Organic Chemistry 2 Reactions
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
81 Terms
Alkyne + H2 + Pt (PtO2)
Alkane
Alkyne + H2 + Pd-BaSO4 (Pd-CaCO3) + quinoline
Z-Alkene
Alkyne + Na + NH3 + Ethanol
E-Alkene (Birch)
Alkene + H2O + H2SO4
Alcohol (markovnikov)
Alkyne + H2O + H2SO4 + HgSO4
Ketone
Enol
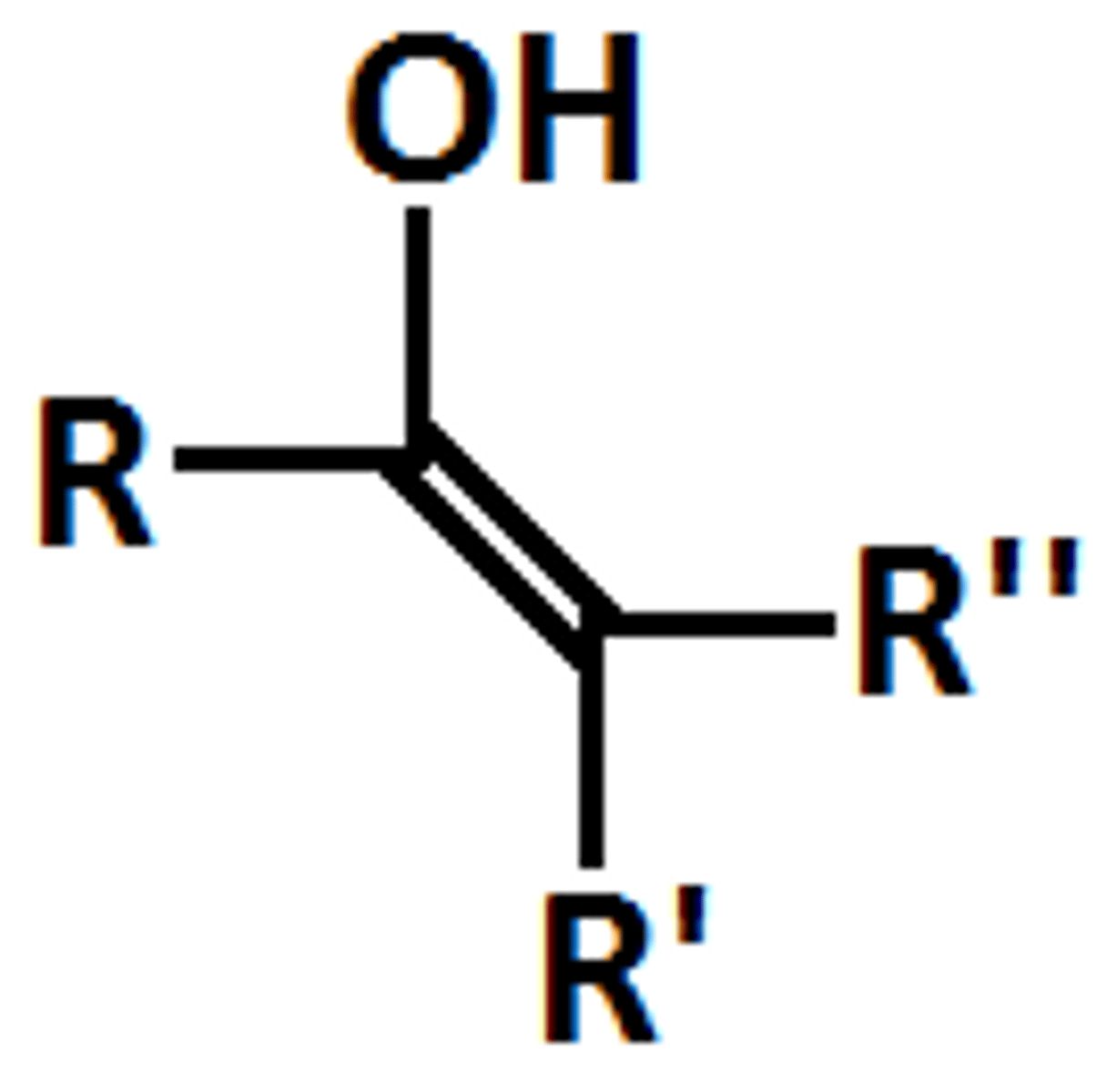
Alkynide Anion properties
Strong Base and Strong Nucleophile
Alkynide Anion + tertiary/secondary alkyl halide
