Atomic structure: Particles: Chemistry: (9:1)
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
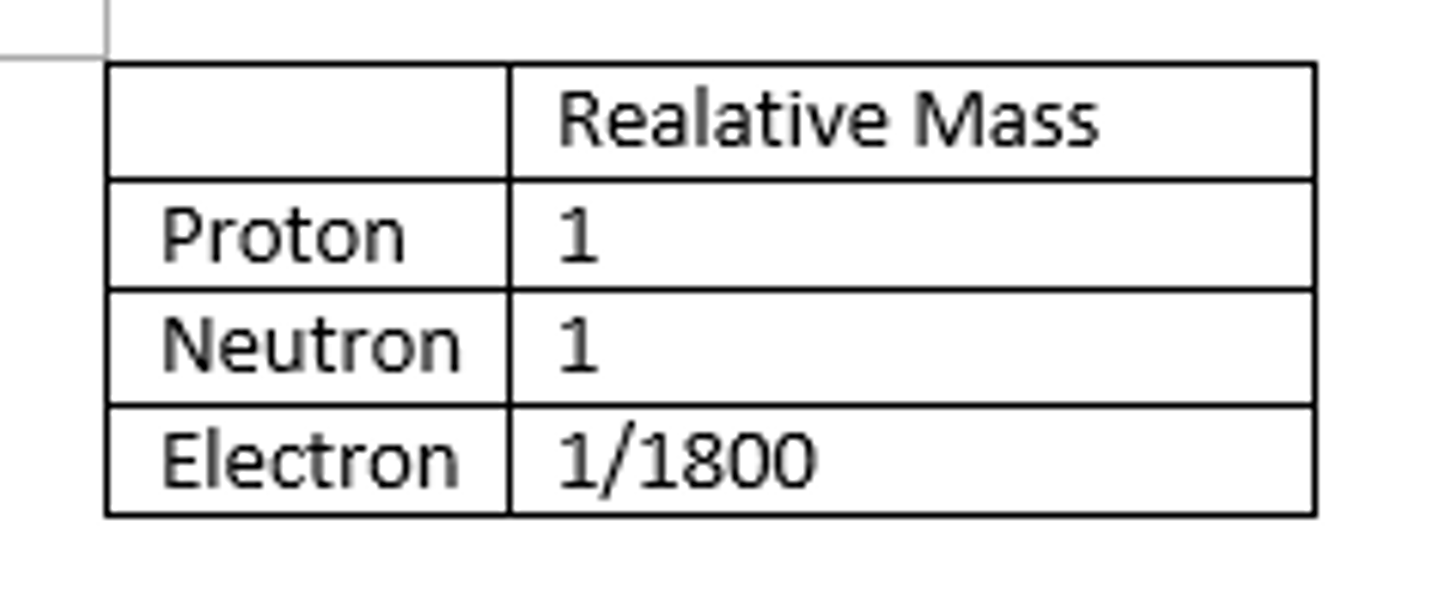
Relative mass of a proton
1
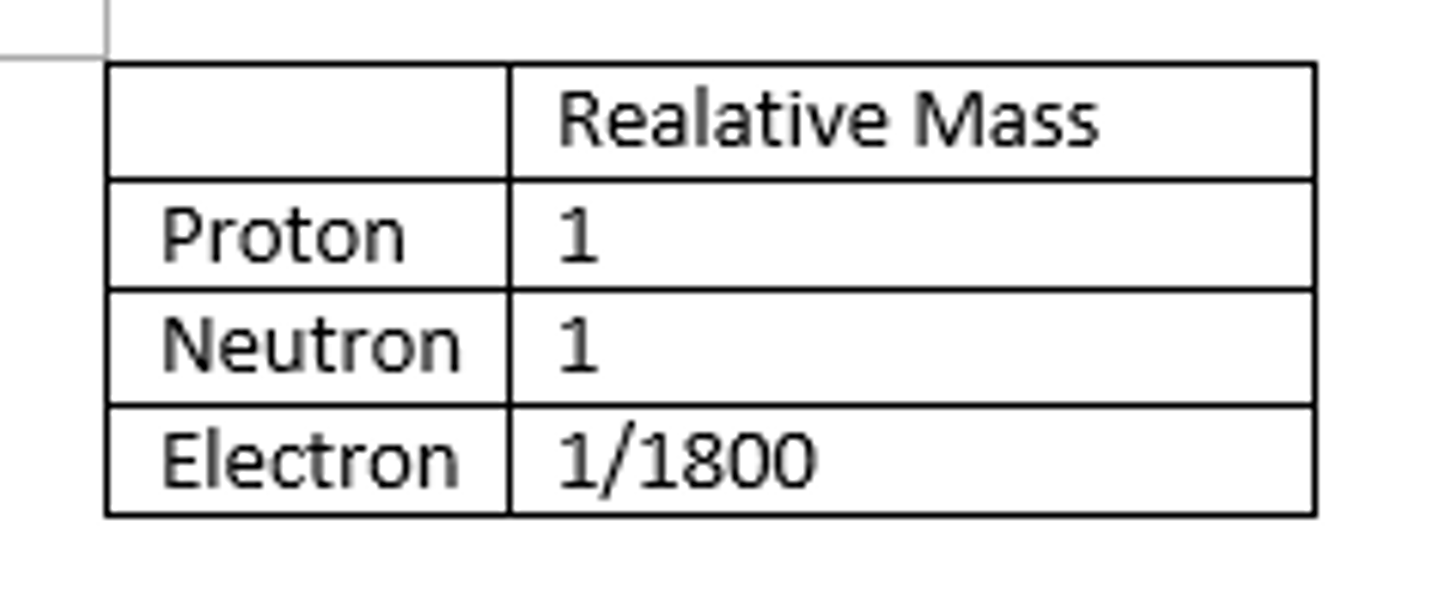
Relative mass of a neutron
about the same as that of a proton
