Organic Chem005 to page 34
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
What must you remeber for dot and cross diagrams?
What are valence electrons?
What is a valence shell?
If N has 5 valence electrons, how many electrons are bonded?
How do you calculate formal charge?
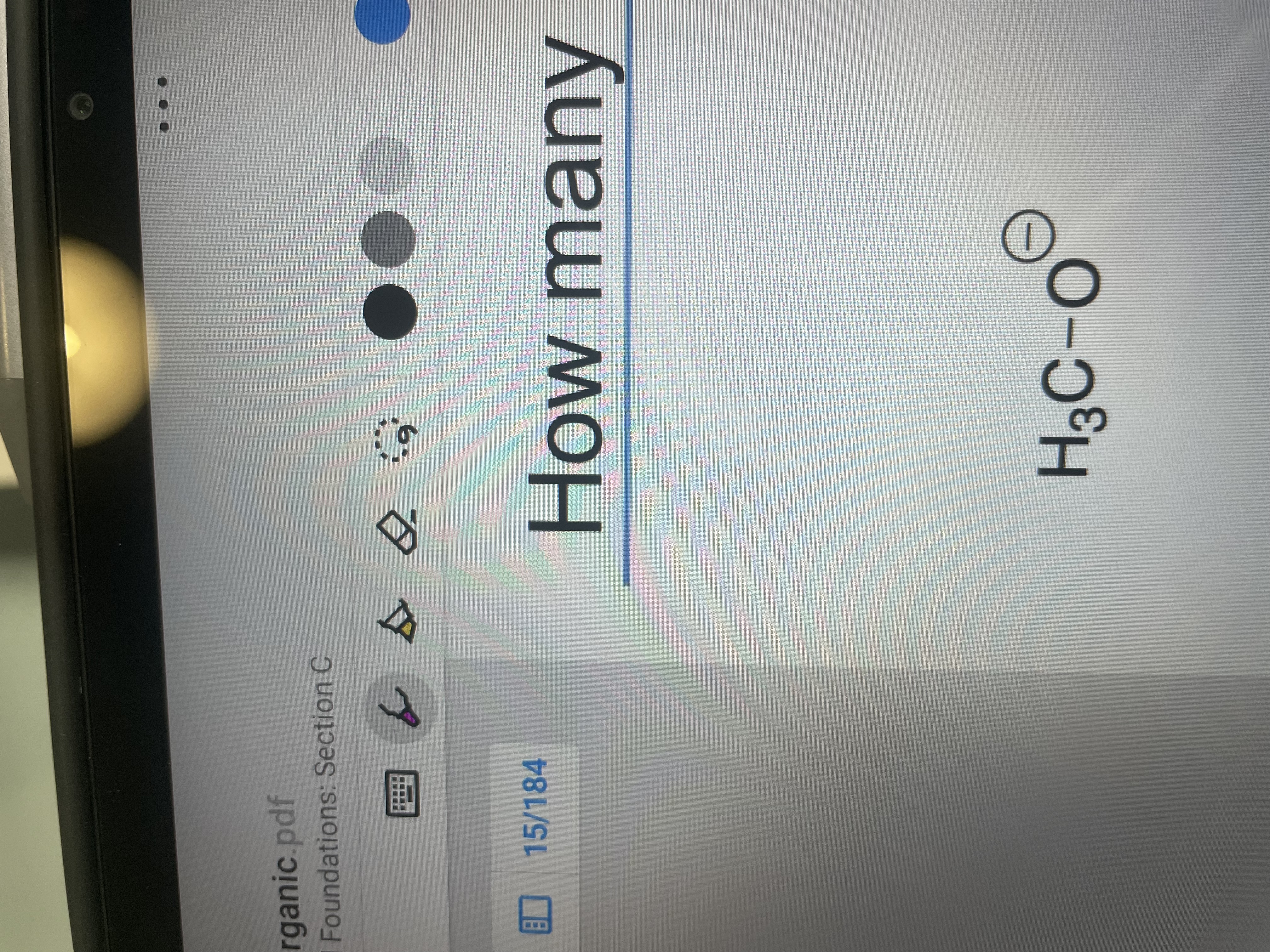
How many lone pairs are on this molecule?
IMAGE
lone pairs of electrons
electrons on outer shell which can form chemical bonds & reactions.
outer shell where valence electrons are found.