E2 reaction
Alkynide Anion + primary alkyl halide
Sn2 reaction
Strong Bases (x4)
NaH
LDA
Butyl-Lithium
NaNH2
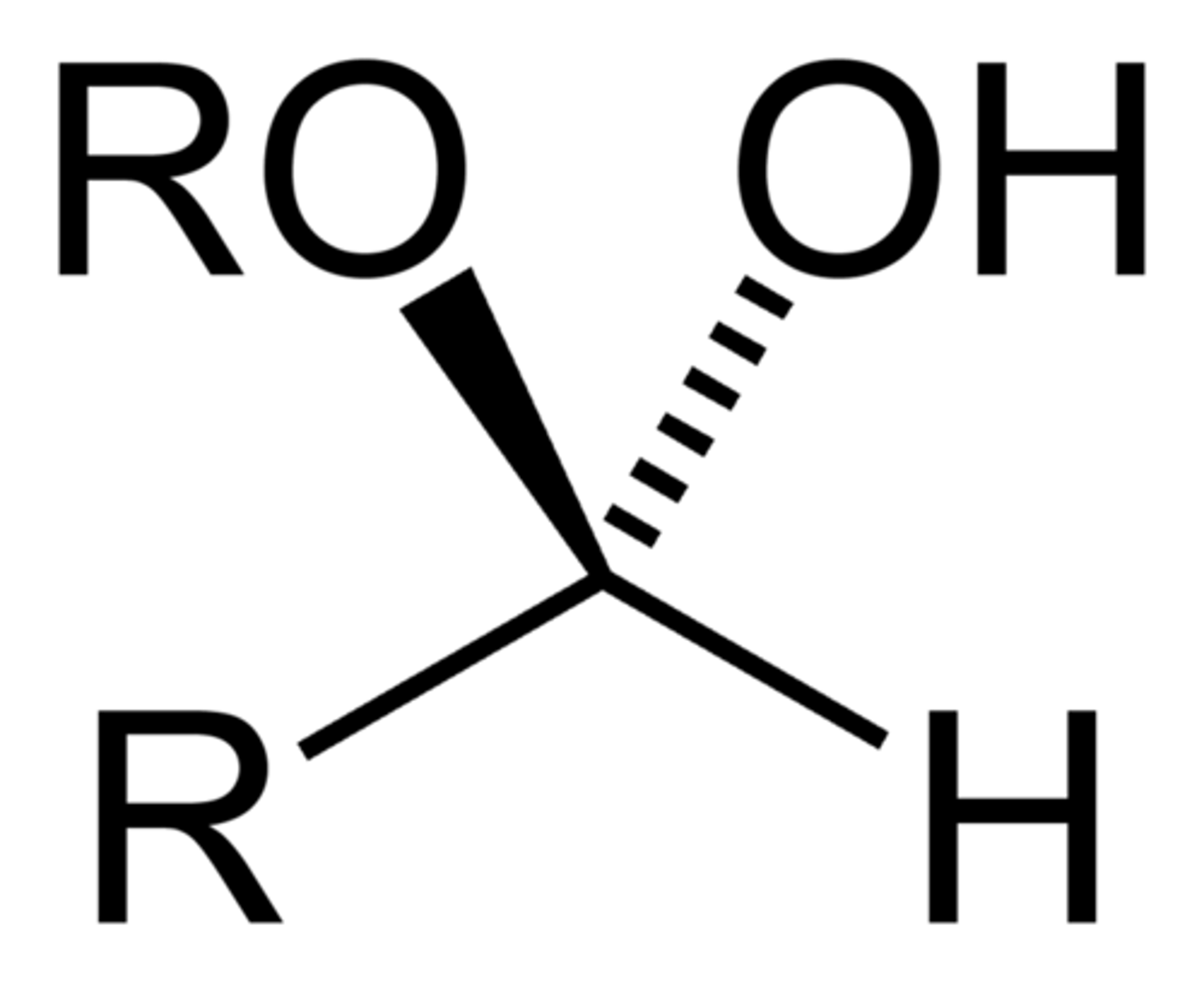
Hemiacetal
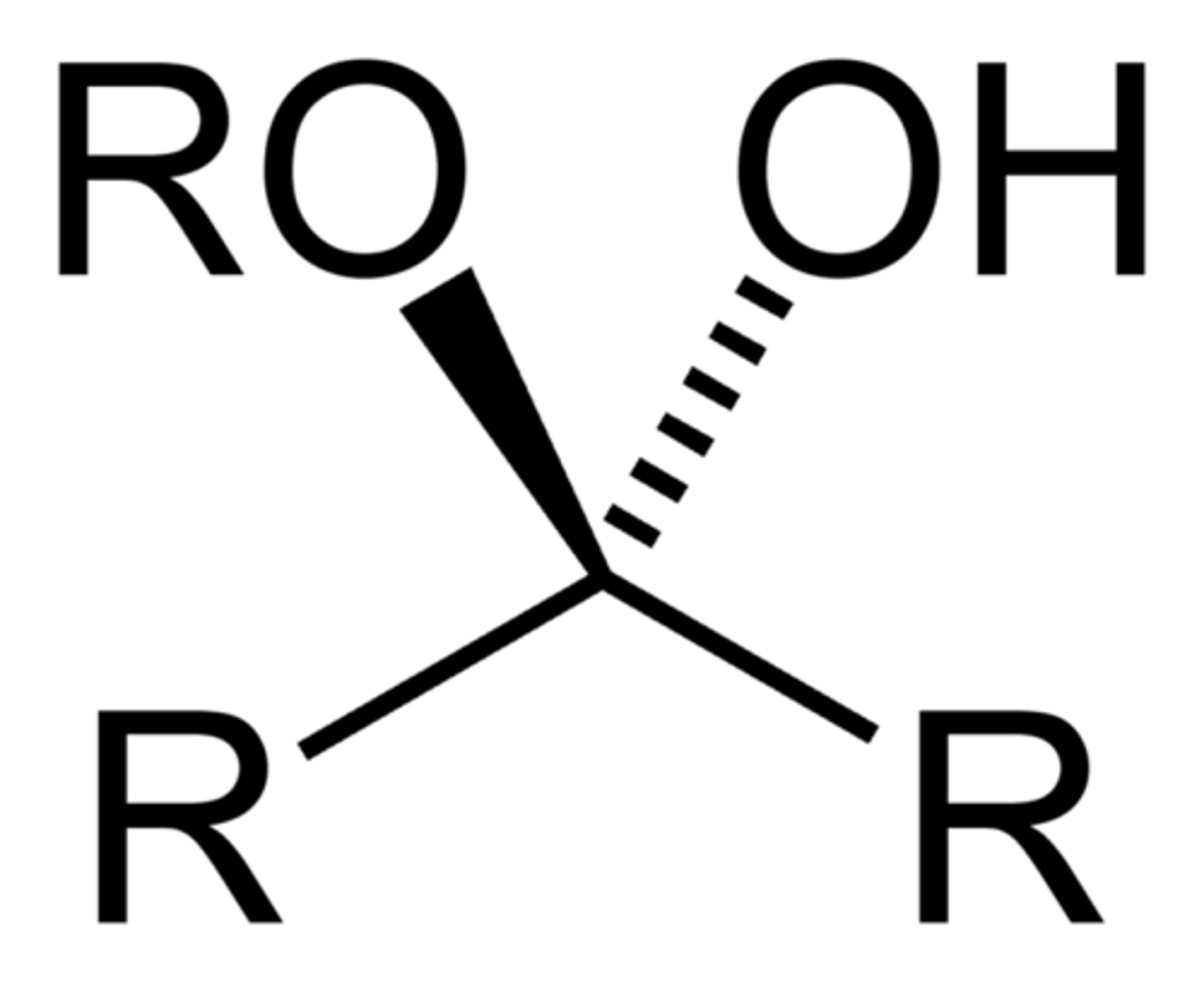
Hemiketal
R-Br + Mg + Et2O
R-Mg-Br (grignard)
Ketone/Aldehyde + 1(R-Mg-Br) + 2(Acid)
Alcohol + R
Nitrile + R-Mg-Br
Ketone + R
R-Br + NaCN + EtOH + heat
R-Nitrile
Saturated Ketone + 1(LiAlH4, EtOH) + 2(HCl, H2O)
Alcohol
Unsaturated Ketone + 1(LiAlH4, EtOH) + 2(HCl, H2O)
