11. The Action Potential Part 3: Ionic Basis of the Action Potential
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
Why is the resting membrane potential around –70 mV, and how does conductance change during an action potential?
Resting Membrane Potential (~ -70 mV):
At rest, the cell is most permeable to K+ (via leak channels)
EK ≈ -80 mV → Vm is closer to EK than ENa (+58 mV) or ECl
So resting potential sits near -70 mV, not exactly -80, because there’s some Na+ and Cl– permeability
During Action Potential:
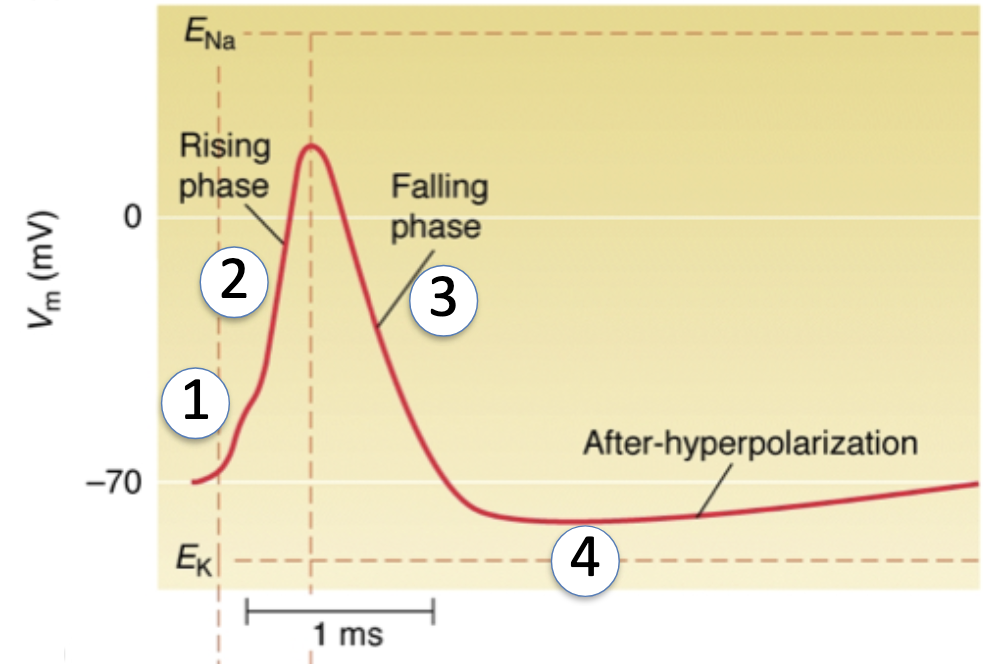
Depolarization (Rising Phase):
Voltage-gated Na+ channels open → ↑ Na+ permeability
Vm moves toward ENa (+58 mV)
As Vm gets closer to ENa, Na+ driving force decreases
Na+ channels begin to inactivate (h-gates close)
Repolarization (Falling Phase):
K+ channels open (n-gates) → ↑ K+ permeability
Na+ channels inactivate → ↓ Na+ permeability
K+ efflux drives Vm back toward EK (-80 mV)
Hyperpolarization:
K+ channels close slowly, so Vm overshoots below resting potential
Eventually, Na+/K+ pump restores ion gradients and returns Vm to -70 mV
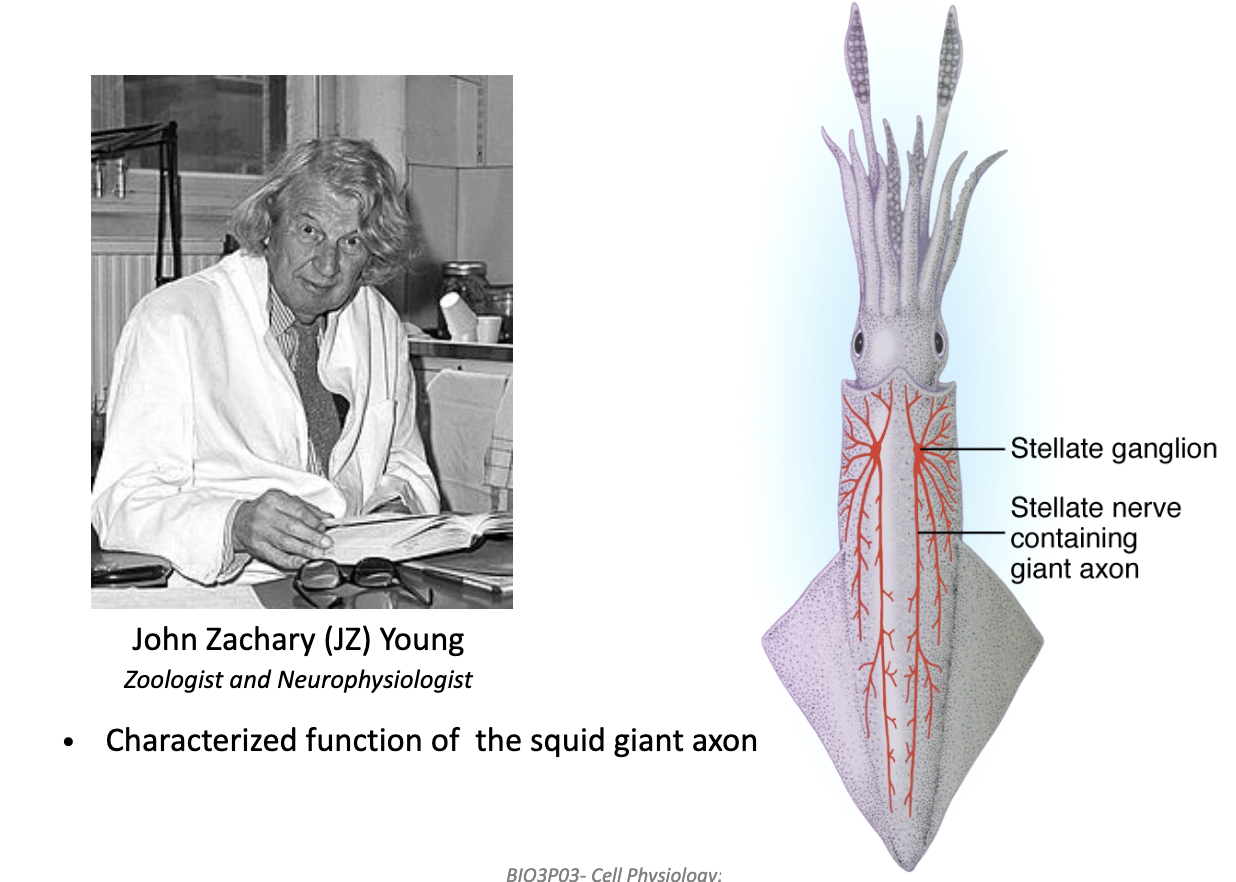
Why was the squid giant axon important for studying action potentials?