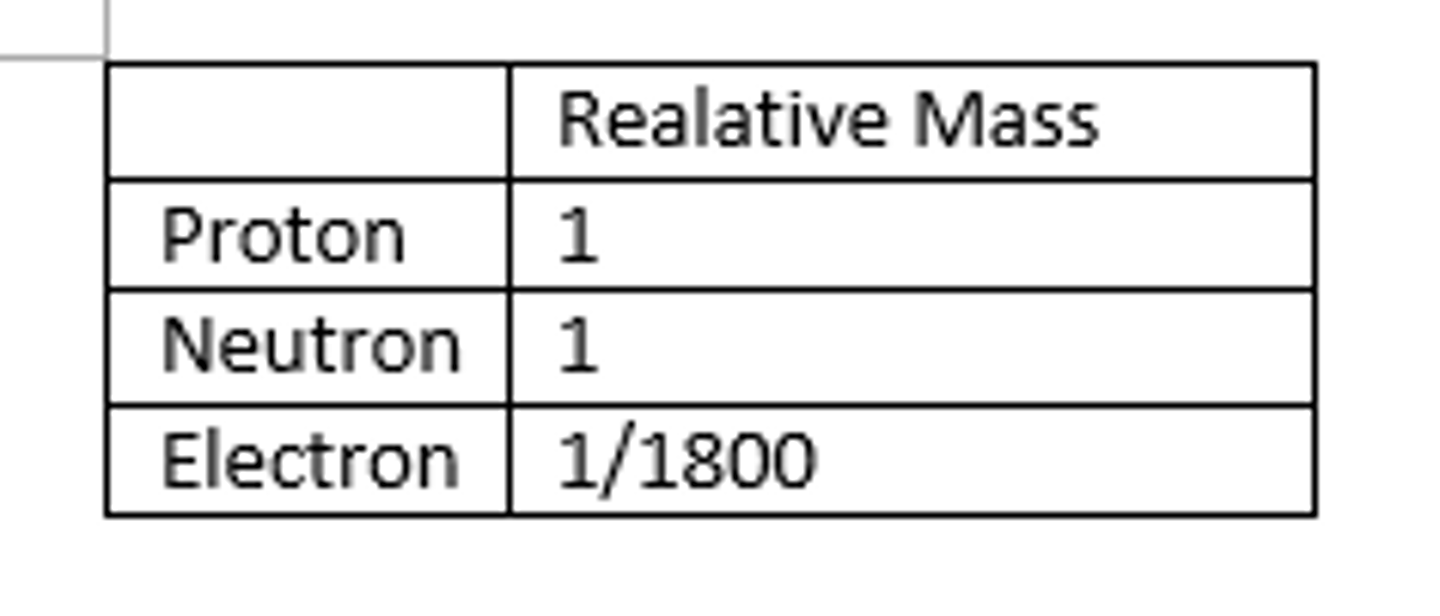
Relative mass of an electron
1/1840
Relative charge of a proton
+1
Relative charge of a neutron
0
Relative charge of an electron
-1
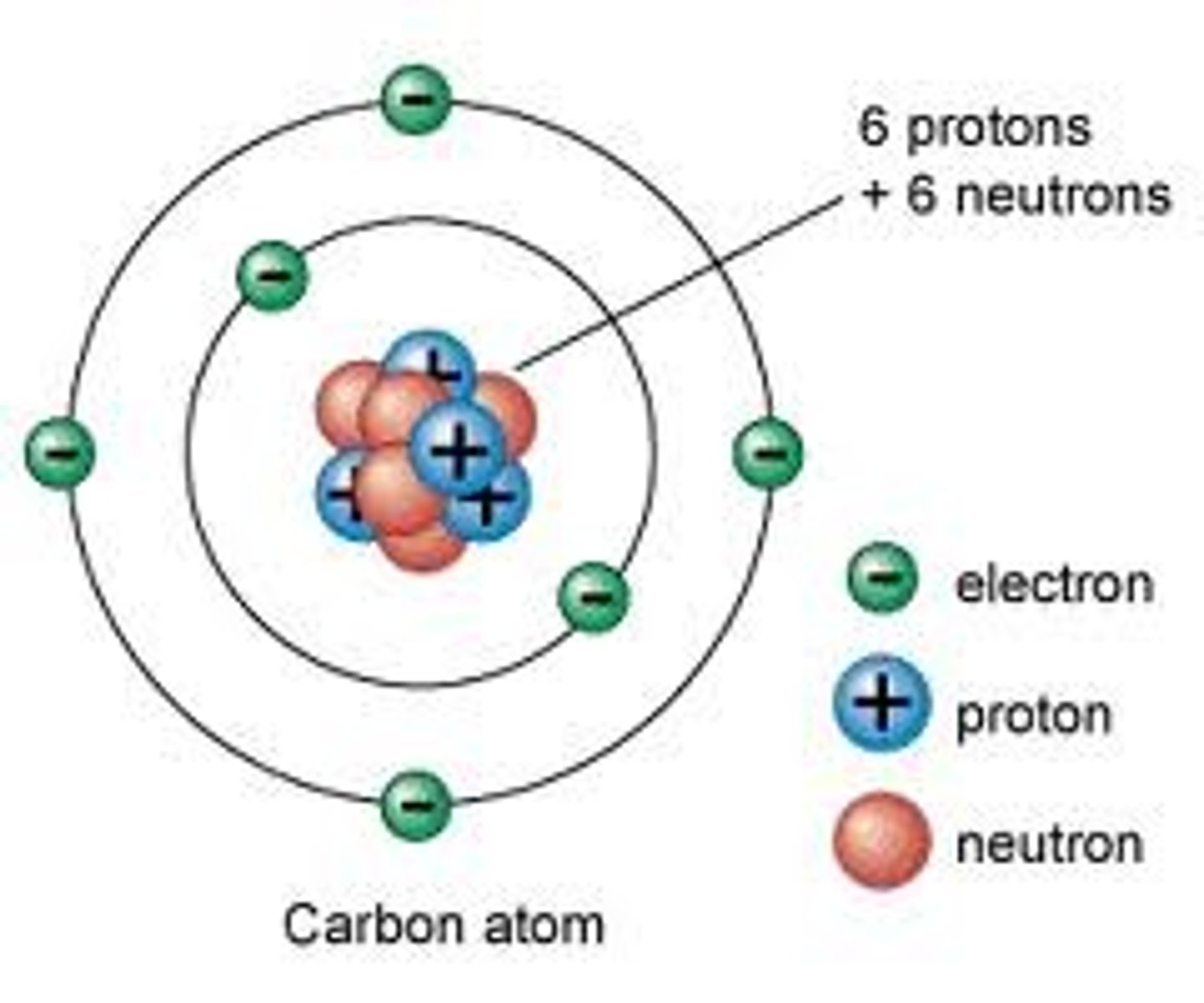
Neutral atoms
