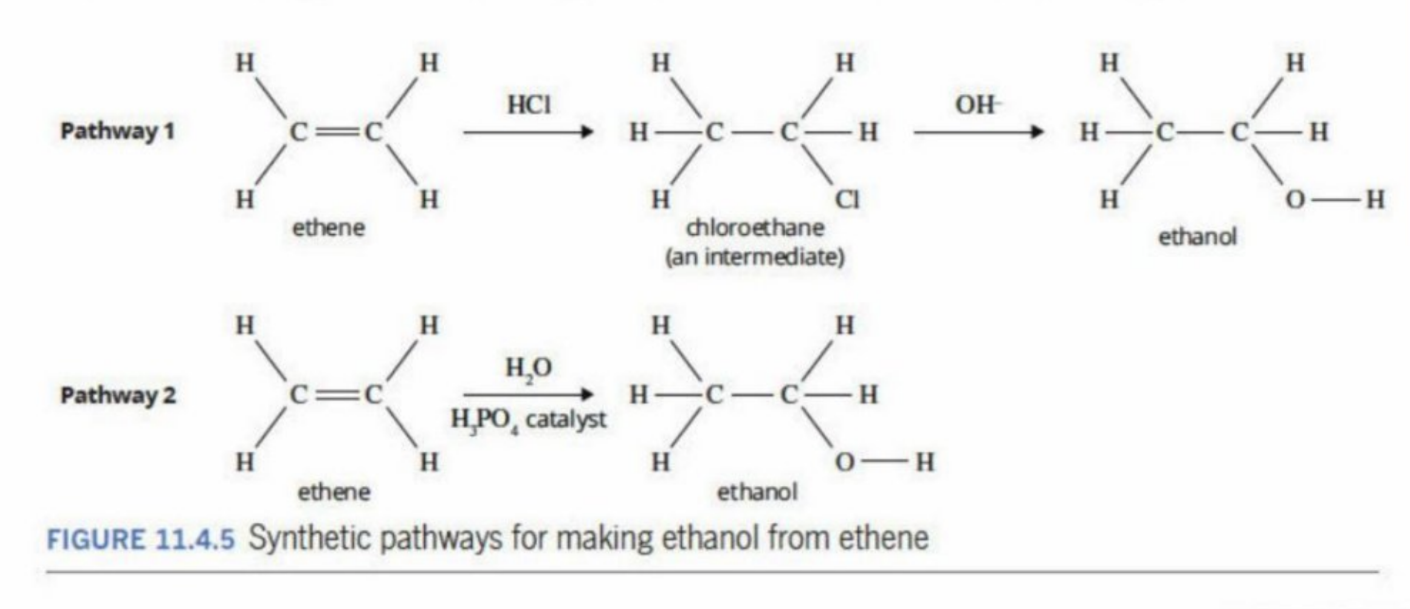
Two pathways in creating ethanol
Uisng HCI to create Chloroethane, then using OH to create ethanol
Using water and phosphoric acid (H3PO4)
Why second method is better
a) Fewer steps = higher yield
Every extra step (like making a haloalkane) loses some product.
Direct hydration avoids the intermediate, so you get more ethanol from the same amount of ethene.
b) Simpler and cheaper
Using just water and an acid is cheaper than buying and handling HCl and a separate OH⁻ source.
Fewer chemicals, less purification needed.
c) Safer and cleaner
Haloalkanes like chloroethane can be toxic and volatile.
Direct hydration avoids creating hazardous intermediates.
d) Less energy-intensive
Direct hydration can often be done at lower temperatures and pressures than the two-step method.
