Biochemical Terminology & Non-Covalent Interactions
1/30
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
Biochemical hierarchy
organizational level of biological systems from simplest to most complex
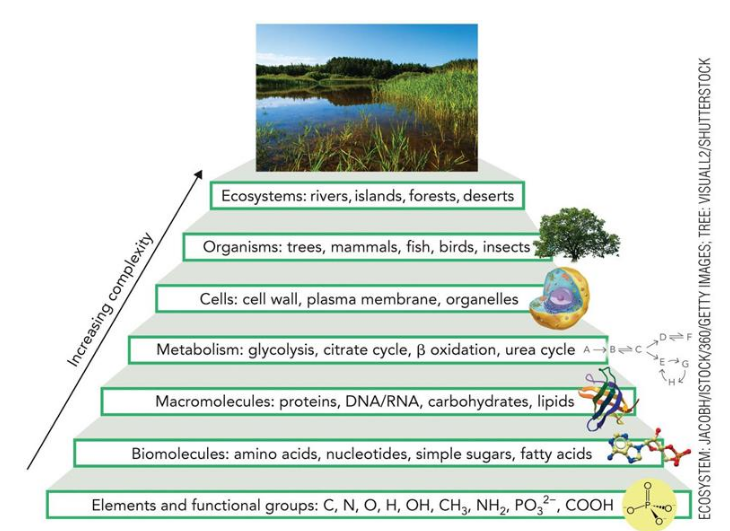
Most Abundant Elements in Organic Matter (6)
>97% weight
CHNOPS (Carbon, Hydrogen Nitrogen, Oxygen, Phosphorous. Sulfur)
Common chemical/functional groups (6)
amino (-NH3)
hydroxyl (-OH)
sulfhydryl (-SH)
phosphoryl (-PO32-)
carboxyl (-COO)
methyl (-CH3)
major classes of biomolecules (4)
amino acids
nucleotides
simple sugars
fatty acids
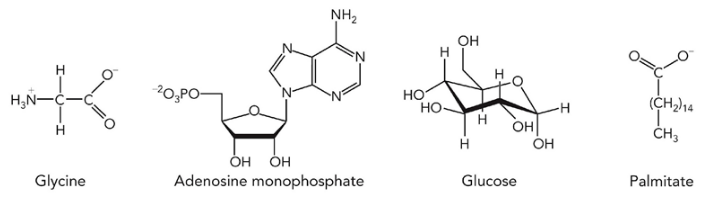
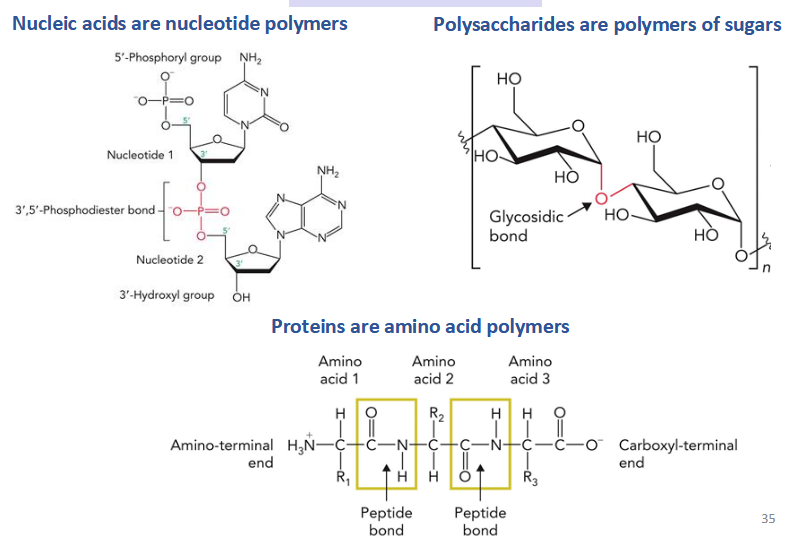
Macromolecules
polymers made up of small molecules
ex. nucleic acids, polysaccharides, proteins
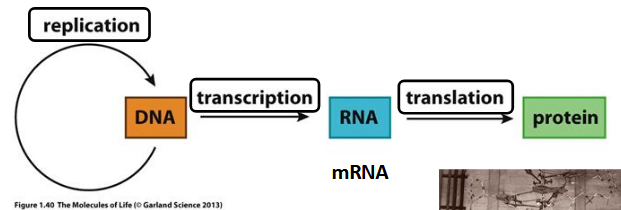
Central Dogma
flow of information in a biological pathway