same number of positive protons as negative electrons
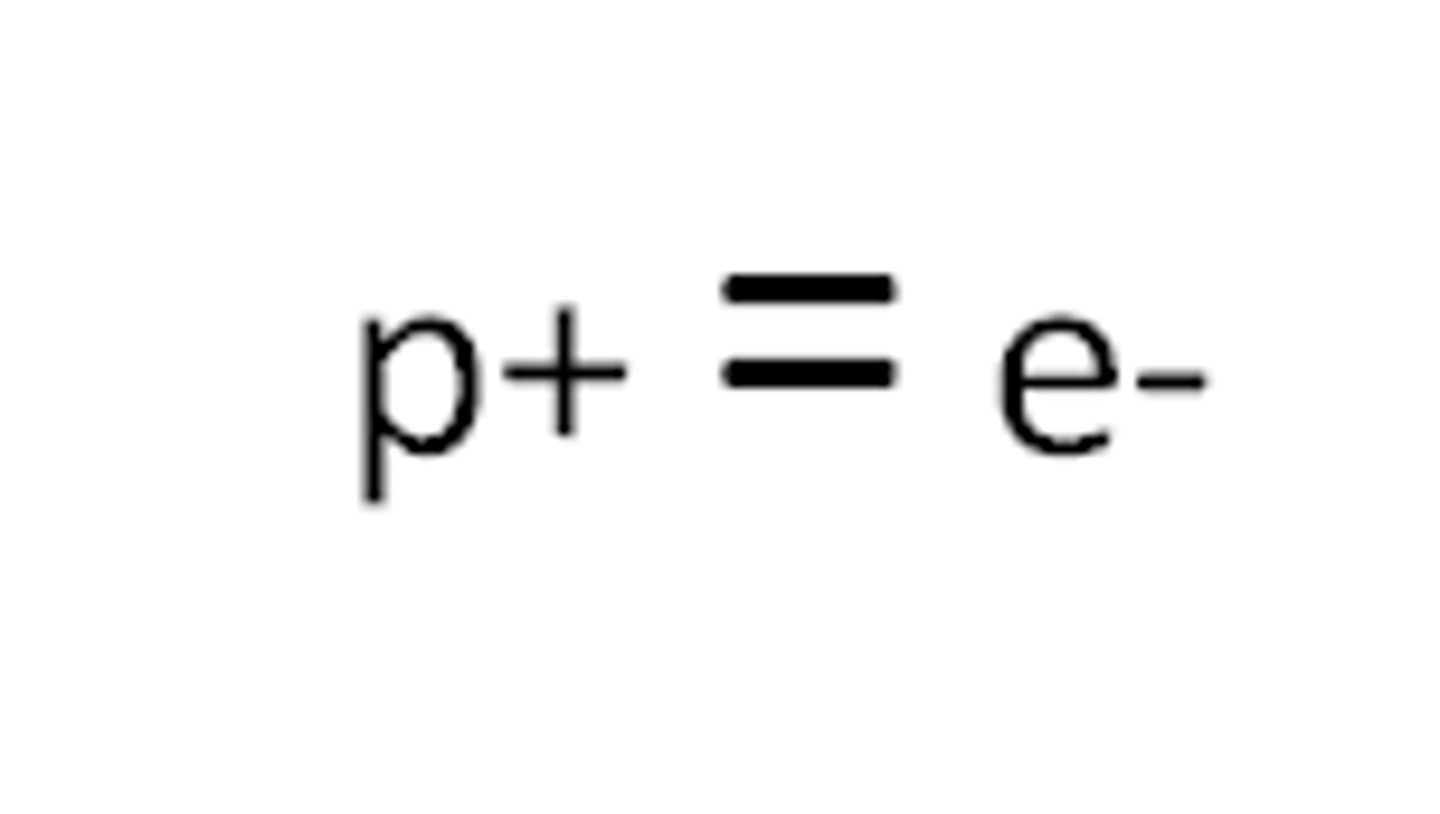
Atomic (proton) number
the number of protons in the nucleus of an atom
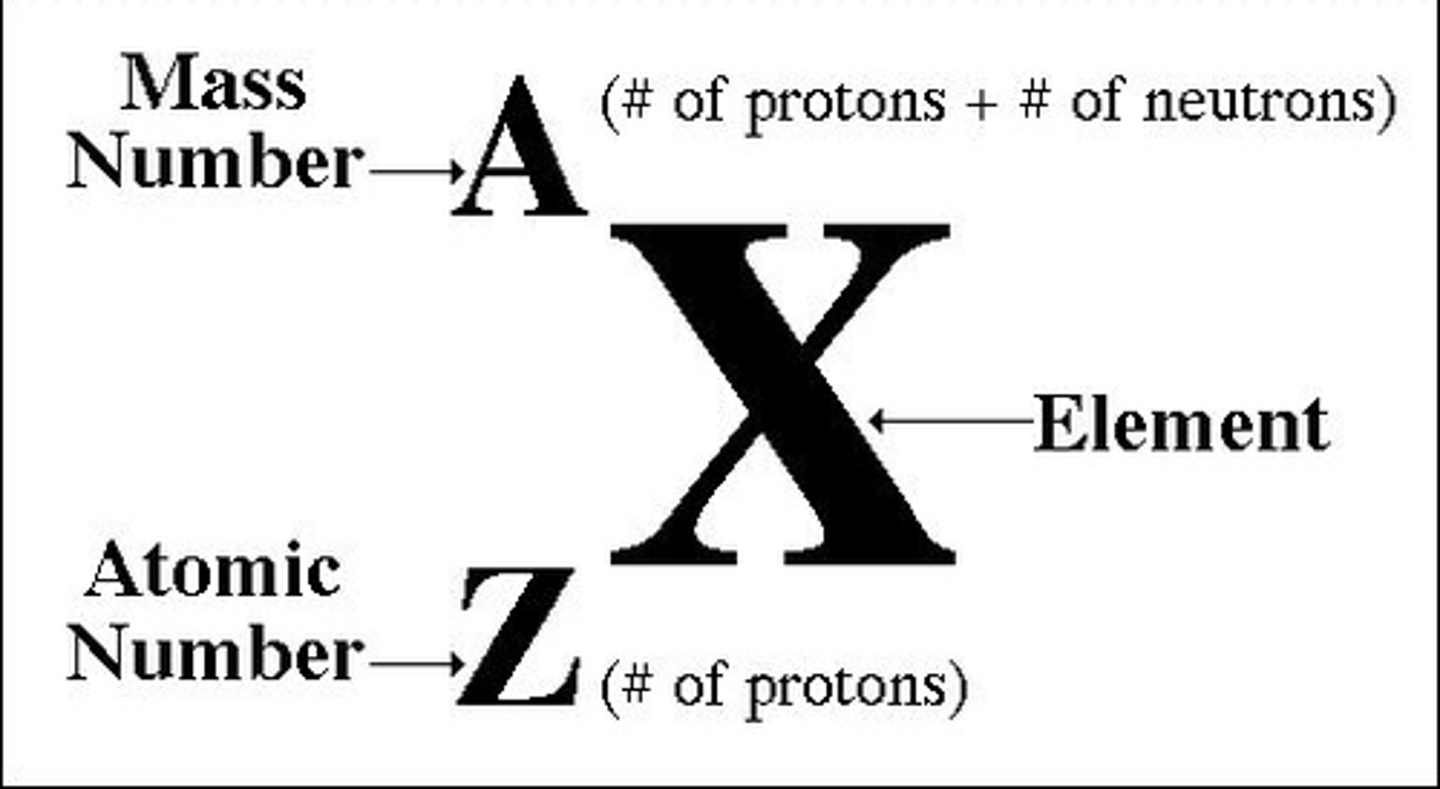
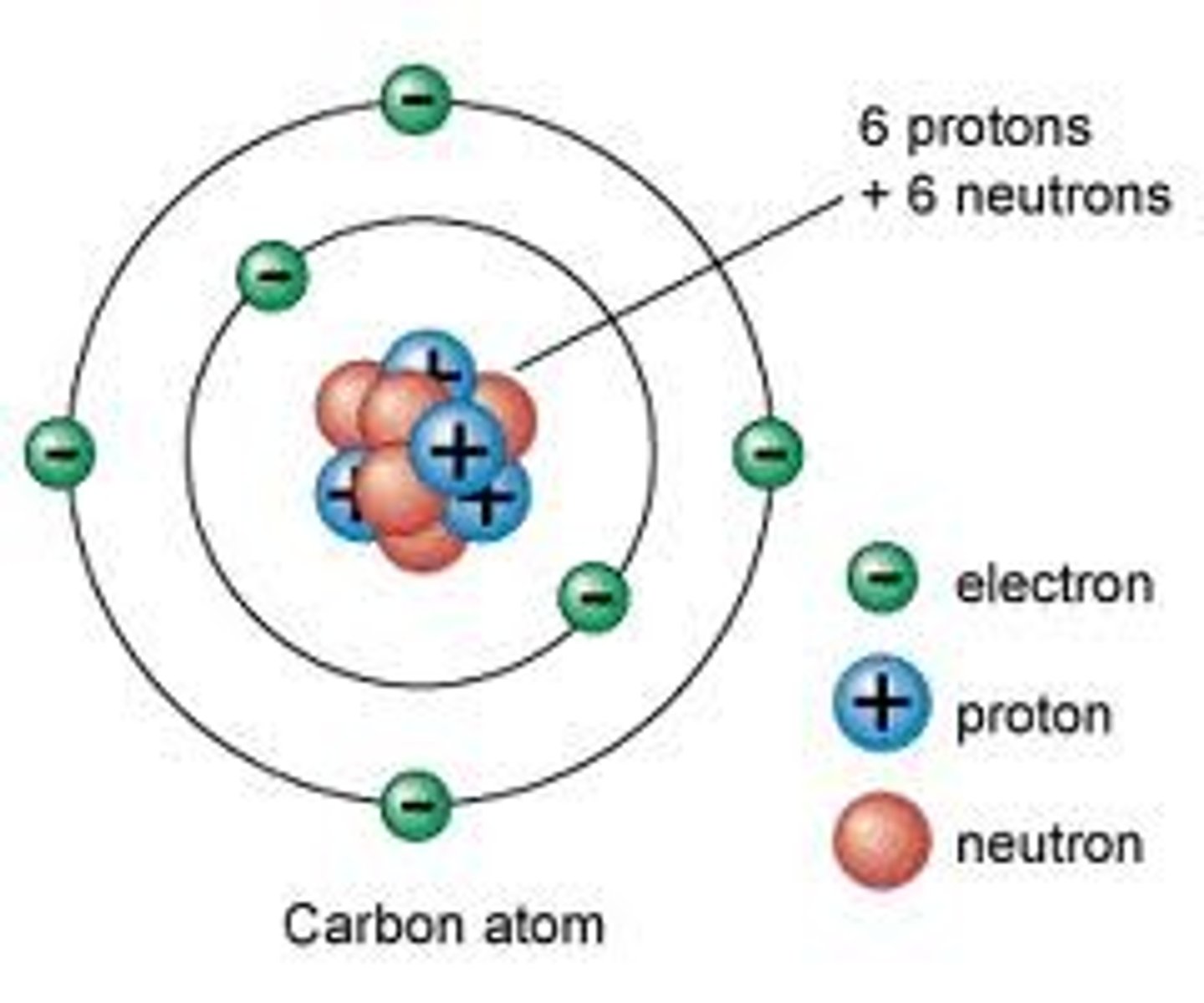
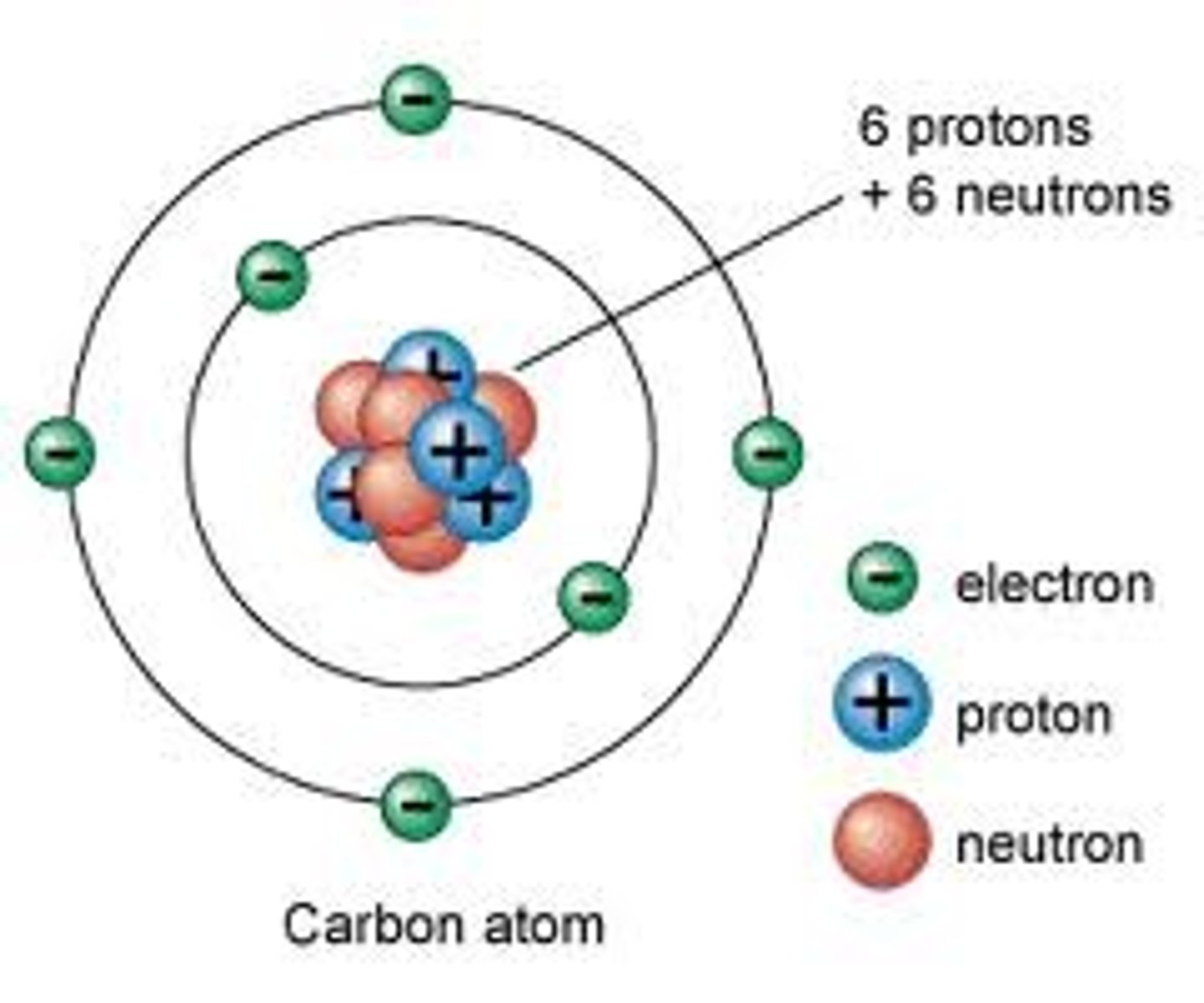