Squid use jet propulsion → need fast escape response.
This required a giant axon, making it easy to insert electrodes.
Discovered by John Zachary Young (1936) — led to key AP experiments.
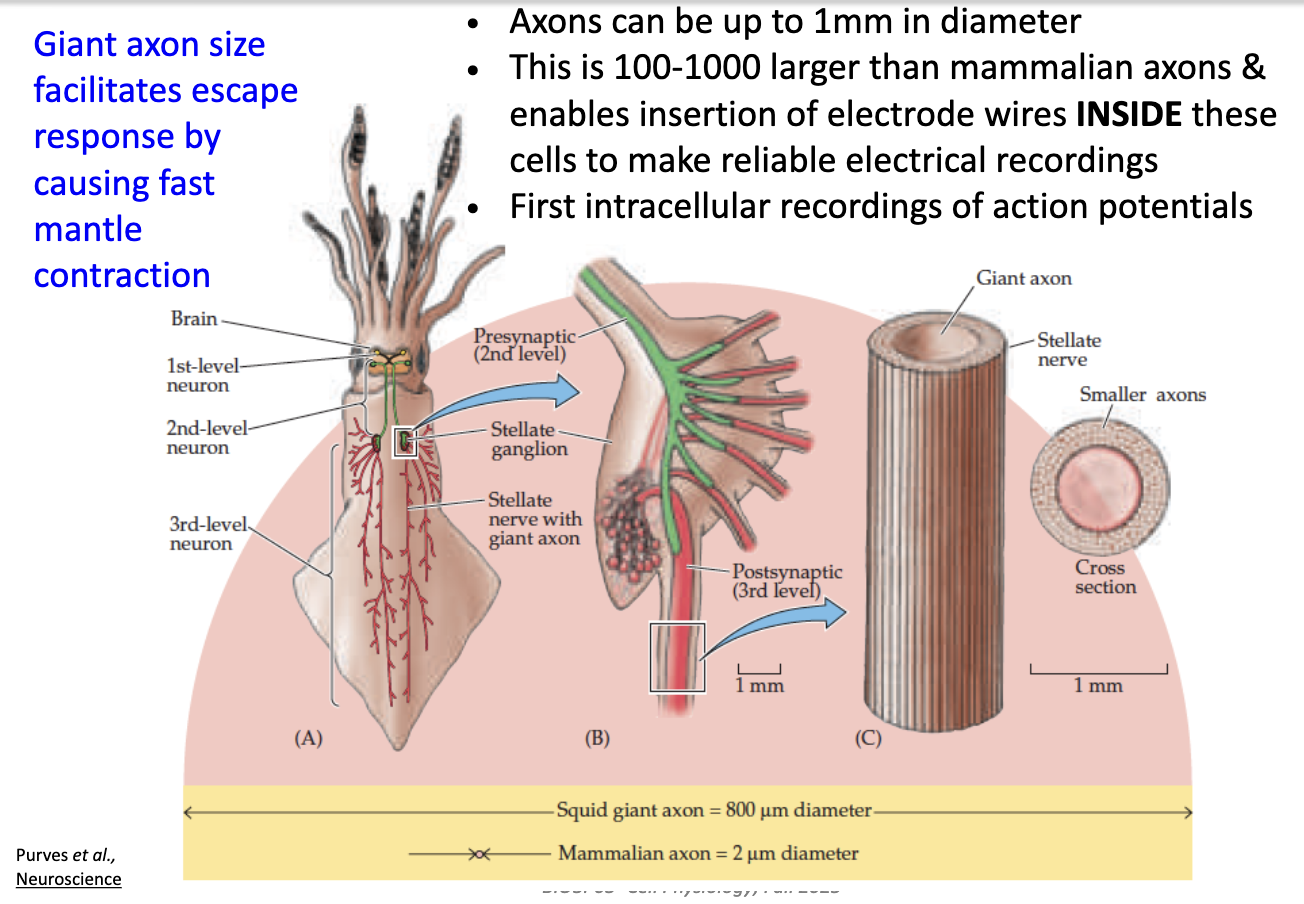
What are the advantages of the squid giant axon for electrophysiology experiments?
Diameter up to 1 mm (100–1000× bigger than mammalian axons).
Large size → can insert electrodes inside.
Allowed first intracellular recordings of action potentials.
Large axon = fast signal conduction → quick muscle contraction.
Large volume + less SA = less resistance
What did Hodgkin and Huxley discover using the squid axon?
Recorded membrane potential (Vm) while axon was in seawater.
Found that during AP, Vm reverses sign (inside becomes positive).
Confirmed that AP = brief polarity reversal across the membrane.
Confirmed after certain threshold AP looks the same.