3 as N has a lone pair
formal charge = number of electrons in neutral atom (valence electrons) - number of electrons in lone pairs - 1/2 the number of electrons bonded
3

Calculate the formal charge on the starred atoms:
what is electronegativity?
What does a greater difference in electronegativity result in? How would we show the polarity on HCN in both bonds?
What is the octet rule?
What are the two main exceptions to this rule? Why do we have acceptions?
no charge, +1, -1
the ability of an atom to attract a shared pair of electrons in a covalent bond.
A higher polar bond. C wouls have a delta + (s+) and N s- as N is more elecronegative and then c is more electronegative than H so same applies.
Rule that atoms share electrons to form full outer shell of 8 electrons.
1) S03 tends to be all bonds shared as double bonds as results in lower formal charge. This is an expanded octet (as more than 8 e-)
2) B compounds aim for 6 valence e- as 8 e- would result in a formal charge.
Want lowest formal charge!

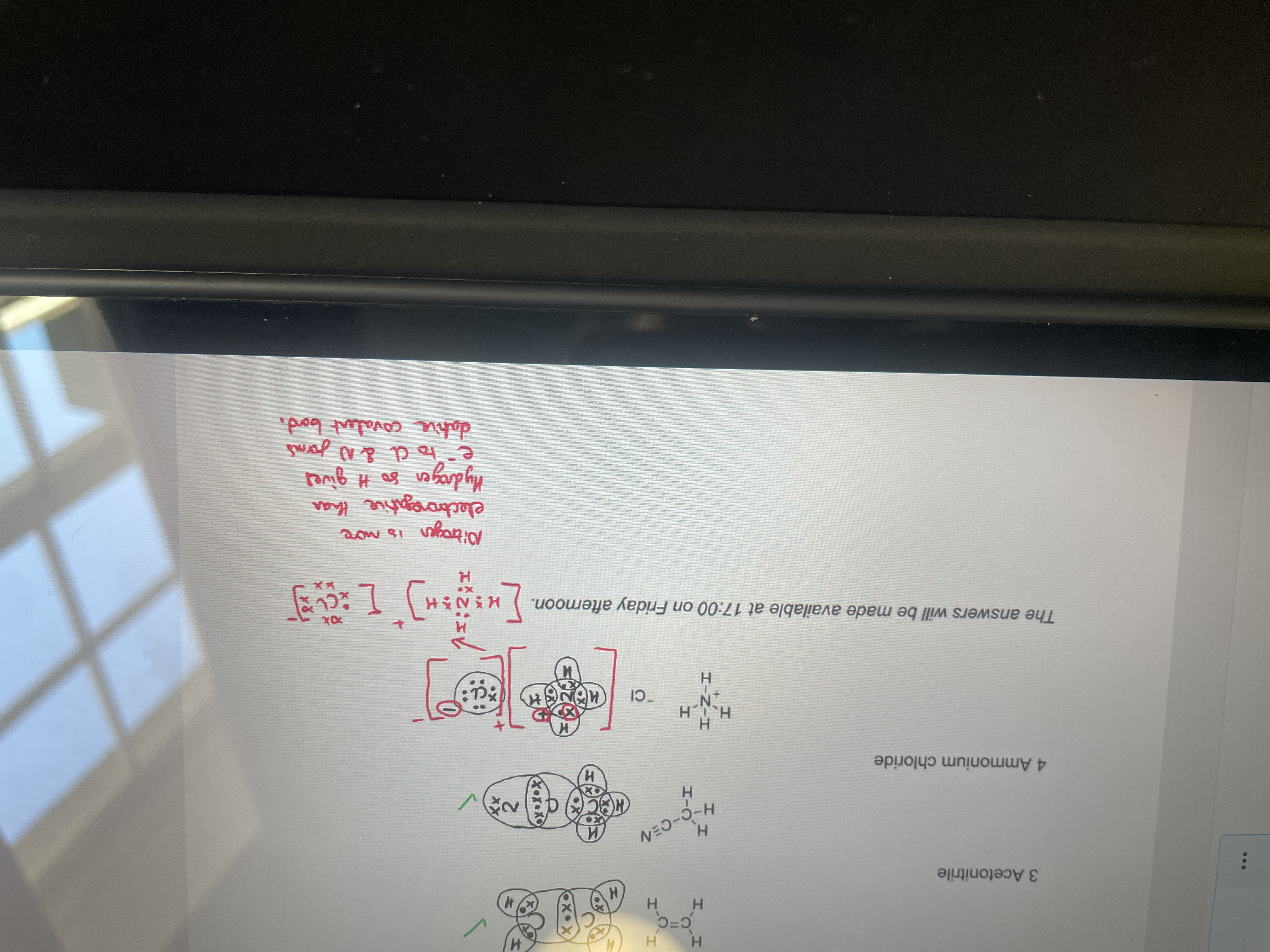
Do the dot and cross diagram for ammonium chloride:
IMAGE
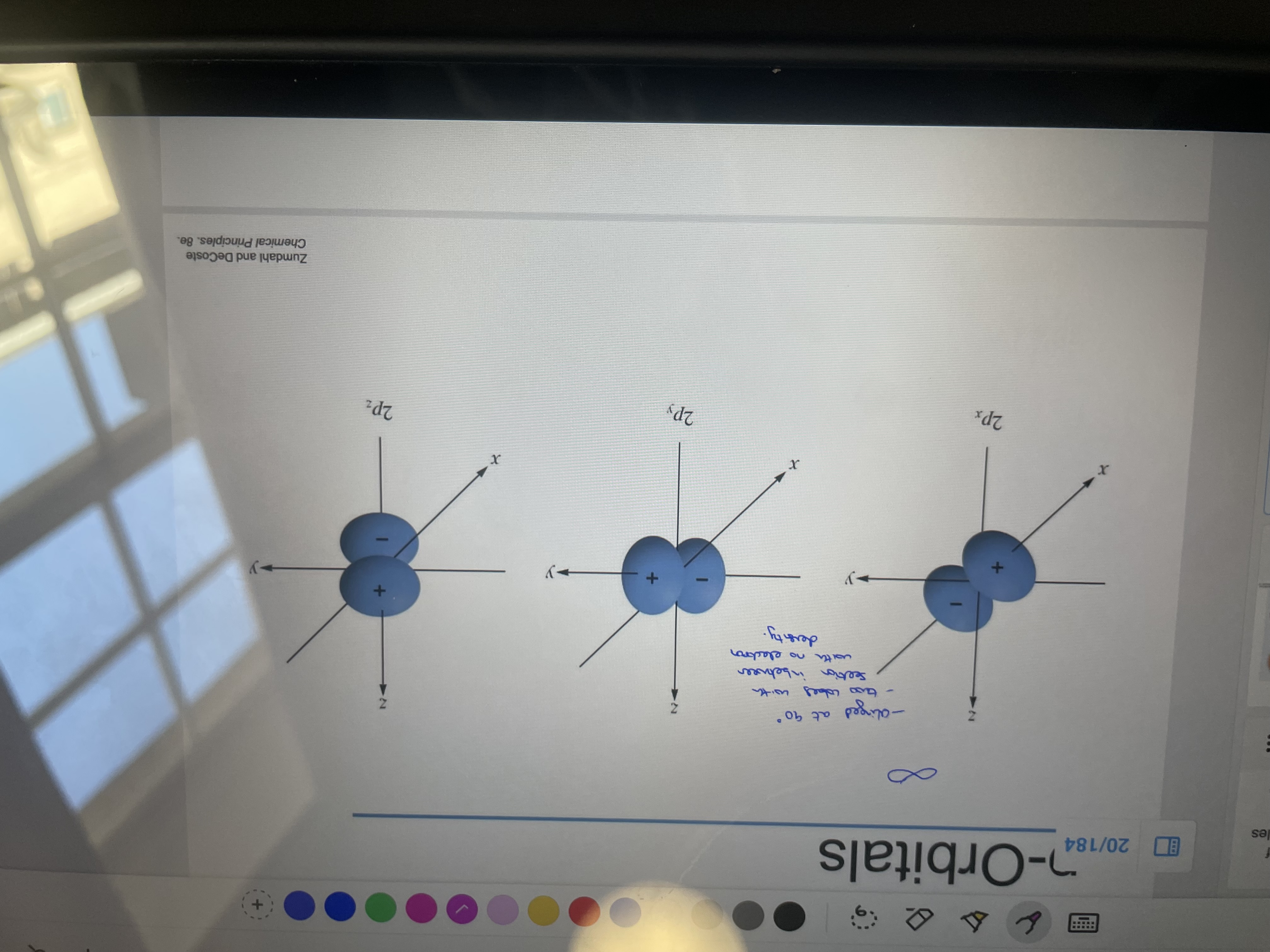
What are the three ways p-orbitals can be aligned? What degree angle would the 3 types be to each other? Describe electron density of the orbitals:
What are the two models of covalent bonding orbitals?