Subatomic particles in the nucleus
protons and neutrons
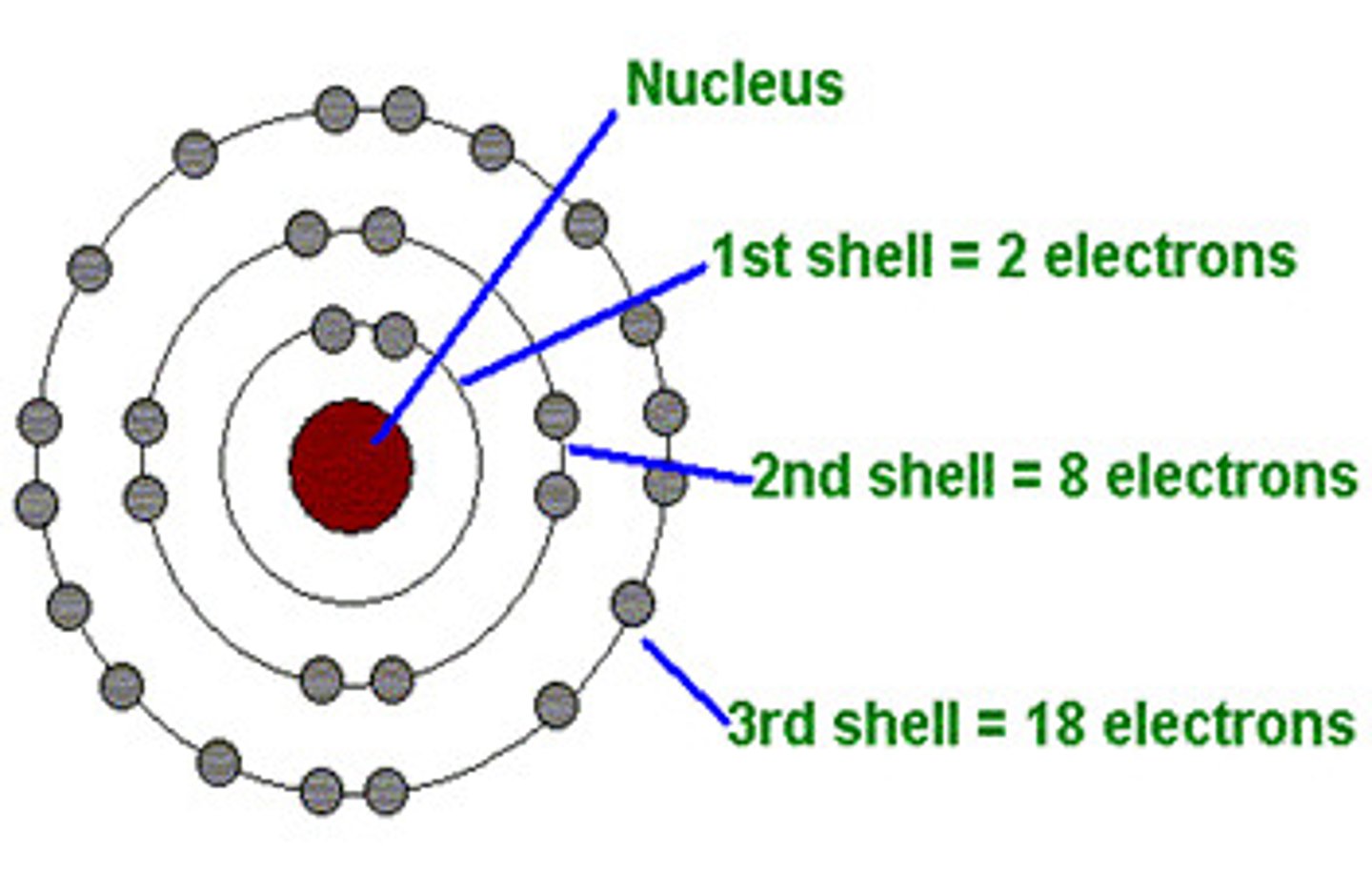
Energy Levels/Electron Shells
The region surrounding the nucleus where electrons orbit the nucleus.
Proton
A subatomic particle that has a positive