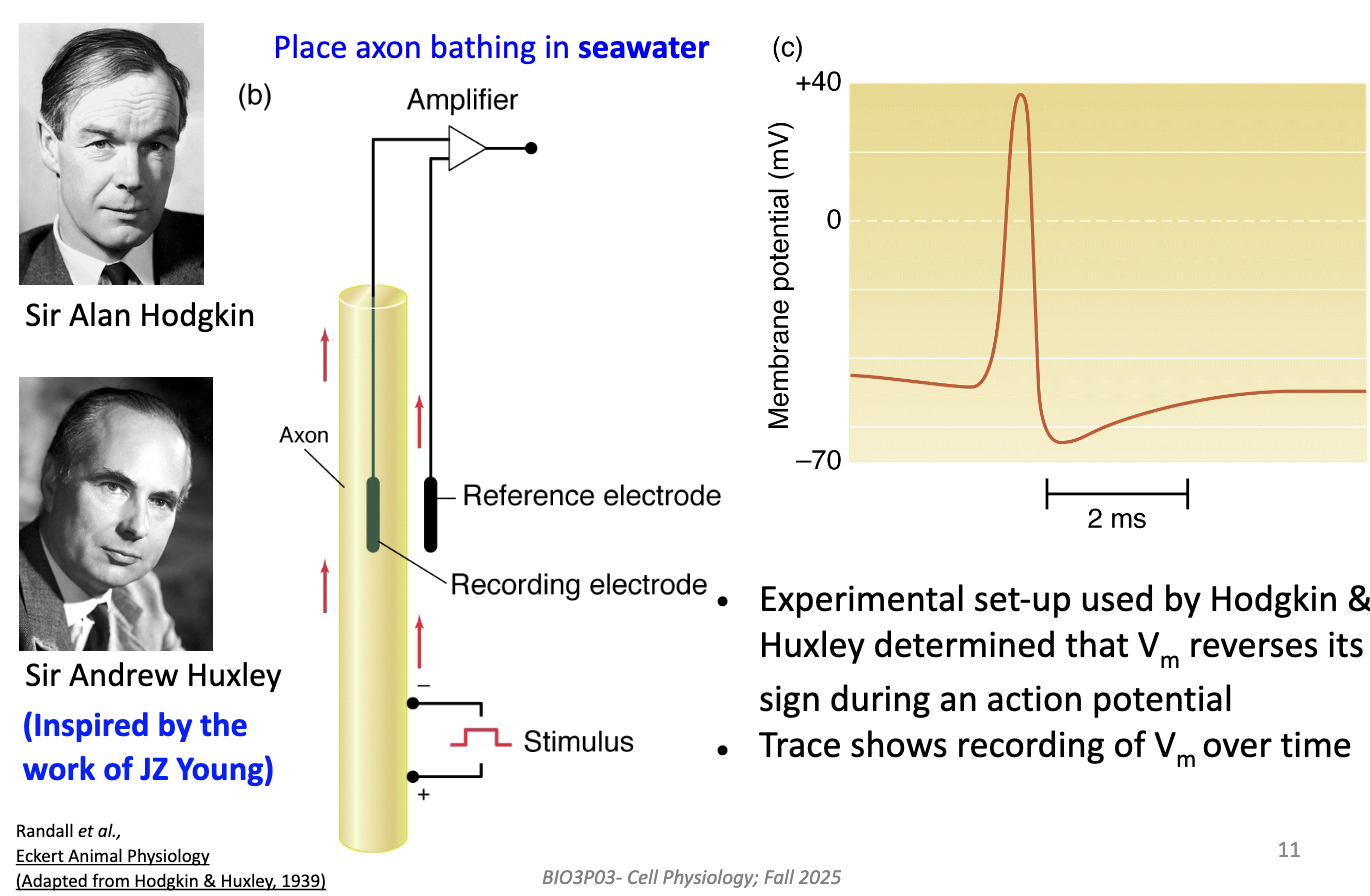
What were Hodgkin and Huxley trying to find out?
What ions carry the current during an AP.
What is the magnitude of these currents and how do they change over time.
Remember: Ion channel activity depends on voltage AND time.
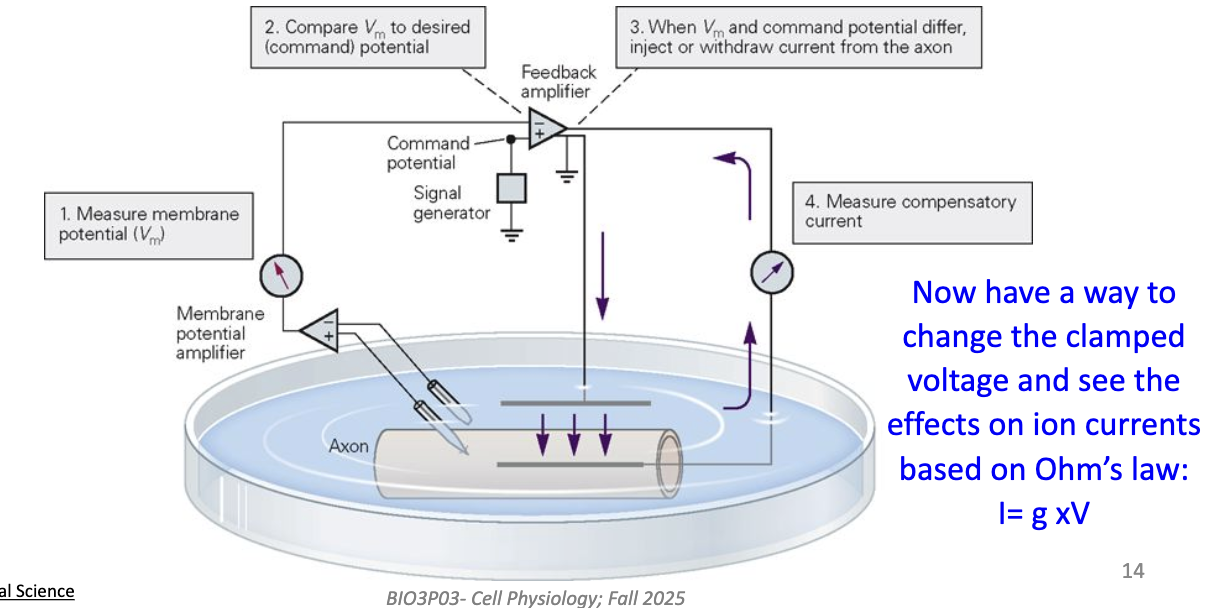
How does the voltage clamp technique (developed by Kenneth Cole) measure ion conductance?
