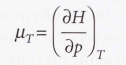Chapter 2 - The First Law
0.0(0)
Card Sorting
1/55
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
56 Terms
1
New cards
Thermodynamics
The study of the transformations of energy
2
New cards
Open system
Can exchange matter and energy with its surroundings
3
New cards
Closed system
can exchange energy with its surroundings, but not matter
4
New cards
Isolated system
No matter or energy is exchanged with the surroundings
5
New cards
Work
Motion against an opposing force
6
New cards
Energy
Capacity to do work
7
New cards
Heat
Energy change due to a temperature change between the system and its surroundings
8
New cards
Exothermic process
Process that releases energy as heat into its surroundings
9
New cards
Endothermic process
Process in which energy is obtained from the surroundings
10
New cards
Thermal motion
Disorderly motion of molecules
11
New cards
Disorderly motion of molecules
It occurs due to heating
12
New cards
Internal energy
The total energy of a system, represented by U.
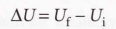
13
New cards
State function
A function where its value only depends on the current state of the system
14
New cards
First Law of Thermodynamics
The internal energy of an isolated system is constant
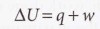
15
New cards
Expansion work
Work arising from a change in volume, whether it is positive (expansion) or negative (compression)
16
New cards
general expression for work
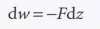
17
New cards
Free expansion
Expansion against zero opposing force, when Pex = 0
18
New cards
Expansion against constant pressure
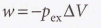
19
New cards
Reversible change
Change that can be reversed by any modification of a variable
20
New cards
Heat transactions
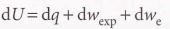
21
New cards
Calorimetry
Study of heat transfer during chemical and physical processes
22
New cards
Calorimeter
Devise used for measuring energy transferred as heat
23
New cards
Adiabatic bomb calorimeter
Used to measure internal energy
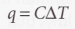
24
New cards
Heat capacity
The slope of the tangent to the curve at any temperature.
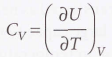
25
New cards
Molar heat capacity at constant volume
The heat capacity per mole of material
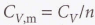
26
New cards
Specific heat capacity
The heat capacity of the sample divided by the mass
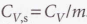
27
New cards
Enthalpy (H)
State function
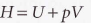
28
New cards
Isobaric calorimeter
A calorimeter used for studying processes at constant pressure
29
New cards
Adiabatic flame calorimeter
Used to measure the temperature change in a combustion reaction
30
New cards
Heat capacity at constant pressure
The slope of the tangent to a plot of enthalpy against temperature at constant pressure
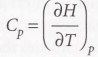
31
New cards
Molar heat capacity at constant pressure
Heat capacity per mole of the material, its an intensive property
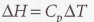
32
New cards
Thermochemistry
Study of the energy transferred as heat during a chemical reaction
33
New cards
Standard enthalpy change
The change in enthalpy for a process in which the initial and final substances are in their standard states
34
New cards
Standard state
Pure form of a substance at a specified temperature and 1 bar
35
New cards
Standard enthalpy of transition
Standard enthalpy change that accompanies a change of physical state
36
New cards
Standard enthalpy of vaporization
Enthalpy change per mole when a pure liquid at 1 bar vaporizes at 1 bar
37
New cards
Standard enthalpy of fusion
Standard enthalpy change accompanying the conversion of a solid to a liquid
38
New cards
Thermochemical equation
A combination of a chemical equation and the enthalpy change
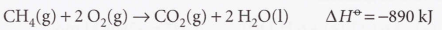
39
New cards
Standard reaction enthalpy
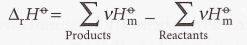
40
New cards
Standard enthalpy of combustion
Standard reaction enthalpy for the complete oxidation of an organic compound to CO2 gas and liquid H2O
41
New cards
Hesss Law
The standard enthalpy of an overall reaction is the sum of the standard enthalpies of the individual reactions into which a reaction may be divided
42
New cards
Standard enthalpy of formation
Standard reaction enthalpy for the formation of the compound from its elements in their reference states
43
New cards
Reference state
The most stable state of an element at the specified temperature and 1 bar
44
New cards
Reaction enthalpy of enthalpies of formation
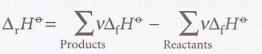
45
New cards
Mean bond enthalpies
Average enthalpy change associated with the breaking of a specific A--B bond.
46
New cards
Kirchhoff’s Law
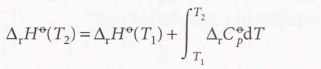
47
New cards
Path functions
Processes that describe the preparation of the state
48
New cards
Exact differential
An infinitesimal quantity that, when integrated, gives a result that is independent of the path between the initial and final states
49
New cards
Inexact differential
An infinitesimal quantity that, when integrated, gives a result that depends on the path between the initial and final states
50
New cards
Internal pressure
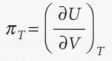
51
New cards
Expansion coefficient
The slope of the plot of volume against temperature at constant pressure
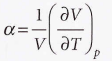
52
New cards
Isothermal compressibility
Measure of the fractional change in volume when the pressure is increased by a small amount
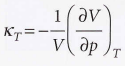
53
New cards
Joule-Thomson coefficient (µ)
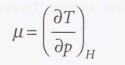
54
New cards
Isenthalpic expansion
Expansion at constant enthalpy
55
New cards
Joule- Thomson effect
Cooling by isenthalpic expansion.
56
New cards
Isothermal Joule-Thomson coefficient
\