charge, defines the type of atom and that is found in the nucleus
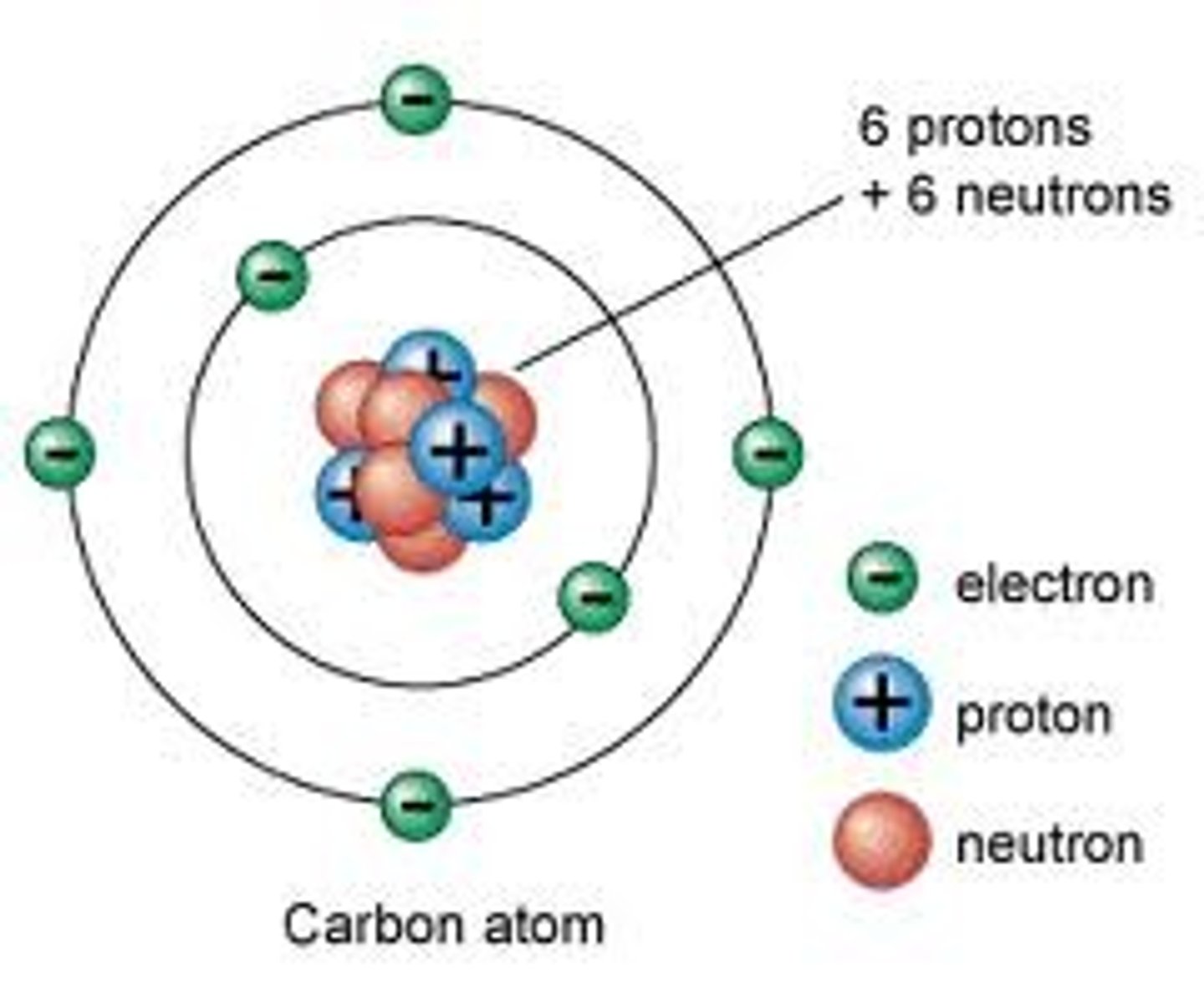
Neutron
A subatomic particle that has no charge and that is found in the nucleus of an atom
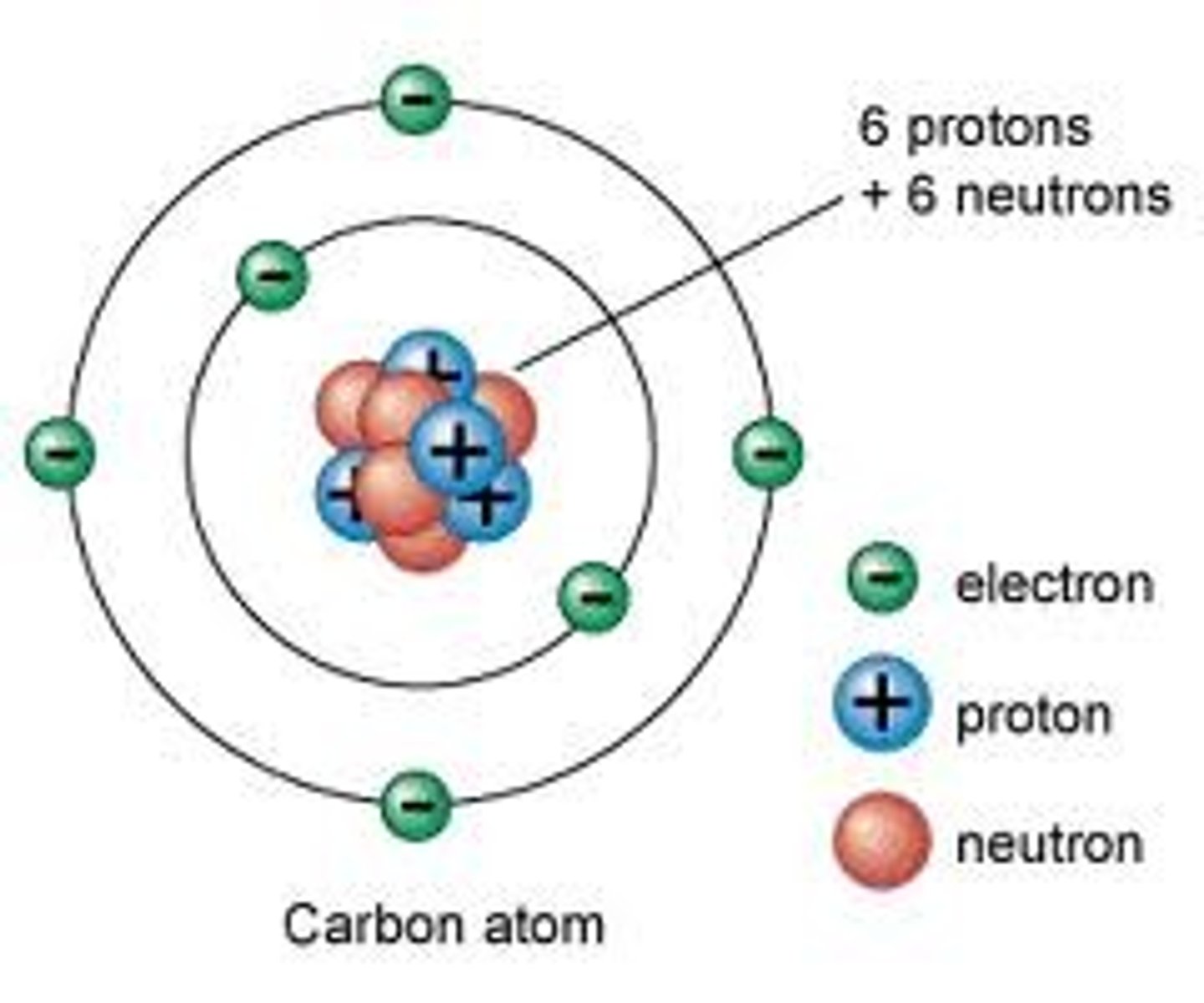
Electron