Uses two pairs of electrodes:
One measures Vm
One passes current to hold Vm constant
The amplifier compares recorded Vm to the command voltage set by experimenter.
If Vm differs → current is injected to keep Vm constant.
This lets us measure ionic currents at specific voltages (using Ohm’s law: current = conductance × voltage).
Shows how ion flow changes with voltage and time.
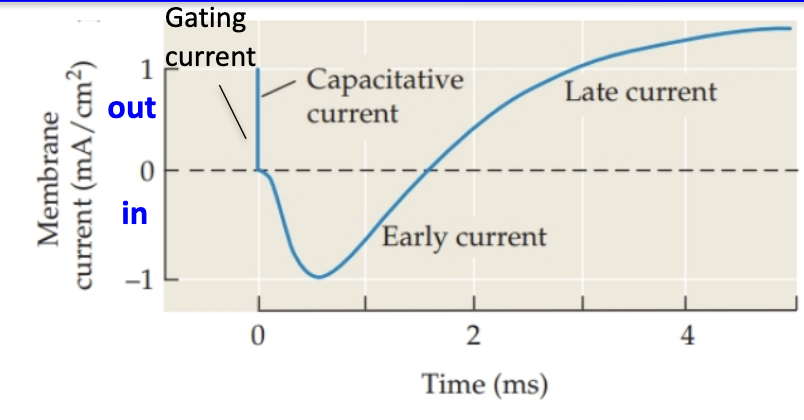
What happens during a voltage-clamp depolarization step?
Vm is “clamped” at a chosen voltage (–9 mV), voltage step was 56 mV
The measured current equals what’s needed to maintain that voltage.
3 phases appear:
Capacitative current: brief sharp positive current
Early inward current: negative current (Na+ entering cell)
Late outward current: positive current (K+ leaving cell)
Negative = current entering cell
Positive = current leaving cell
These match what happens during an actual AP (Na+ in → K+ out).
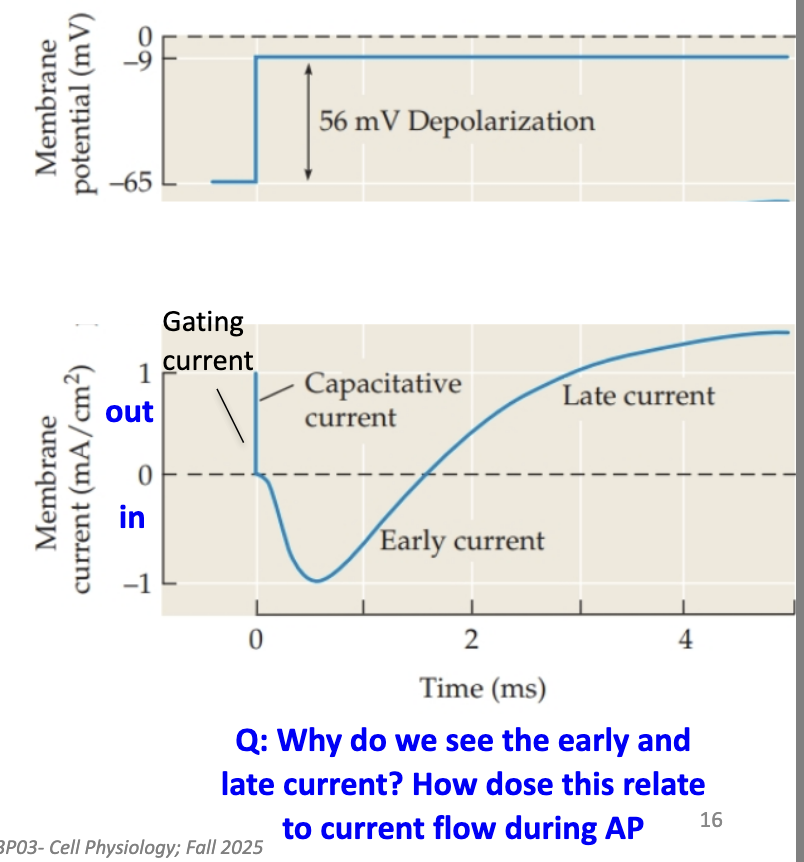
What is gating current and what causes it?
A tiny current caused by movement of charged parts of channel proteins.
Specifically, movement of positively charged S4 segments during channel opening.
Build up of positive charge on inner leaflet repels positive charge on S4 → moves to extracellular side → channel opens
Reflects voltage sensor movement, not ion movement.
What is the capacitative current seen during depolarization?
Occurs as charges move to change the voltage across the membrane capacitor.
Very brief/transient→ only happens while voltage is changing.
Once voltage is steady (clamped), capacitative current stops.
Represents charge displacement, not ion flow.
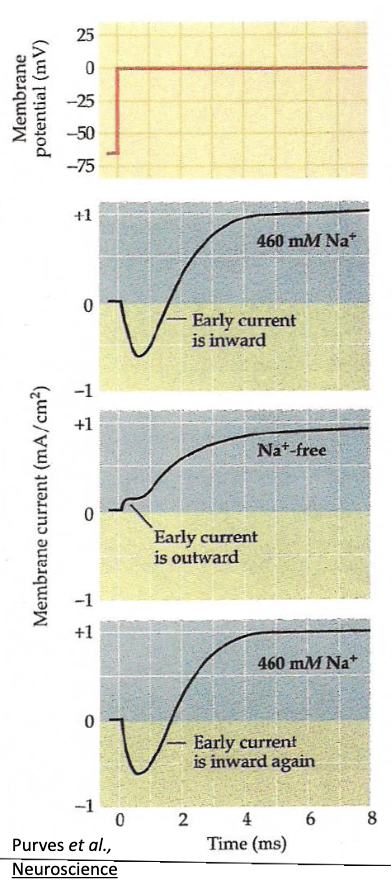
How did Hodgkin & Huxley confirm that Na+ carries the early inward current?