Making propanoic acid (in order to make an ester)
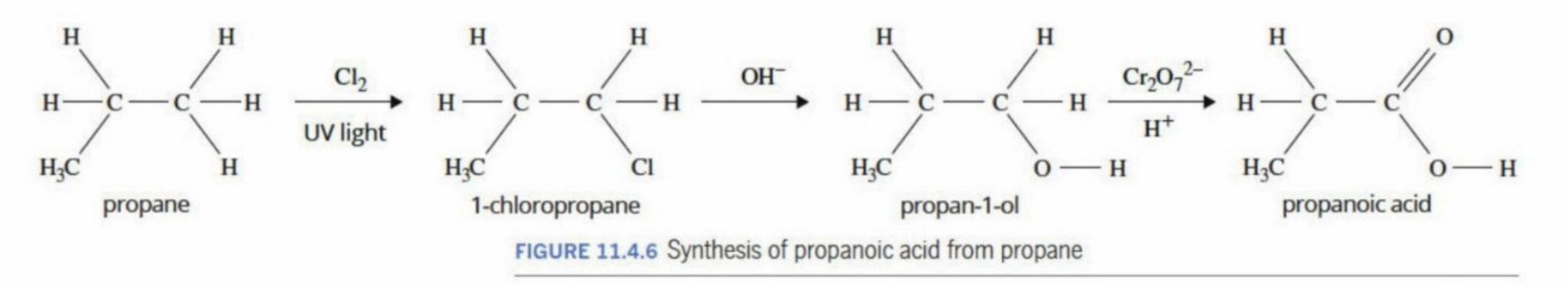
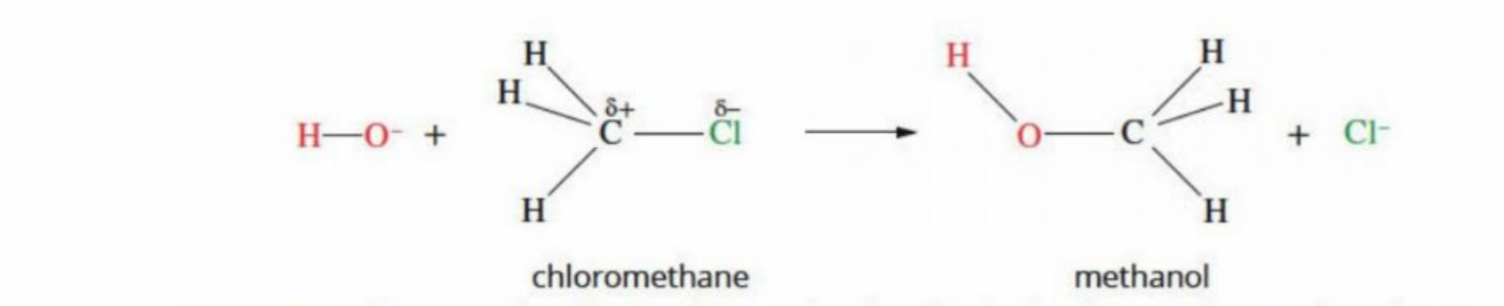
Why does OH displace CI
Chlorine is displaced because it is a good leaving group, and OH⁻ is a nucleophile that can attack the carbon and form a stronger, stable bond with oxygen.
Electrophile
An “electron lover” which is attracted to electrons
Must be positive in charge, or deficient in electrons itself
In the same reaction: the carbon in C–Cl is partially positive → it is the electrophile
It accepts a pair of electrons to form a bond
Nucleophile
An atom that is negative in charge and is attracted to positive charge
is either negative or neutral
Must have a lone pair to donate
Ethyl Propanoate being formed through creating the acid and the alcohol (condensation reaction)
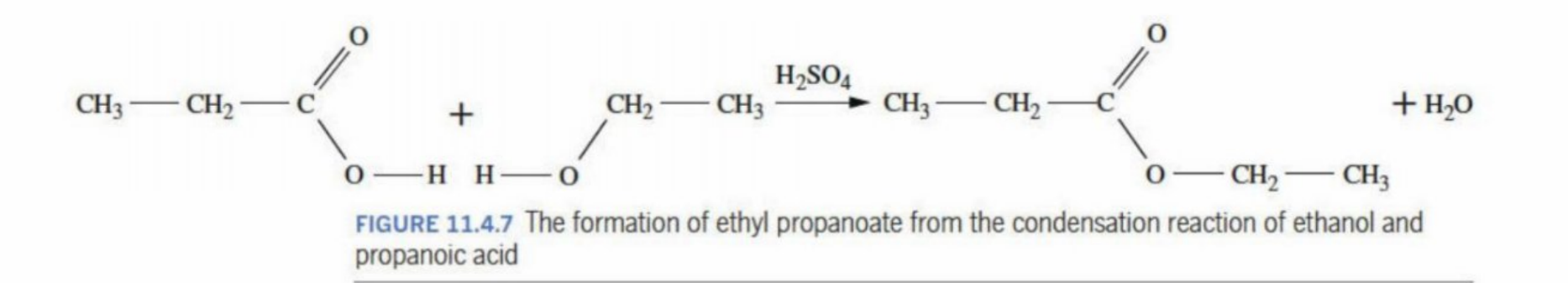
Purpose of a reaction pathway
To show the reactions required to produce a product from simple starting materials (to create a visual representation)
Full Reaction pathway of ethyl propanoate
Reaction pathways - Overview of what can be made using different reagents and catalysts