A subatomic particle that has a negative charge and is found in electron shells, orbiting the nucleus
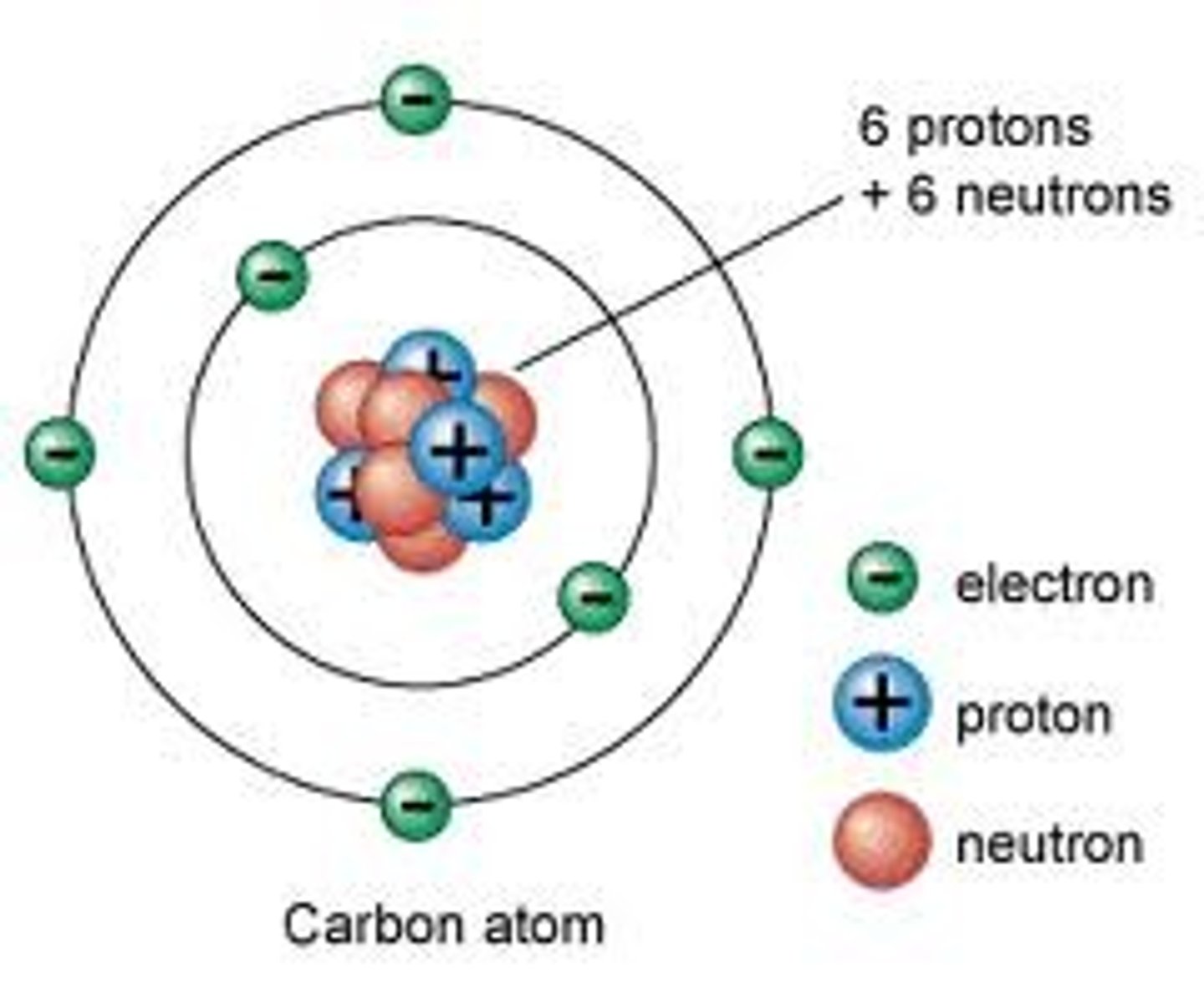
Mass number (atomic mass)
number of protons plus the number of neutrons in an atom of an element
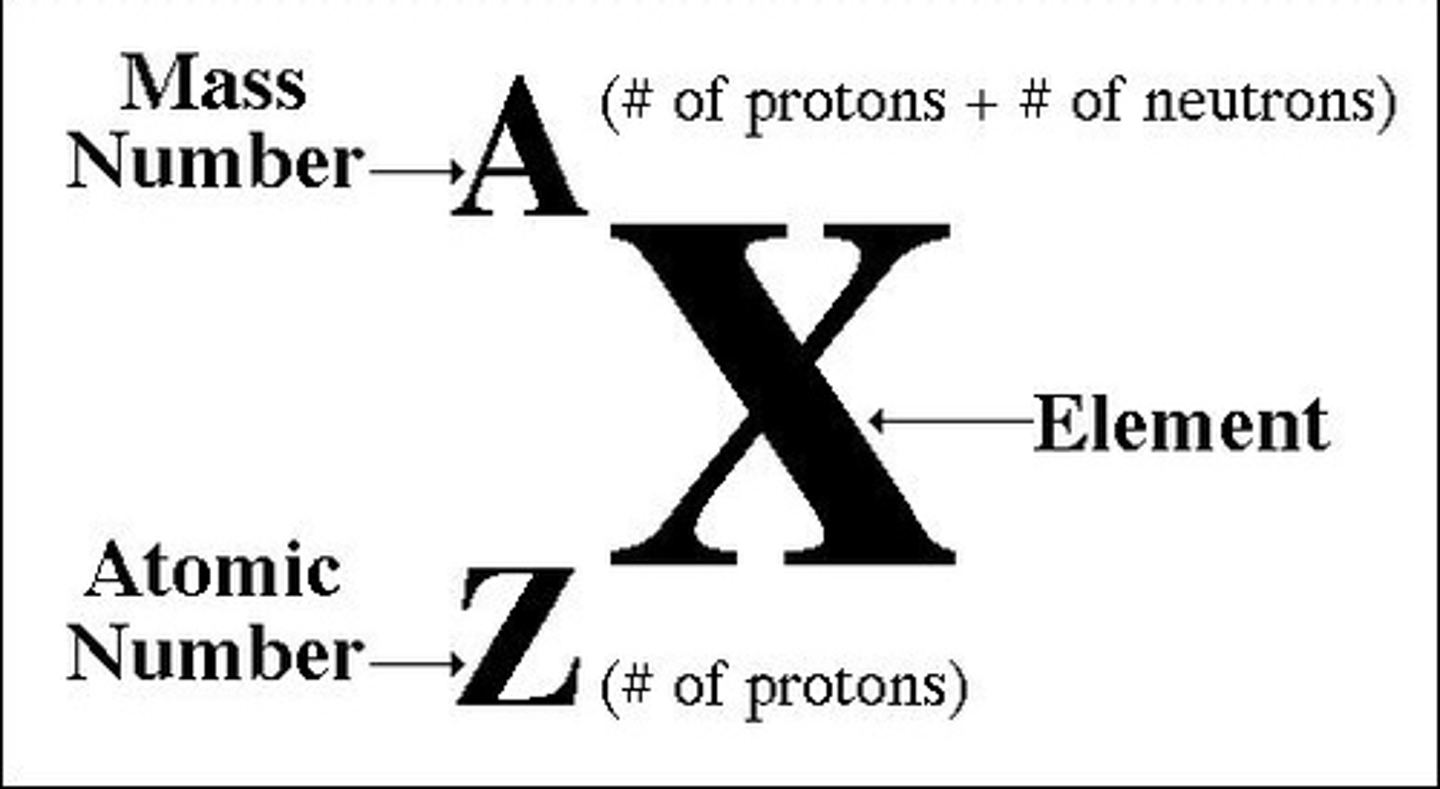