In seawater → early inward (Na+) and late outward (K+) currents observed.
Replace Na+ with choline → inward current disappears, only K+ outward current remains.
Return to seawater → inward current returns → confirms Na+ entry causes it.
Na+ does not contribute to delayed outward current.
If Vm is clamped to ENa, the inward current = 0, since no driving force for Na+.
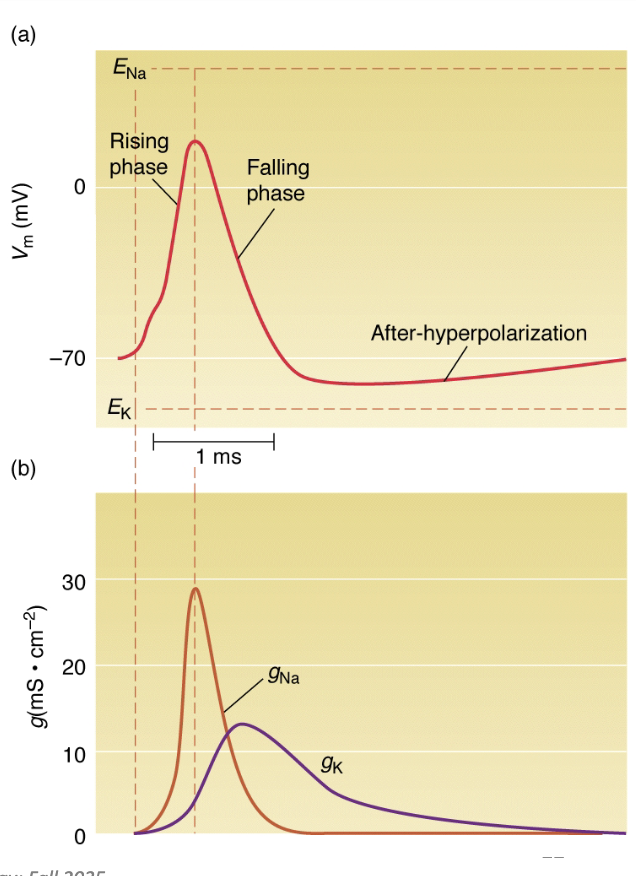
What did Hodgkin and Huxley discover about Na+ and K+ conductance changes?
By applying different voltage steps, they measured gNa and gK at each.
gNa: rises rapidly then falls (inactivation).
gK: rises slowly and stays elevated.
Conductances depend on voltage and time — they change dynamically during AP.
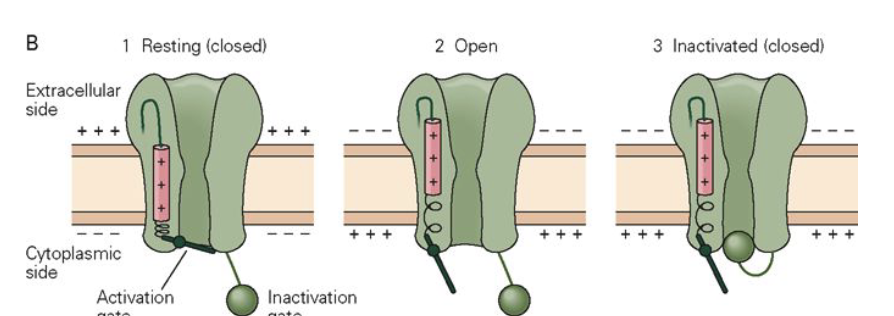
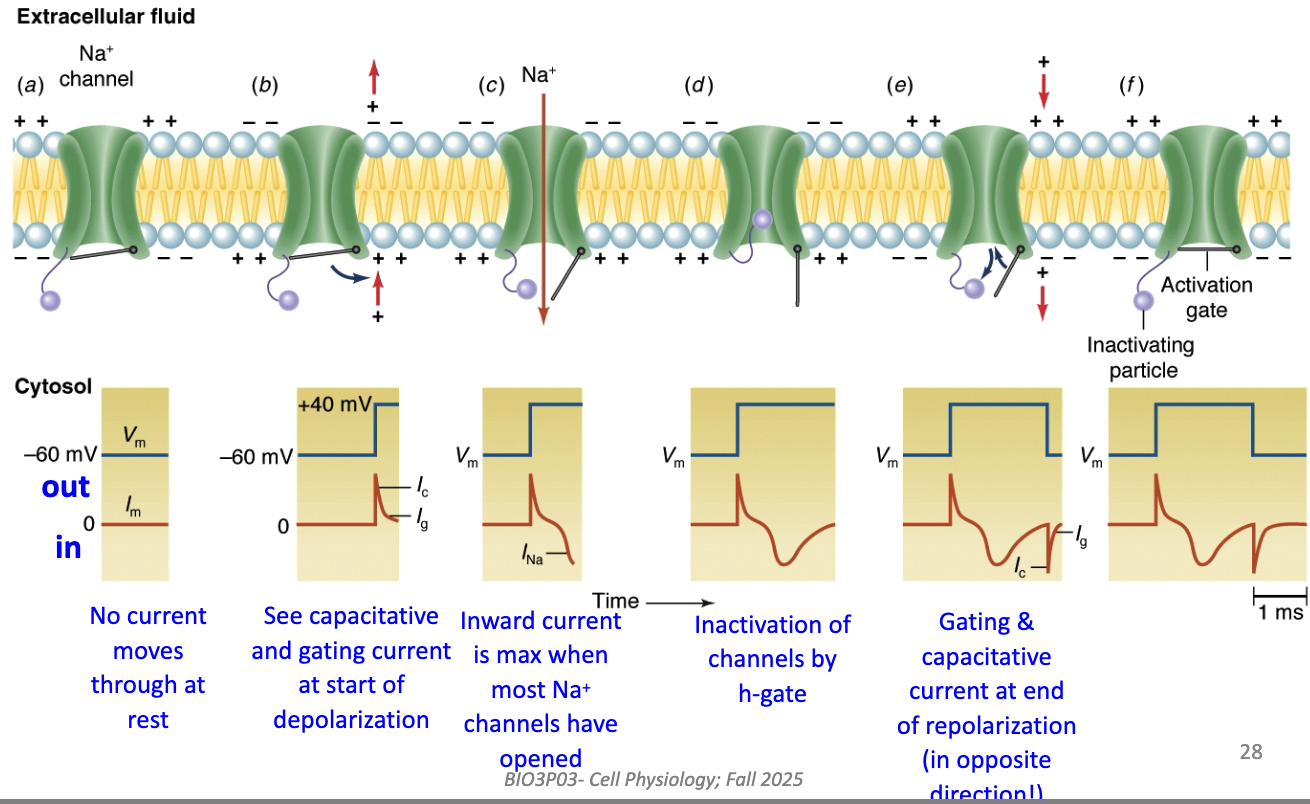
What structural change occurs when Na+ channels open during depolarization?