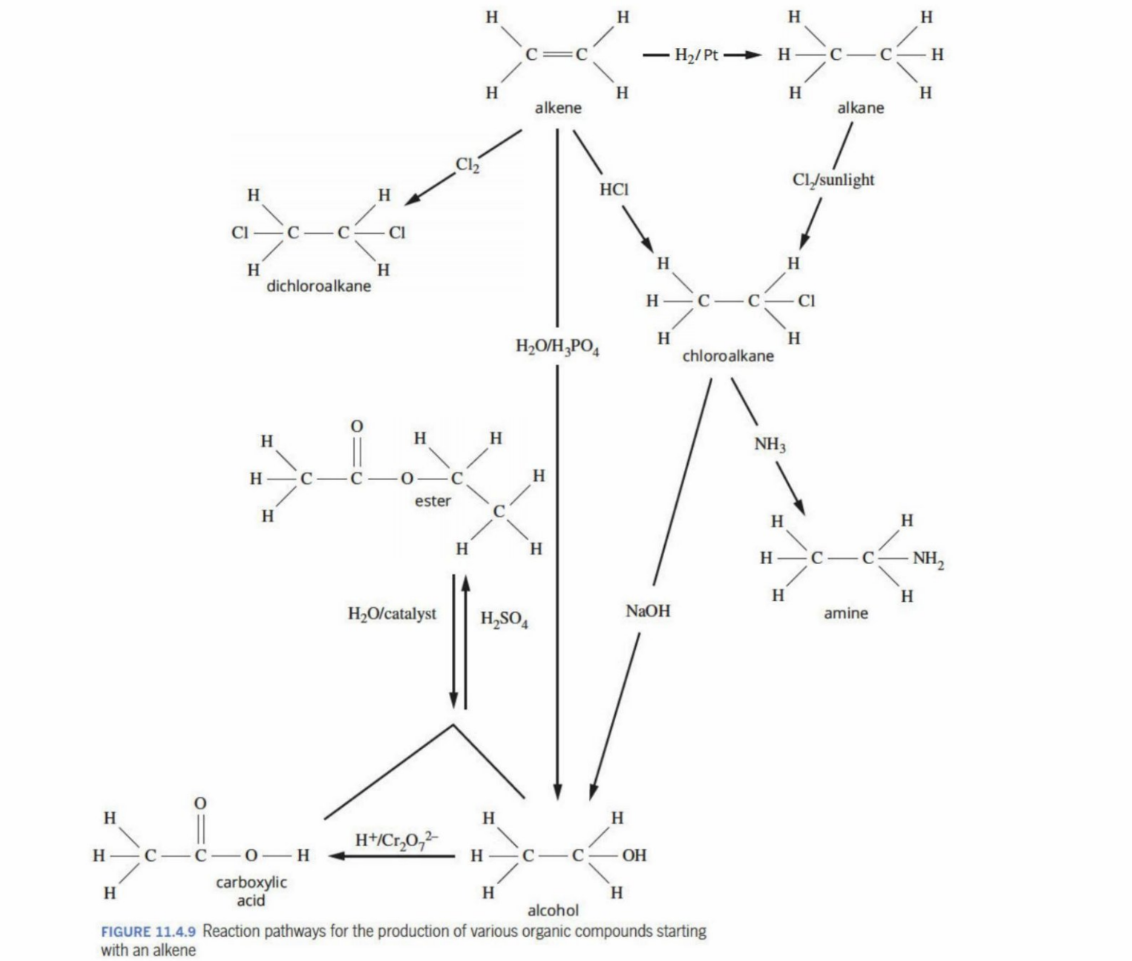
Steps in devising an appropriate plan for a reaction sequenc
1) Functional groups and the required molecule are identified
2) A synthetic pathway is made based on the existing knowledge of functional groups - may require intermediate stages (compounds)
3) Isomers need to be considered as they could also be produced (you may aim for one specific molecule, but isomers may form as well) - more than possible pathway is to be made - product can be made through many different ways
4) Methods of separation from intermediates and other isomers must be determined
5) Final product must be purified and evaluated
6) Must consider yield - not all reactants may turn into products
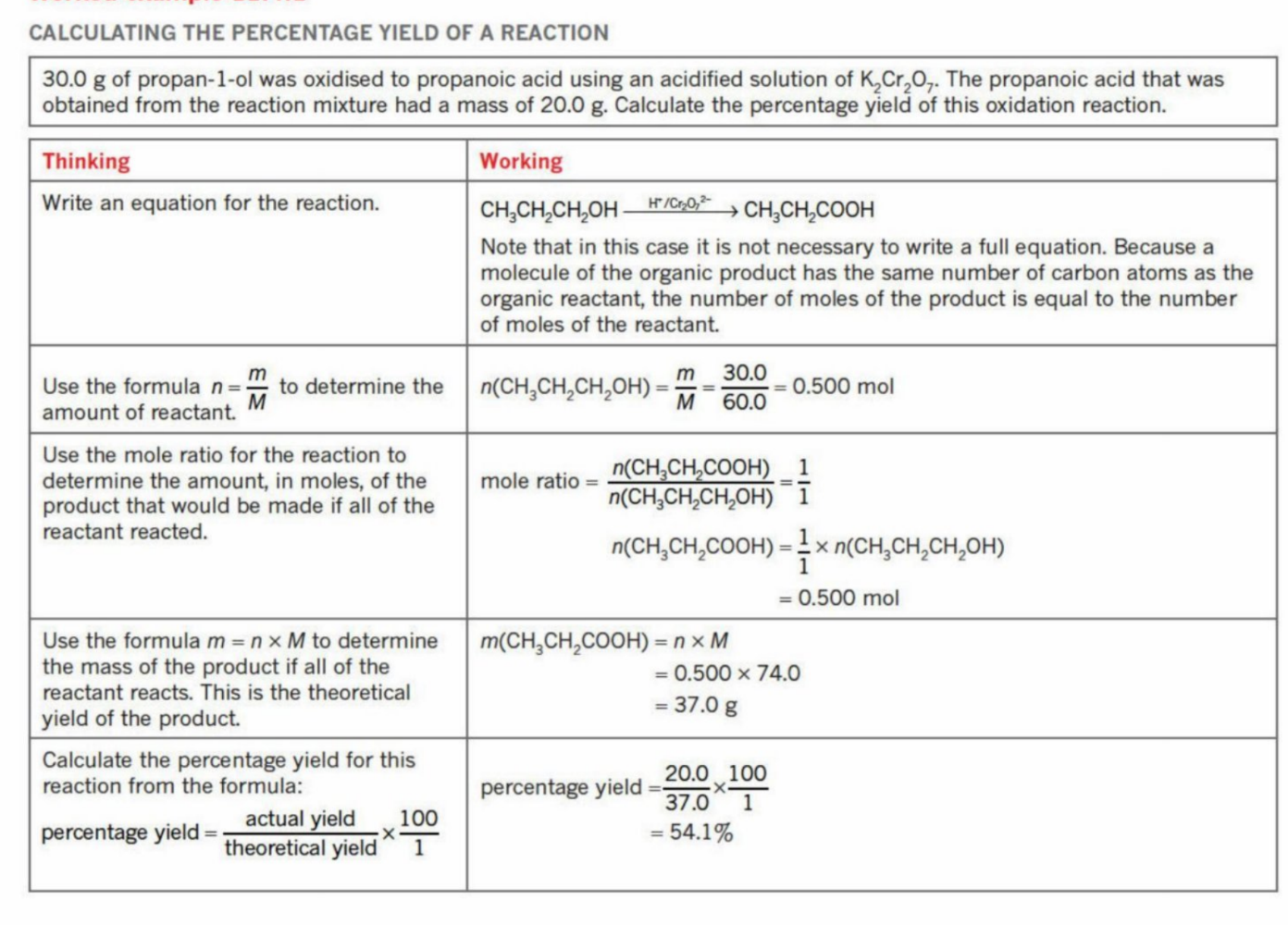
Yield
The amount of product obtained through respective reaction pathways
Efficiency - can be derived through calculations
3 types: Theoretical yield, Actual yield, Percentage yield
Theroretical yeild
The mass of product obtained If the limiting reagent reacts according to the stoichiometric ratios of the equation - the theoretical output of product (reactants 100% convert into products)