Why would each one be used?
x,y,z don't have to be the one they are above its just used to distinguish.
90 degrees
Electron density shown in photo but also inbetween lobes there is a section with no electron density.
Localised electron model (valence shell electron repulsion (VESPR) theory) and molecular orbital theory.
VESPR - good to show molecule shape
oribital - good to understand molecule reactivity.
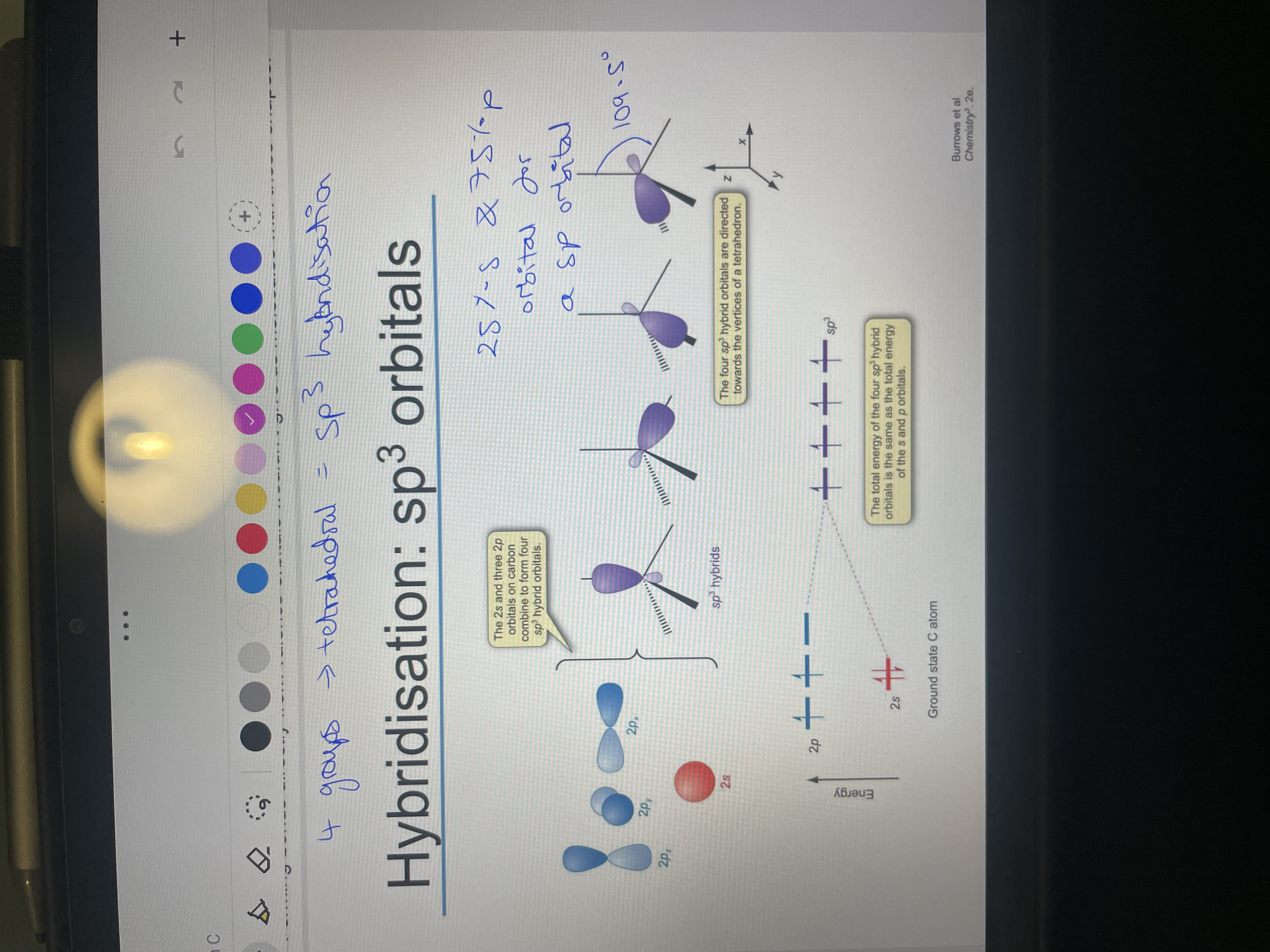
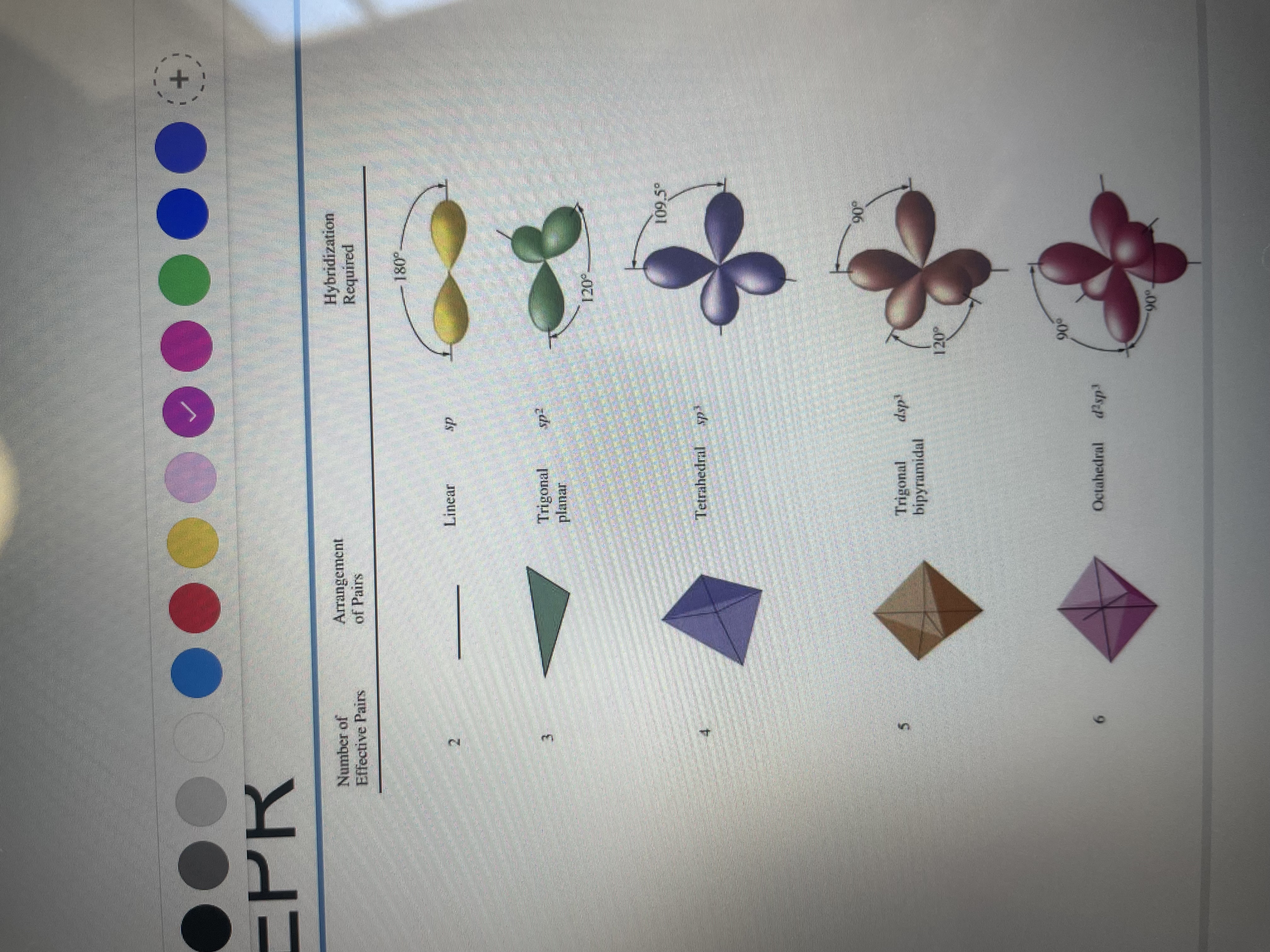
How do we arrange the groups in VESPR? What is the issue with lone pairs?
Is a molecule has 2,3,4,5,6 bonding pairs what would be the shape name, orbitals used and bonding angle?
For sp3 hybridisation what is the orbitals used? How many groups is it for?