Voltage step → S4 sensors move outward → m-gate opens.
Na+ flows inward → depolarization.
Over time → h-gate closes, stopping current (inactivation).
This matches observed inward and outward current traces.
How do structural channel changes correspond to ionic currents?
At rest: channels closed → no current.
Start of depolarization: gating + capacitative currents (small, brief).
Peak depolarization: max inward Na+ current (many channels open).
Later: Na+ channels inactivate (h-gate closes) → inward current stops.
End of repolarization: opposite gating & capacitative currents occur.
Understand capacitance for test.
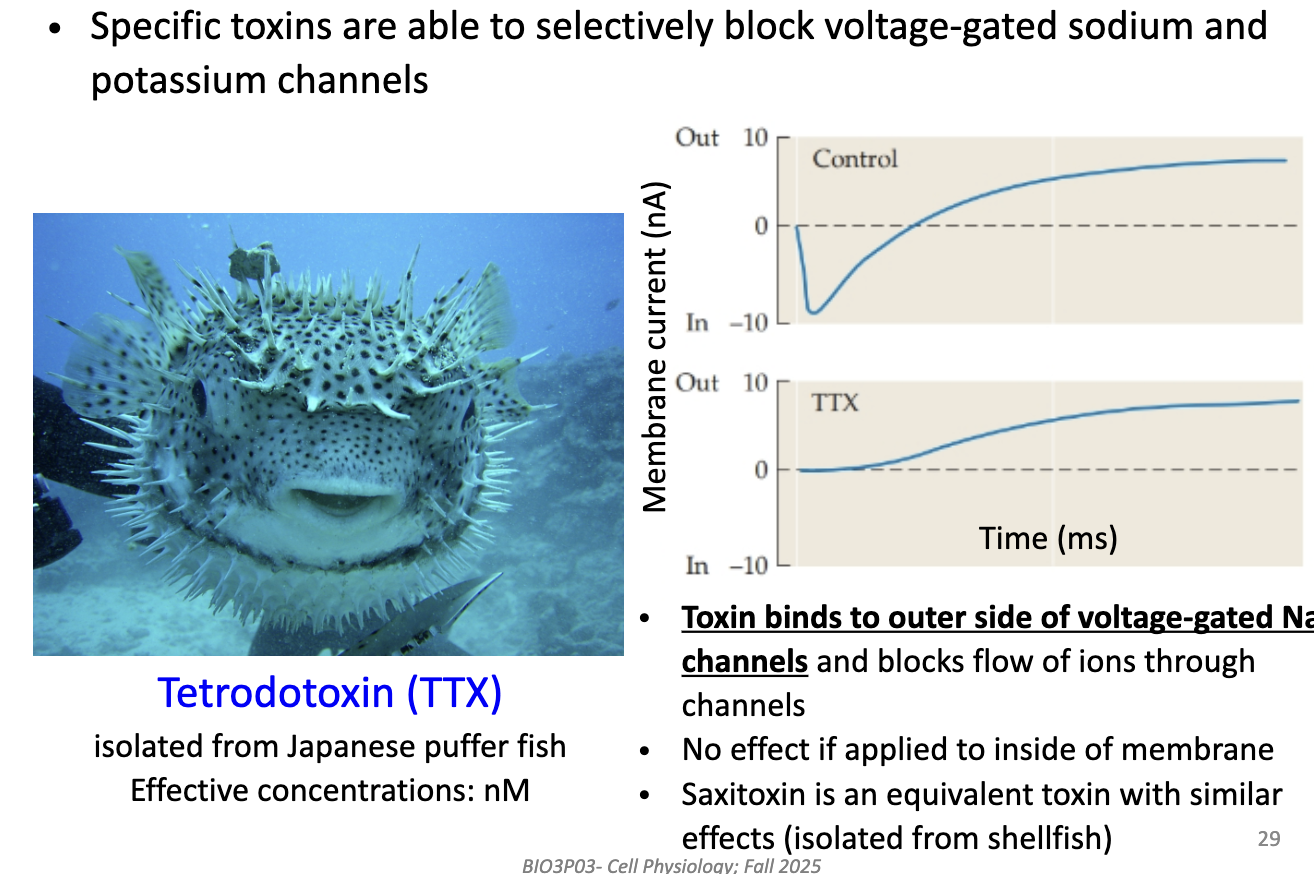
How does Tetrodotoxin (TTX) help separate Na+ and K+ currents?
TTX (from puffer fish) binds to outer side of voltage-gated Na+ channels.
Blocks Na+ flow → removes inward current.
Has no effect if applied inside the cell.
Equivalent toxin: saxitoxin (STX) from shellfish.
Used to isolate K+ current by blocking Na+ channels.