Actual Yield
Lower than theoretical yield (what is desired) due to a number of factord
Reasons why actual yield is lower than theoretical yield
1) If reaction reaches equilibrium - reactants are being turned into products; however, products are also being turned into reactants (never attains full product output)
2) If the reaction rate is slow - cannot be fully completed in the bound time, creating less product
3) Changing between vessels, separation, purification, and filtration result in smaller amounts of product formed
4) Competing reactions - reactants may form another reaction instead of the intended one.
Percentage Yield
Compares actual yield to theoretical yield

Example
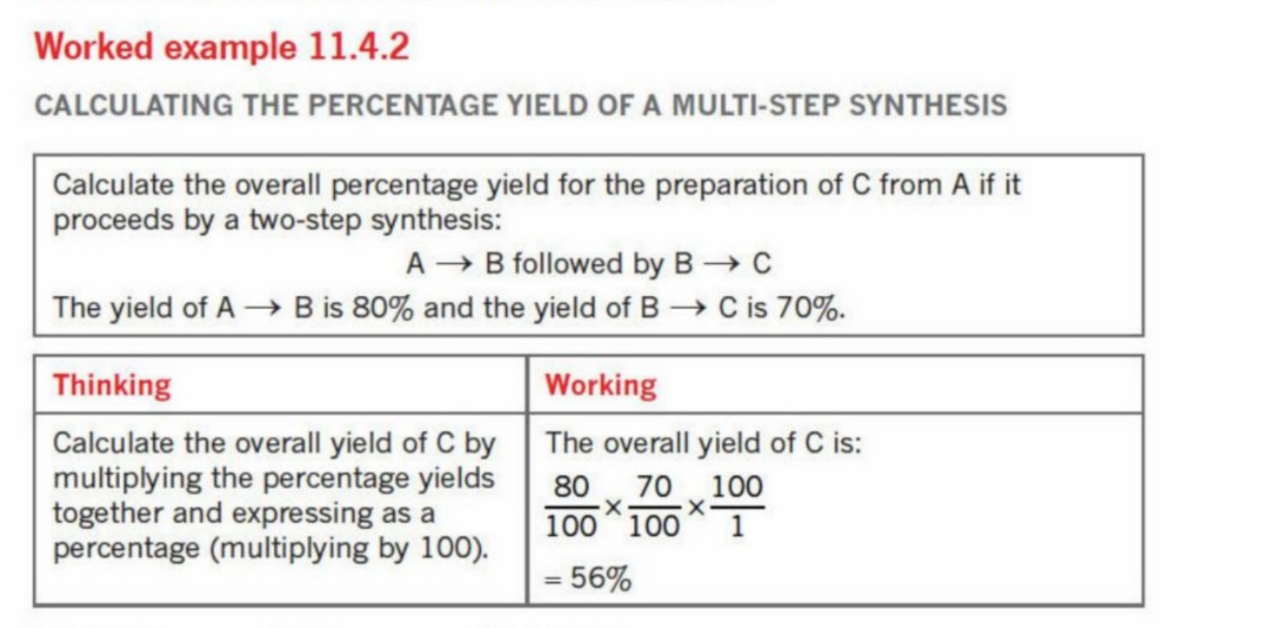
Percentage Yield in multi-steps
Yield is lost in every step of a reaction pathway
A lower yield in one of the intermediate reactions can cause a massive effect in the amount of product obtained
Comparison of percentage yields can indicate which pathway is most efficient - is paramount to prevent wasting of valuable reactants
Example