What is the ratio of s:p orbitals and bond angle?
What is the total sp3 orbital energy equal to?
so that they are as far away as possible from each other..
L.P. take up more room than bonding pairs.
2px, 2py, 2pz and 2s which result in 4 sp3 orbitals. 4 groups so tetrahedral
25% s and 75% p, 109.5
equal to the energy of the 3 p orbitals and s orbitals.
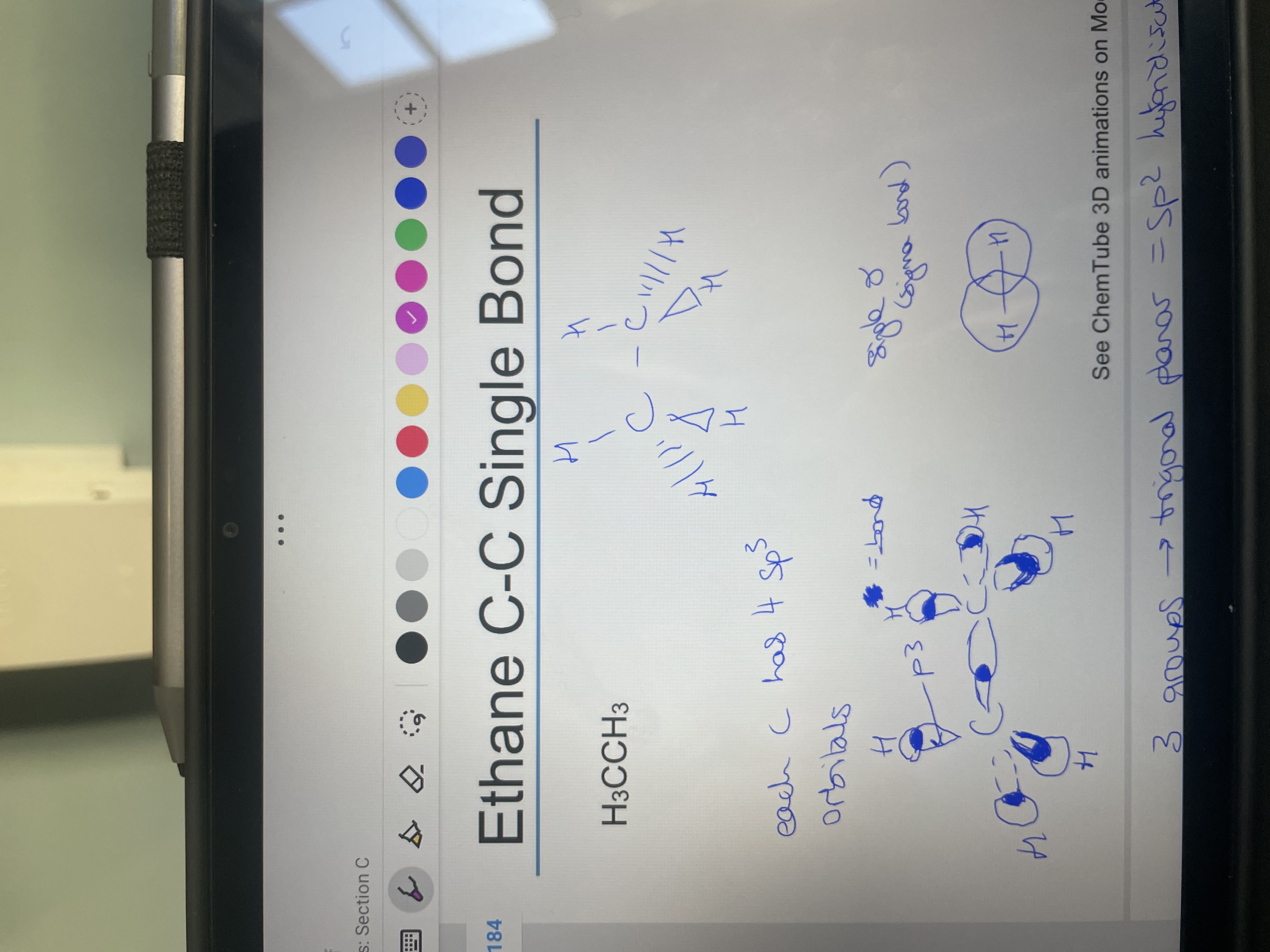
Draw H3CCH3 (ethane) showing 3D structure then draw with sp3 orbitals shows bond:
What type of bond is it?