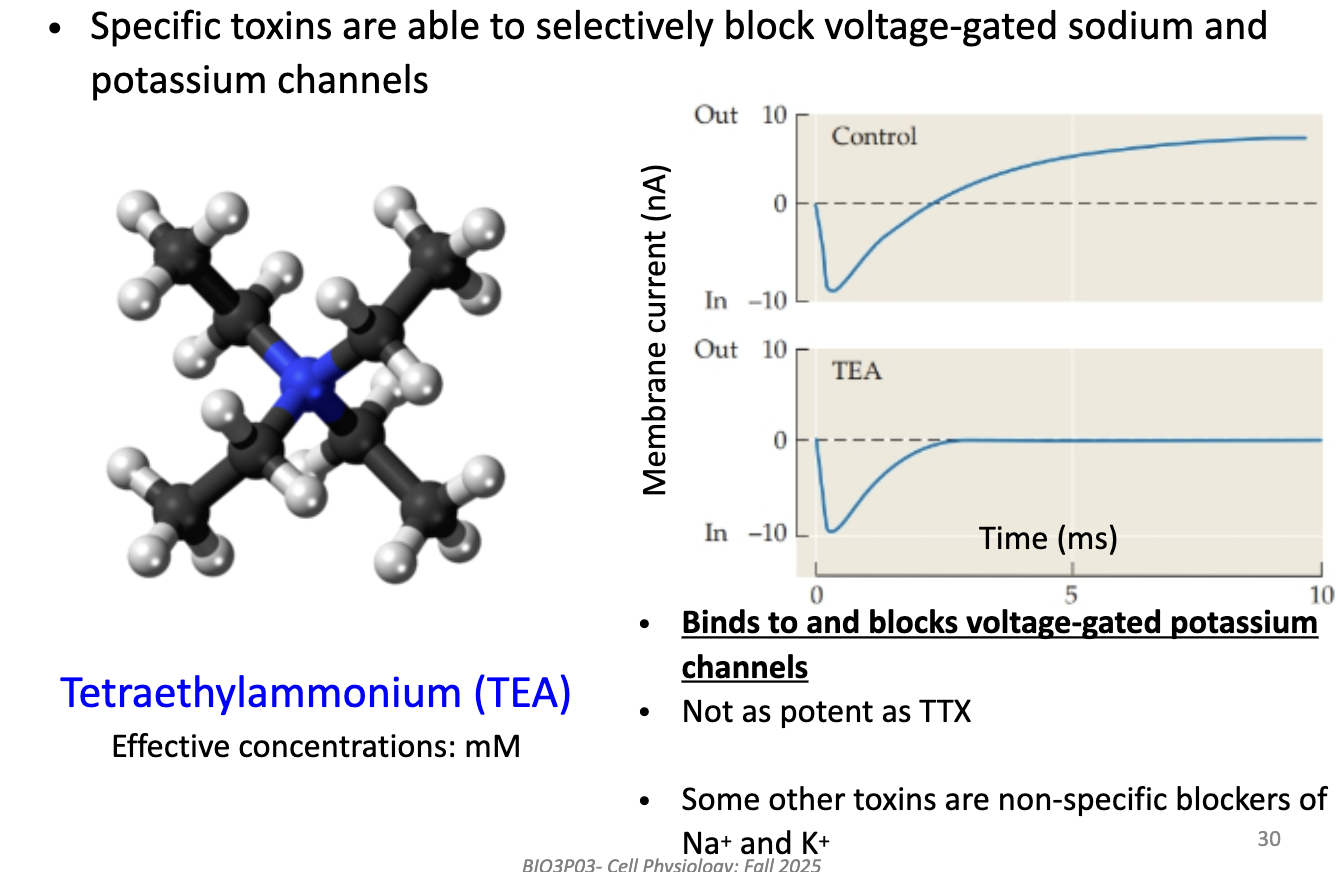
How does Tetraethylammonium (TEA) help study K+ currents?
TEA blocks voltage-gated K+ channels (binds on outside).
Used in mM concentrations (less potent than TTX).
Leaves Na+ current intact → isolates Na+ current recordings.
Some other toxins block both Na+ and K+ (non-specific).
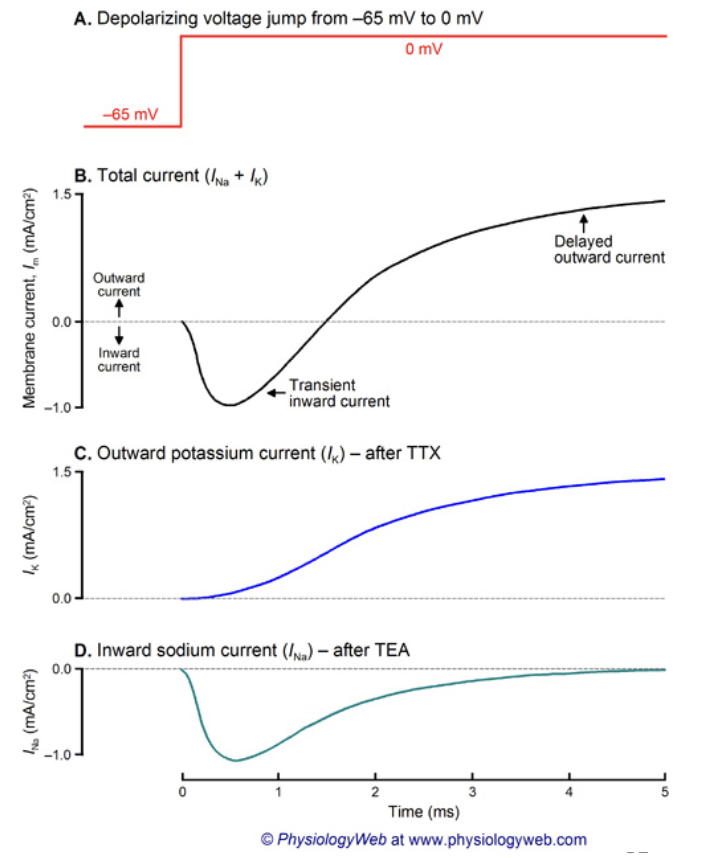
What information does voltage clamp data provide about ion channels?
Reveals macroscopic currents (sum of all channel activity).
Can test drug effects on Na+ and K+ currents.
But can’t tell whether a drug:
Blocks channels directly, or
Changes channel open time or gating.