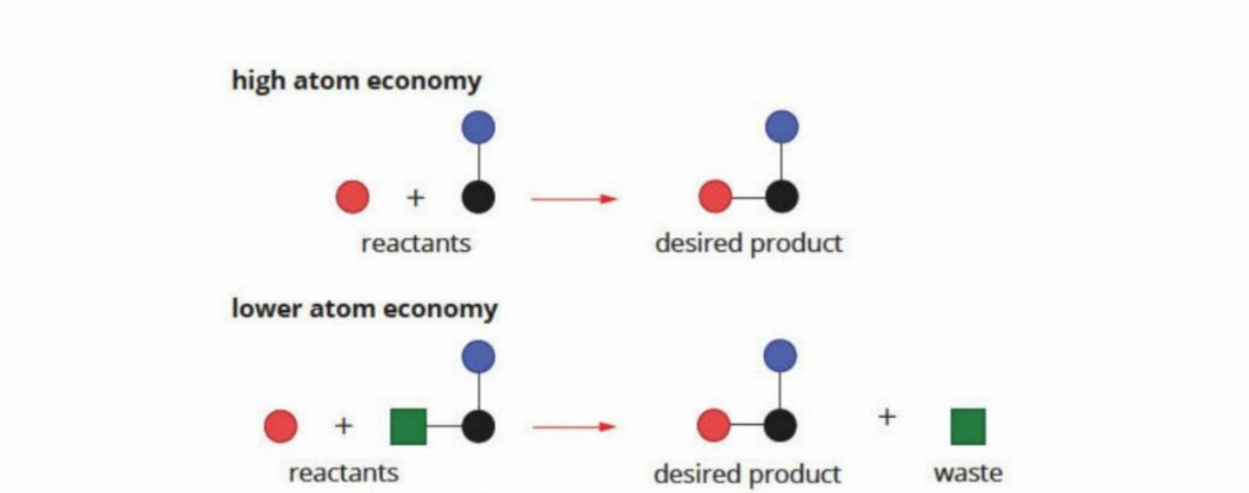
Atom economy
A measure of how many atoms in the reactants end up in the products
The higher the atom economy, the less wastage produced and higher yield is established
Visual Representation
If only one product, then atom economy is 100%
Finding atom economy
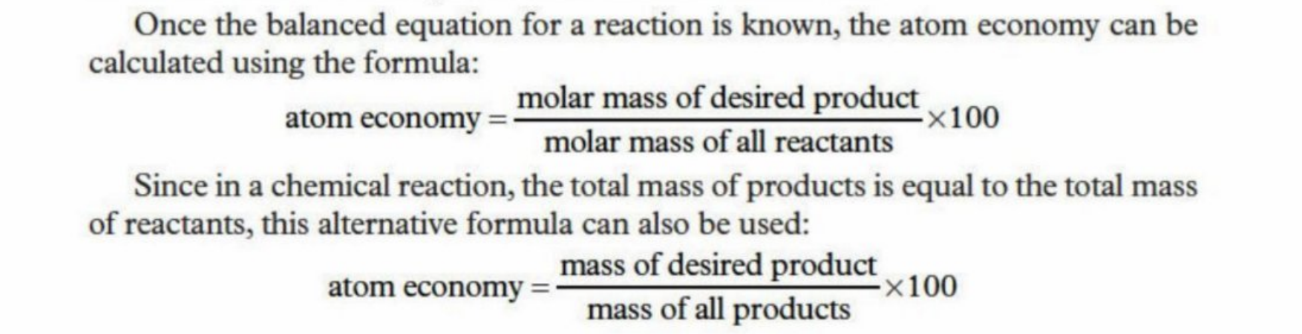
Example
Benefits of high atomic economy
Produces less waste
Uses reactants more efficiently
More atoms end up in the desired product
Lower costs for raw materials
Lower disposal costs for waste
Easier purification of product
Reduced environmental impact
Aligns with green chemistry principles
Radical
An atom or molecule with a single unpaired electron
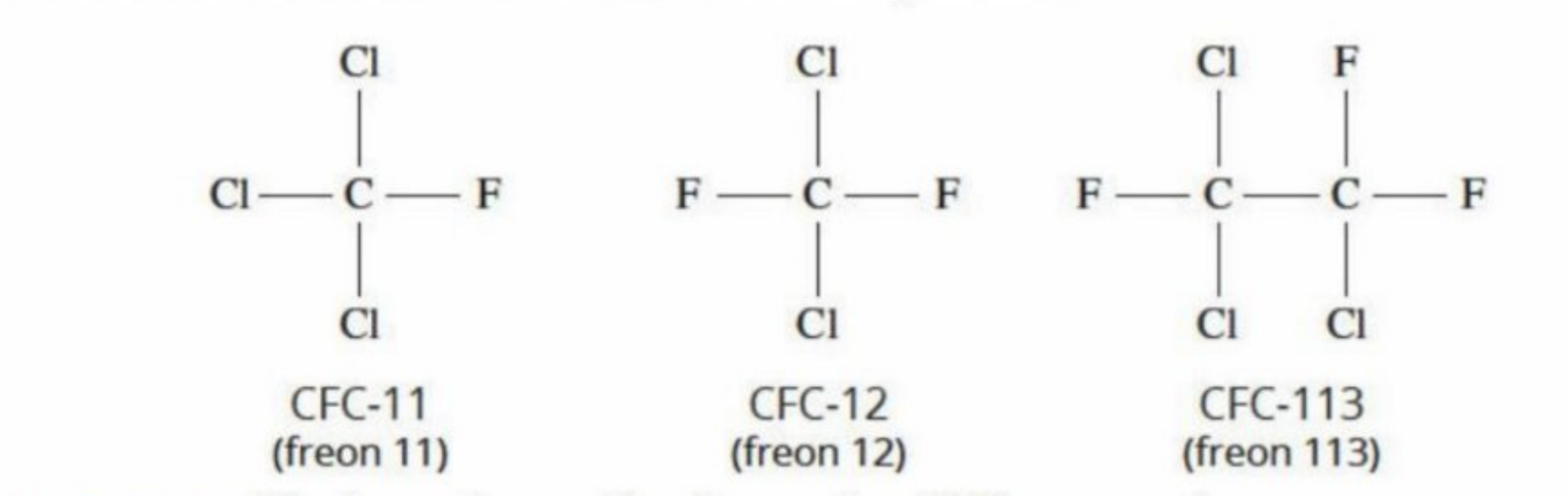
CFC Compounds
Chlorofluorohydrocarbons (hydrocarbons contained fluorine and chlorine)
Were responsible for the decrease in the concentration of the ozone
Structure —→
Montreal Protocll
The agreement of scientists and authorities from around the world is slowly phasing out the usage of CFC’s in aerosols and refrigerants
Occurred in 1987
Now, levels have stabilized through using alternatives - by 2050 ozone levels will have stabilized to pre-1987 levels