DNA → RNA → proteins
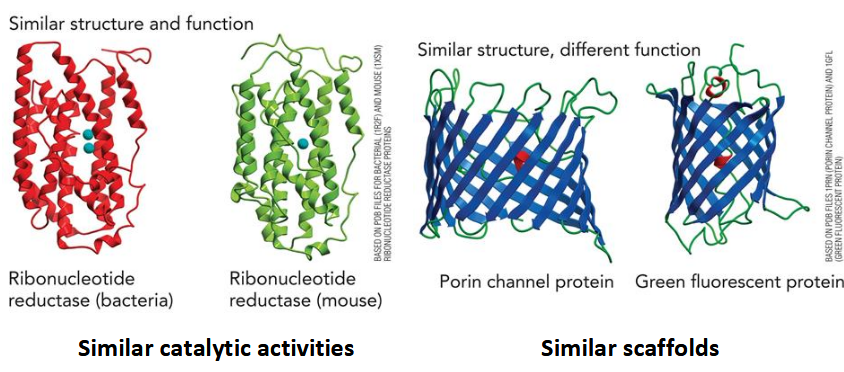
structure-function relationships
Sequence of polymer → 3D structure → function
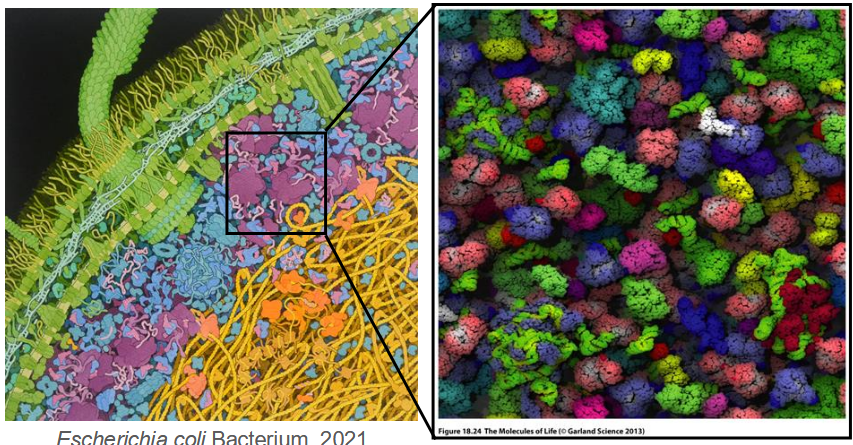
Crowding
biochemical systems are packed tightly together
Effective macromolecular concentration in cytoplasm ~300-400 mg/mL
Intermolecular interactions
within the same molecule
Intramolecular interactions
between more than one molecule
Covalent bonds
connecting the atoms that form macromolecules; stronger than non-covalent interactions
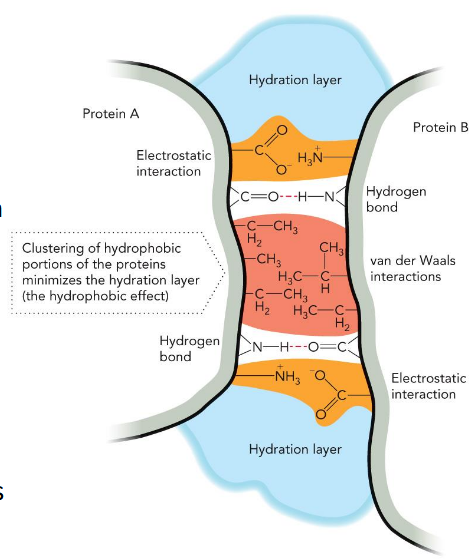
non-covalent interactions
dictates stability/function of the 3-D architecture of macromolecules
Are Non-Covalent Interactions stronger or weaker than covalent bonds?
weaker; individually weak but together strong
WHy does nature often rely on Non-Covalent Interactions?