So clamp data shows total current, not single-channel behavior.
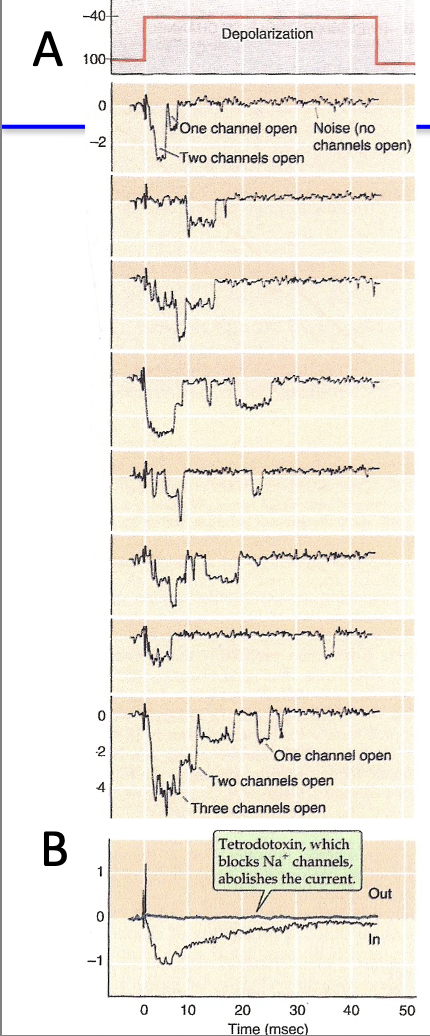
How does patch clamp improve on voltage clamp recordings?
Patch clamp measures currents through single ion channels.
Shows:
Each opening produces a tiny, discrete current step.
When multiple channels open → currents add up → looks like voltage clamp trace.
Openings are random (stochastic), but probability is highest just after depolarization (~4 ms).
TTX can be used to confirm Na+ channel identity because theres no inward current.
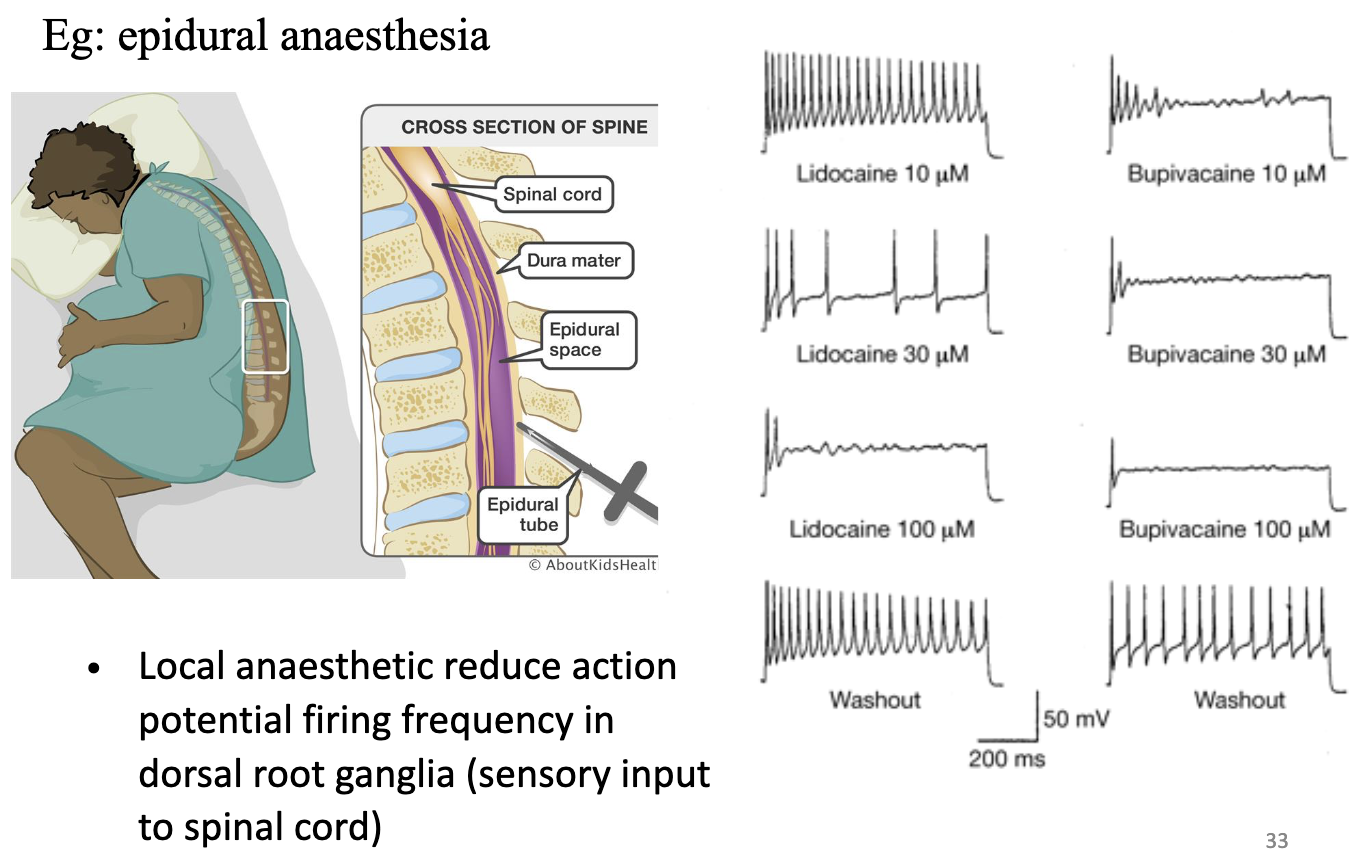
Why is it important to understand the ionic basis of the action potential?
Explains how drugs (e.g ., anesthetics) affect neural activity.
Local anesthetics (like epidural drugs) block Na+ channels → reduce AP firing.
Washout makes it reversible.
Reduce AP firing frequency in dorsal root ganglia (sensory input to spinal cord)