Green Chemistry
Creating a new approach to the design and manufacturing processes of chemicals required, mitigating the effects upon the environment and human health
Examples Of Fundamental aims - green chemistry

Feedstock
A raw material used in the preparation of other products
e.g. oil is used for biodiesel
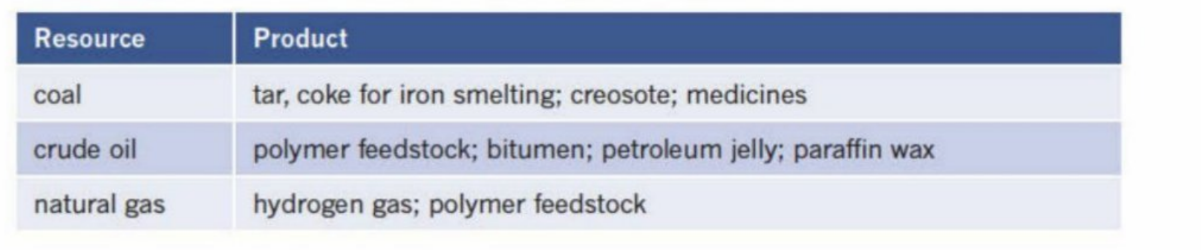
Uses of coal crude oil and natural gas
Are non-renewable - are pollutants and cannot be replenished for constant manufacturing
Are also non-biodegradable - will persist in environment
There are alternatives being developed
Biopolymers
Are made from feedstocks (the raw materials) that are derived from plants and celluloid and cellophane - both were derived from cellulose
Were used in 1920s - now are primarily made from fractional distillation of crude oil (non-renewable)
The polymers do not decompose easily
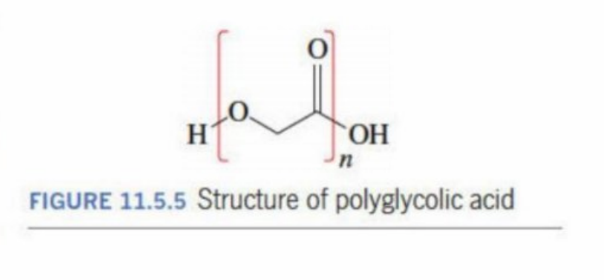
Polyglycolic acid
Is a biopolymer that is good at biodegradability
Has a fast degradation rate
Has a high stiffness (performs like heavy-duty plastic but is lightweight)