Average atomic radius
0.1 nm (1 x 10^-10 m)

Average radius of an atomic nucleus
10,000 times smaller than an atom (1 x 10^-14 m)
Number of protons in an atom
atomic number
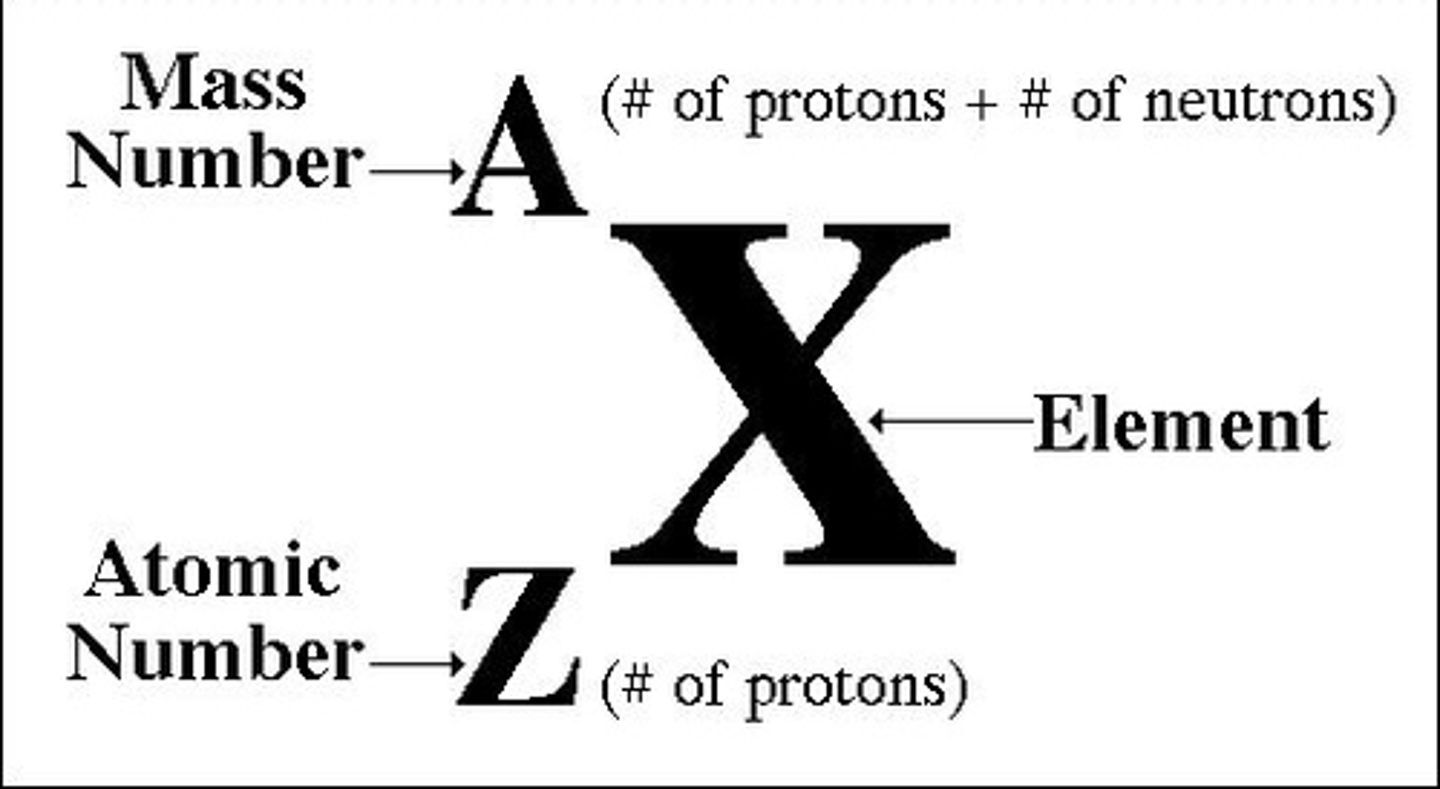
Number of neutrons in atom