sigma - direct atomic overlap of orbitals
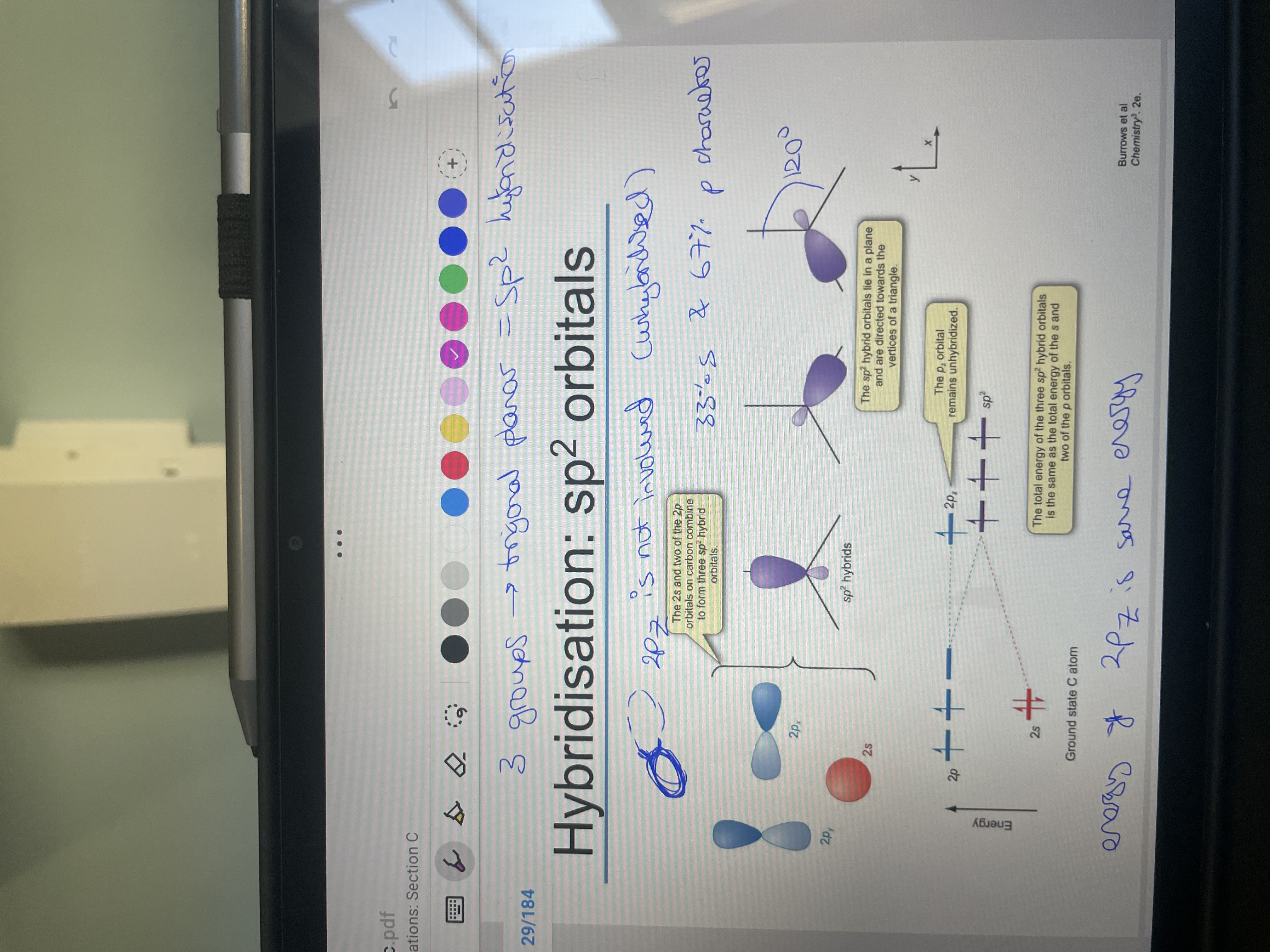
For sp2 hybridisation what is the orbitals used? How many groups is it for?
What is the ratio of s:p orbitals and bond angle?
What is the total sp2 orbital energy equal to? What else would be on the energy diagram?