facilitate transient and reversible interactions
Bond energies
describe energy used to break a bond for released to form a bond
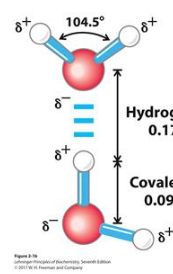
Hydrogen bonds
attractive interaction between hydrogen and electronegative atoms (FON); dipole-dipole interaction that depends on distance and direction (preferred 180°); essential in forming biomolecular structures
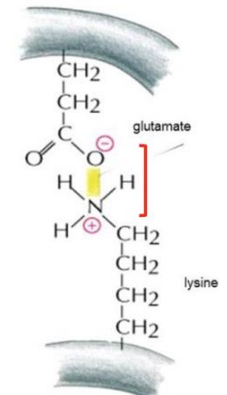
electrostatic/ion-pairs/salt bridges
interaction between two ions close to each other; governed by Coulomb’s Law; most stable non-covalent interaction
Van der Waals force
force between neutral atoms based on distance; weak
types of non-covalent interactions
Hydrogen bonds
Ion-pairs (salt bridges)
Van der Waals force
Aromatic interactions
Hydrophobic effect
dipoles
separation of positive and negative charges in a molecule; can be caused by a redistribution of electron density
dipole moment
sum of the difference in electronegativity over the molecule; depends on atom electronegativity and orientation
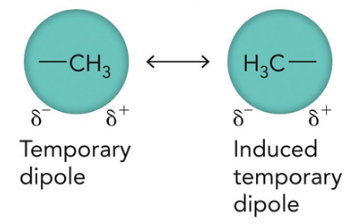
induced dipole interactions
occur due to transient fluctuation in electron clouds which set up transient dipoles, which mutually reinforce each other
electron repulsion
limits the vicinity of two atoms
Van der Waals radius (rvdw)
radius of the “hard sphere:; half the distance between closest approach of two nonbonded atoms of a given element; limited by electric repulsion; Atoms interact optimally when the distance between them is equal to the sum of their rvdw
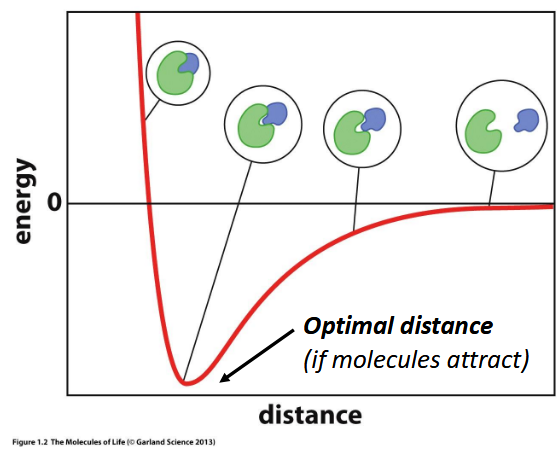
optimal distance
preferred distance for two atoms to interact; equal to the sum of their rvdw
steric effect
Repulsion between atoms at short distances; constrains the 3-D structures of biomolecules
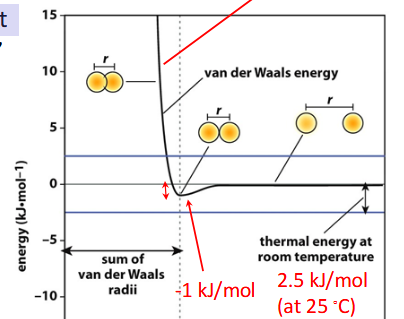
stabilization energy
minimum in ATOMIC INTERACTION graph; when two atoms are in van der Waals contact
shielding
The environment/medium that an ion pair is in can weaken electrostatic interactions
electric permittivity (ε)
influences how charges move across a medium; influences strength of electrostatic interaction
π-stacking
derive from dipole/dipole interaction; pi bonds are more polarizable than sigma bonds; positively charged regions interact favorably with negatively charged areas above/below
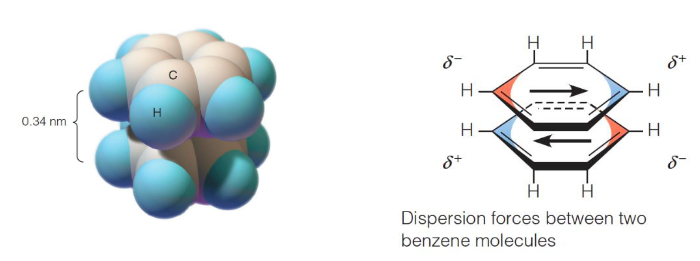
energy between two molecules
estimated based on additive pairwise non-covalent interactions; approximate b/c doesn’t include three-body effects