mass number - atomic number
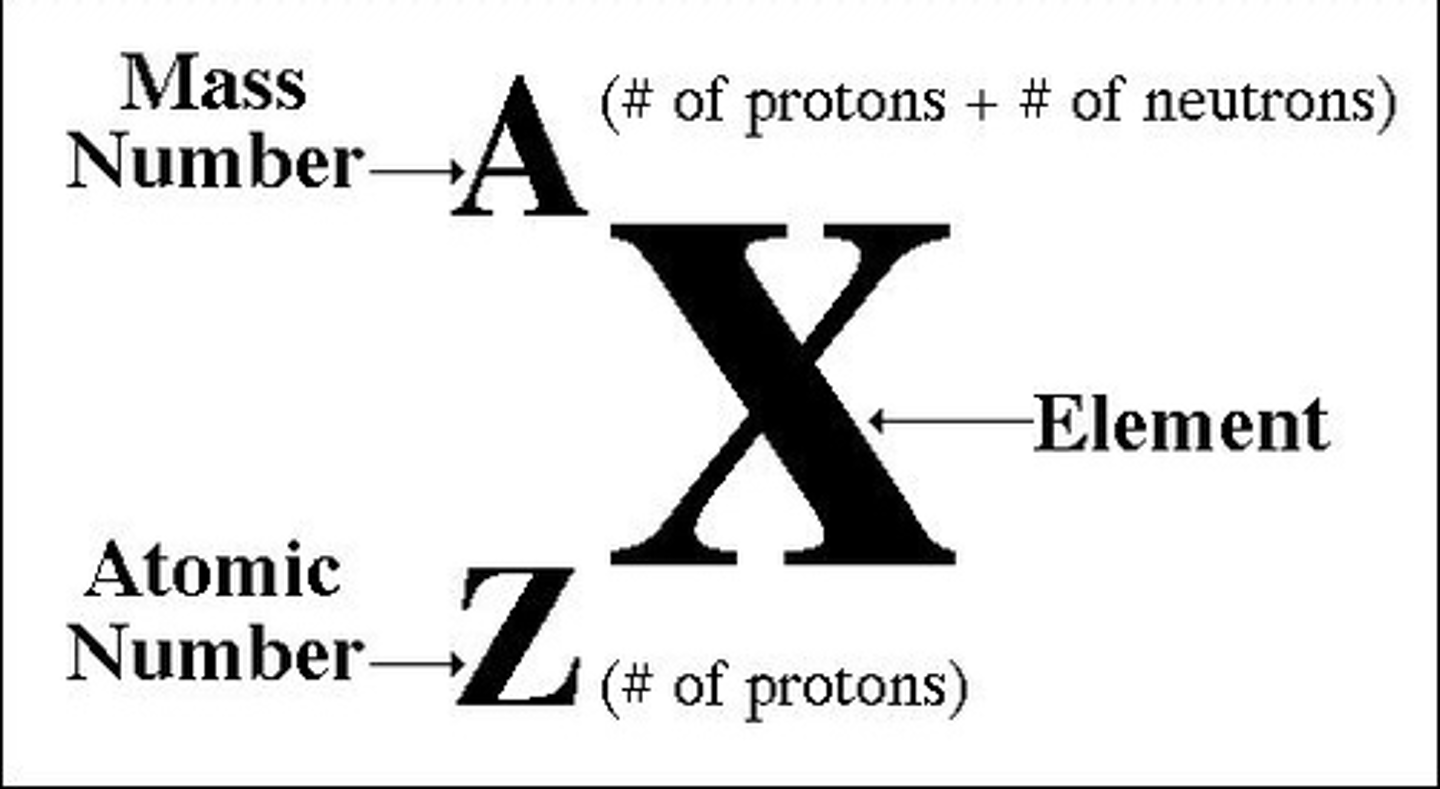
Number of electrons in an atom
is the same as the number of protons
Relative atomic mass
The weighted average mass of an atom of an element compared with one-twelfth of the mass of an atom of carbon-12.