Is biocompatible (used in surgeries as stitches)
does not leak -Prevents leakage
Polyglycolic acid (structure)
Biosolvents
Are derived from biomass and use renewable feedstocks as opposed to non-renewable feedstocks
Example of a bio solvent
Glycerol - Used in the production of biodiesel - also used in cosmetic space
Turpentine - a grease solvent and paint thinner - is derived from the resin of trees (renewable)
Solketal - Polar compound and is used in ink and paint manufacturing - derived from glycerol (a renewable feedstock)
Advantages of biopolymers and biosolvents

Haber Process - Catalysts
Used to make ammonia from nitrogen and hydrogen gas in the presence of an iron catalyst
Is an exothermic reaction
High pressure is used to favor the reaction that produces the lower amount of atoms (the forward reaction - produces ammonia)
A medium (450 degrees) temperature is used to speed up the reaction, despite not attaining all of the yield
A catalyst - Iron - is used to lower the activation energy, resulting in much lower energy required for teh energy to occur
Enables enormous savings in heating - reduces consumption of fossil fuels
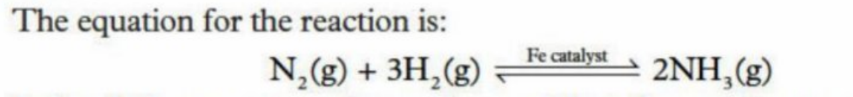
Haber process (link)
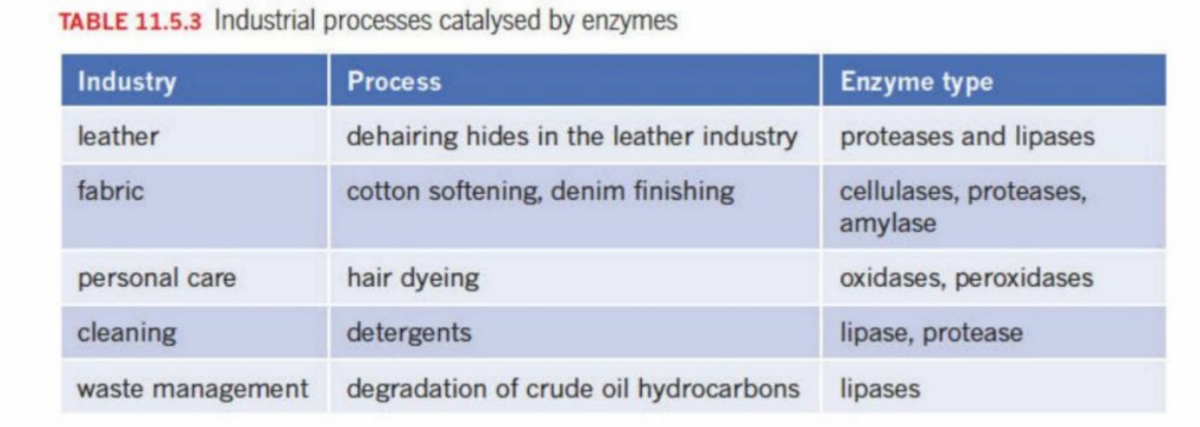
Enzymes
Are biological catalysts
Reduce the need for chemicals to carry out reactions - resources are saved and heating cost and wastes are reduced
Industrial Processes using enzymes
Catalyst Advantages (Overall)

Designing safer chemicals
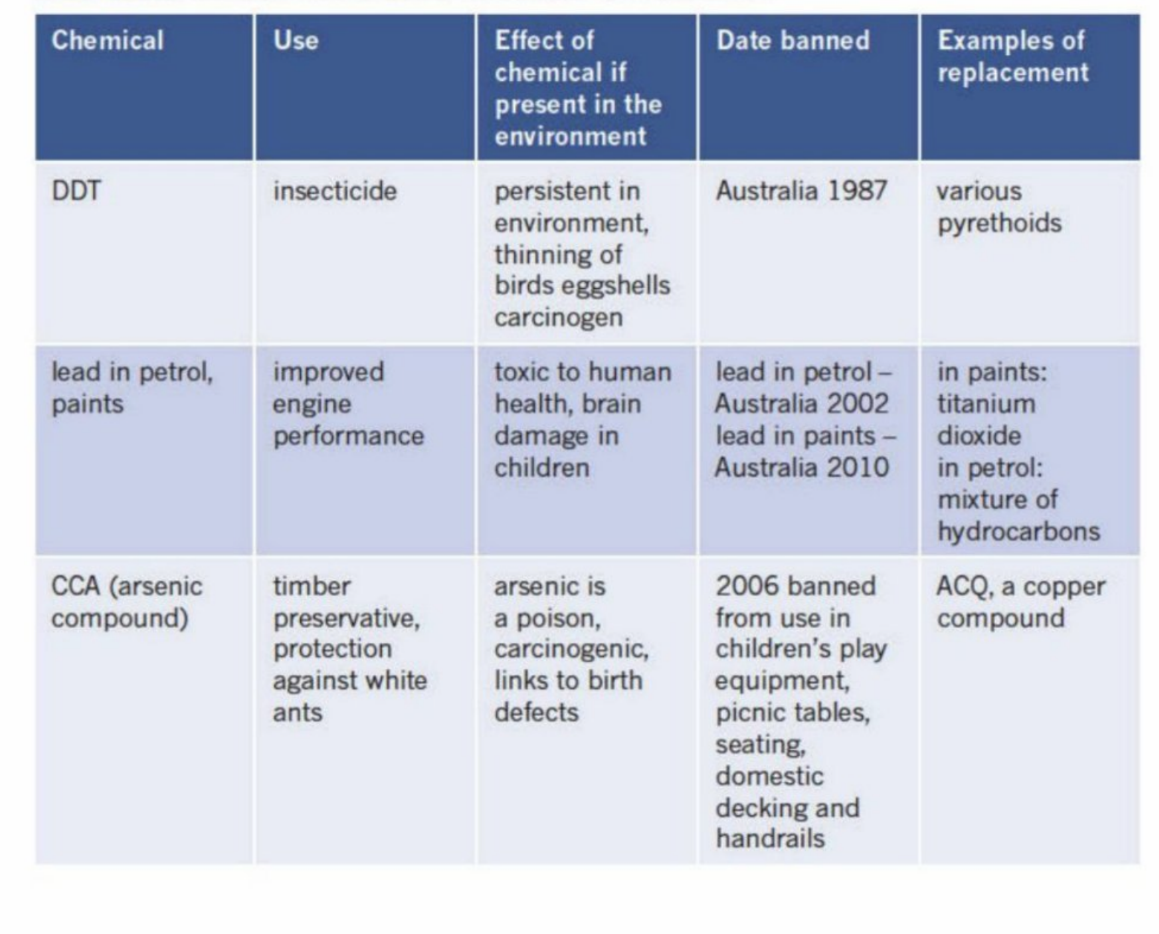
Banning/prohibiting certain chemicals due to the damaging impact they have on the surrounding environment and the harm they pose to human health
Chemicals that have been banned
PFAS - Perfluoroalkyl compounds (fire-fighting foams)
Have a long chain of carbons bonded to fluorine atoms
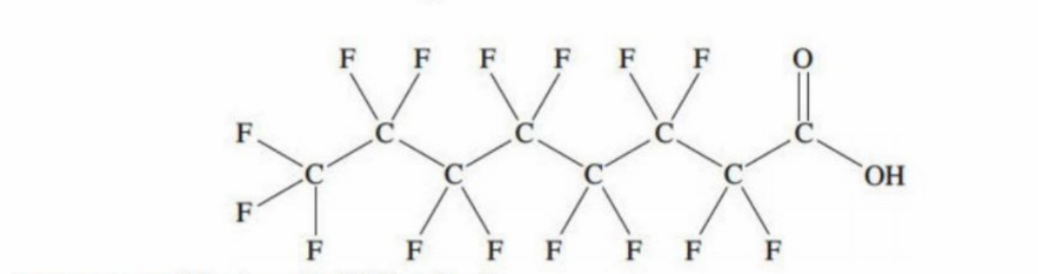
Why PFAS is banned
The C-F bonds are very strong
Have high heat resistance and stability
Are banned as they don’t break down readily and can build up concentration in waterways, soil and groundwater - become pollutants to the environment
Alternatives to PFAS
Using non-fluoro containing mixtures - non-toxic and biodegradable - will break down in the environment in a short time