Alcohol (not conjugated)
Saturated Ketone + NaBH4 + EtOH
Alcohol
Unsaturated Ketone + NaBH4 + EtOH
No Reaction
Ester + 1(excess R-MgBr) + 2(Acid)
Alcohol with 2R
Terminal Alkyne + Strong base
Alkynide Anion
Ketone + Ph3P=R + THF
=R (Wittig)
Ketone + KCN + HCN
Alcohol with CN
Ketone + 1(R-Li2 + Et2O) + 2(H2O)
Alcohol + R
Ester + 1(R-Li2 + Et2O) + 2(H2O)
Alcohol + 2R (alkylithium)
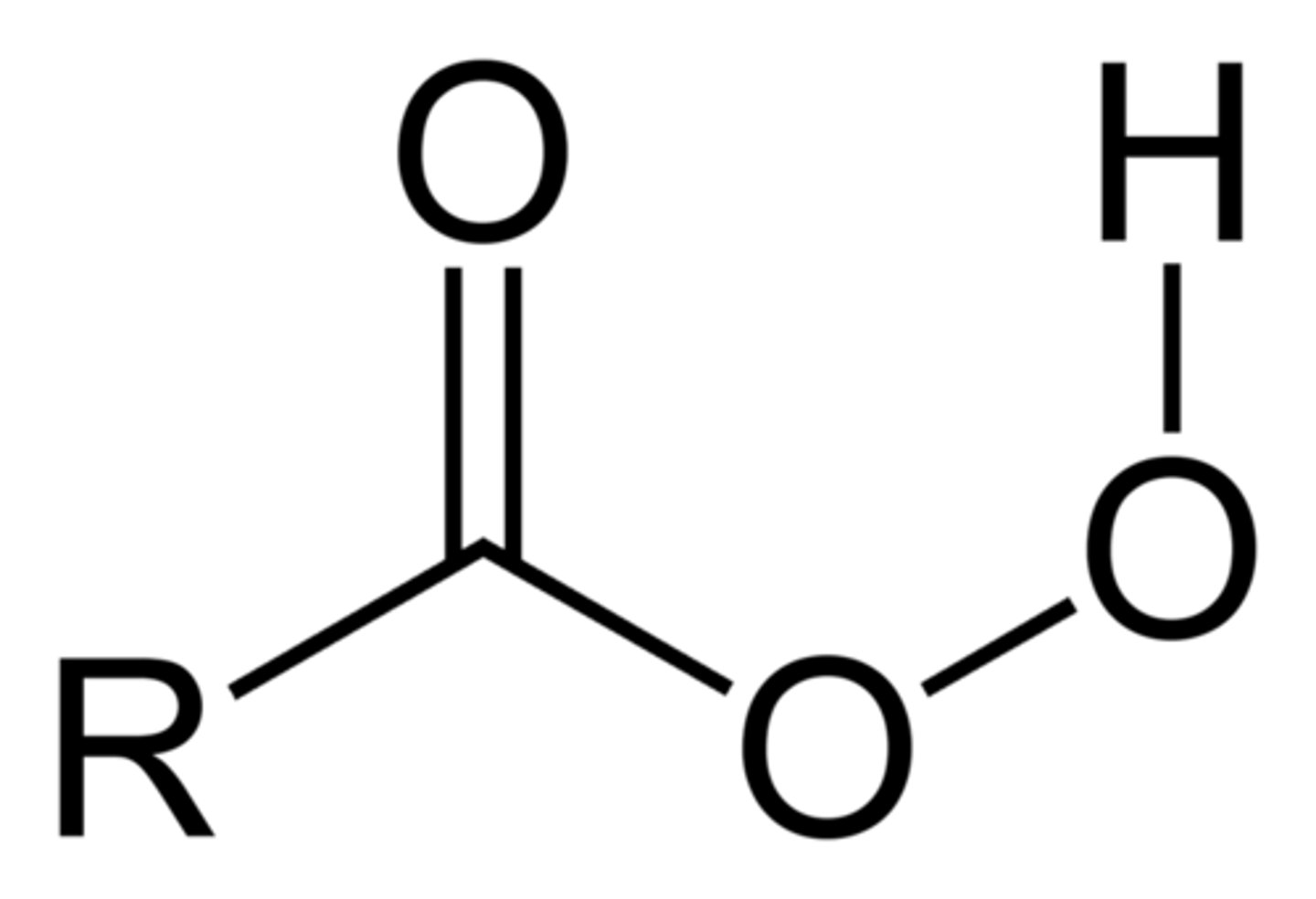
ketone + peroxyacid
(stereochemistry)
Ester (Baeyer-Villiger)
Retain Stereochem
Ketone + H2N-OH + Acid
Amide (C=O, N)
(Beckmann)
Ketone + 1(H2N-NH2 + EtOH) + 2(KOH, heat)
No functional group
(Wolff-Kishner)
Ester + 1(LiAlH4, EtOH) + 2(HCl, H2O)
Alcohol
Amide + 1(LiAlH4, EtOH) + 2(HCl, H2O)
Amine
Carboxylic Acid + 1(LiAlH4, EtOH) + 2(HCl, H2O)
Alcohol
Conjugated Ketone + 1(DIBAL-H) + 2(MeOH)
Alcohol (conjugated)
Ester + 1(DIBAL-H) + 2(MeOH)
2x Alcohol (cleavage)
Will DIBAL-H affect a double bond?
No
Will LAH affect a double bond?
Yes (will react with it in a conjugated ketone)
Nitrile + 1(DIBAL-H) + 2(MeOH) + 3(Acid)
Aldehyde
List the Carboxylic Acid derivatives in order of decreasing reactivity.
Acid chloride, acid anhydride, aldehyde/ketones, ester, amide, carboxylate
Carboxylic acid + SOCl2 (COCl2) + heat
acid chloride
Ester + Acid + H2O
Carboxylic acid + alcohol
Carboxylic acid + alcohol + acid
Ester
Amide + Acid + heat
Carboxylic acid
Nitrile + Acid + H2O
Carboxylic acid
Nitrile + 1(base + H2O) + 2(Acid + H2O
Carboxylic acid
Ester + base
Carboxylic acid
Amide + base
Carboxylic acid
R-MgBr + 1(CO2) + 2(Acid)
Carboxylic acid
Acid chloride + Me2CuLi + Et2O
Ketone + Me
conjugated ketone + LiCu(CH3)2 + THF
Ketone with CH3 added to double bond
Ketone (saturated) + LiCu(CH3)2
No reaction
Ester + LiCu(CH3)2
No reaction
m-CPBA
peroxyacid
Aldehyde + NaBH4 + EtOH
Alcohol
Alkene + KMnO4 + KOH + H2O
Diol (syn)
What will KMnO4 react with first: aldehyde or alkene?
Aldehyde
Me3SiCl + Et3N
TMS-Cl + Et3N
Protecting group for alcohol
TBAF
Deprotection for alcohol
Alcohol + 1(NaH) + 2(R-X)
Ether
(William ether synthesis)
Alkene + H2O + Acid
Alcohol (on more substituted C and possible rearrangement)
Alkene + 1(Hg(OAc)2 + nucleophile) + 2(NaBH4)
Nucleophile on most substituted C and H on less substituted (markovnikov)
(Oxymercuration-demerucuration)
Alkene + 1(BH3-THF) + 2(H2O2, NaOH)
OH on less substituted C and H on more substituted (anti-markovnikov)
(Hydroboration-oxidation)
Alkene + OsO4 + NMO
Syn diol
Primary alcohol + K2Cr2O7 + H2SO4
Carboxylic Acid
(Jones)
Secondary alcohol + K2Cr2O7 + H2SO4
Ketone
(Jones)
Primary alcohol + PCC + CH2Cl2
Aldehyde
Secondary alcohol + PCC + CH2Cl2
Ketone
Primary alcohol + Me2SO
Aldehyde
(Moffatt)
Primary alcohol + (CoCl)2 + Me2SO
Aldehyde
(Swern)
Aldehyde + NaClO2 + CH2Cl2
Carboxylic Acid
(Kraus-Pinnick)
Ether + H-X + heat
X-R + X-R' + H2O
Aromatic Rule
4n+2
Antiaromatic Rule
4n
Alkene + MCPBA
Epoxide
Alkene + 1(Br2, H2O) + 2(NaOH)
Epoxide
Epoxide + basic nucleophile + H2O
Opening
(Inverts stereochemistry)
(Nucleophile attacks less substituted C)
Epoxide + Acid
Opening
(Retains stereochemistry)
(Nucleophile attacks more substituted C)
Alcohol + TsCl + Py
Sulfonate Ester (R-O-Ts)
(good leaving group)
Alkene + 1(O3) + 2(DMS)
Ozonolysis (double bond becomes two Carbonyl groups)
Diol + HIO4
Cleavage and formation of two carbonyl groups
Alkene + OsO4 + NaIO4 + H2O/Acetone
Cleavage and formation of two carbonyl groups
(Lemieux-Johnson)
Ketone/Aldehyde + Enolate
Addition
(Carbonyl attack carbonyl)
(a ketone from the enolate is also formed)