2py, 2px (zyx interchangable! so actually just not the p orbital that goes foraward and back facing) and 2s
3 GROUPS SO TRIGONAL PLANAR
33% s and 67% p, 120
total energy = total energy of the s and 2 p orbitals.
2pz but at higher energy than sp2 orbitals as energy remains unchaged as not hybridised.
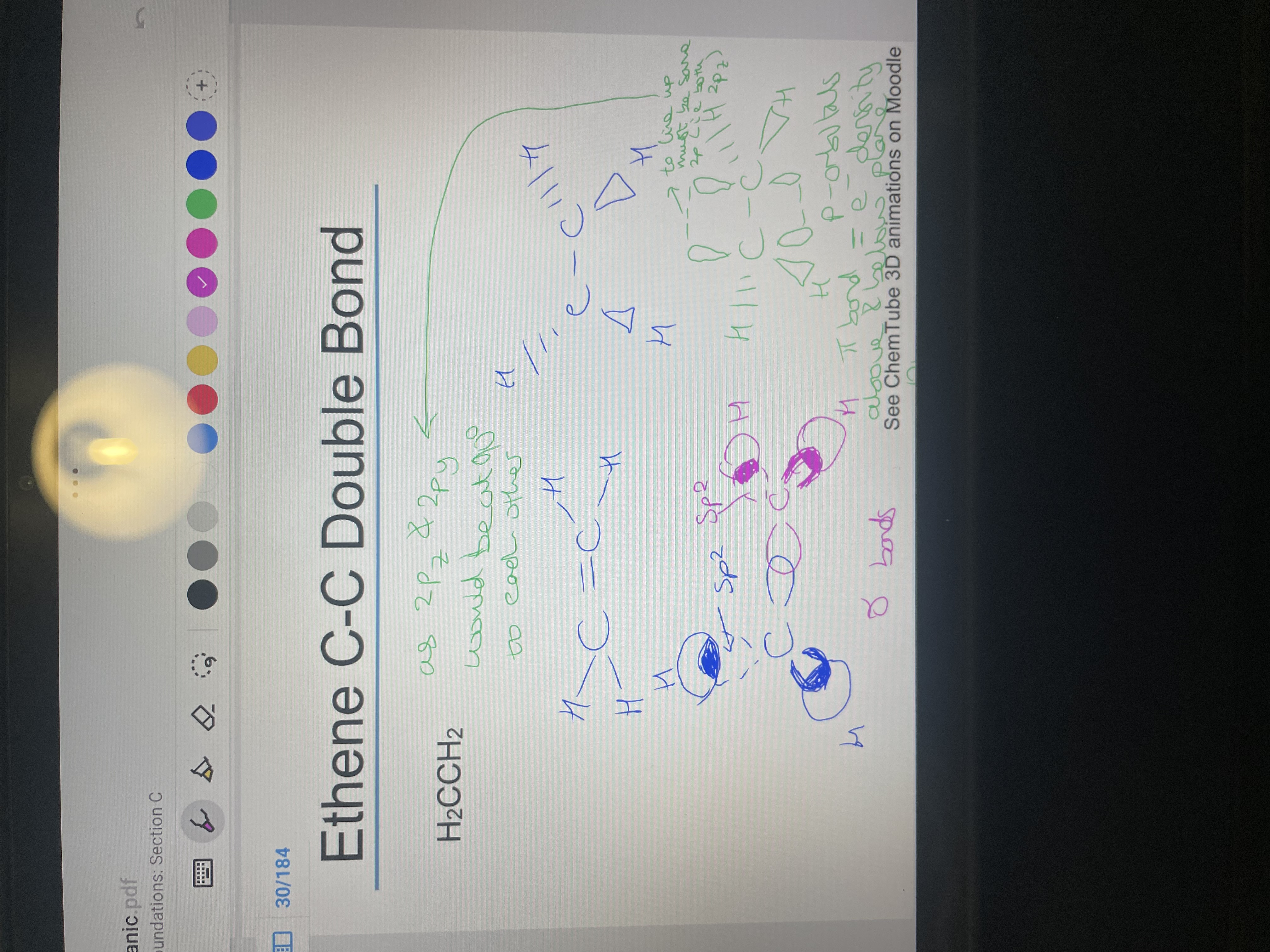
Draw ethene with double bond in hybridised form with sp2 orbitals showing 3D aspects and where bonds overlap:
Where is the energy density of the pi bond found?
above and below the plane of the bond
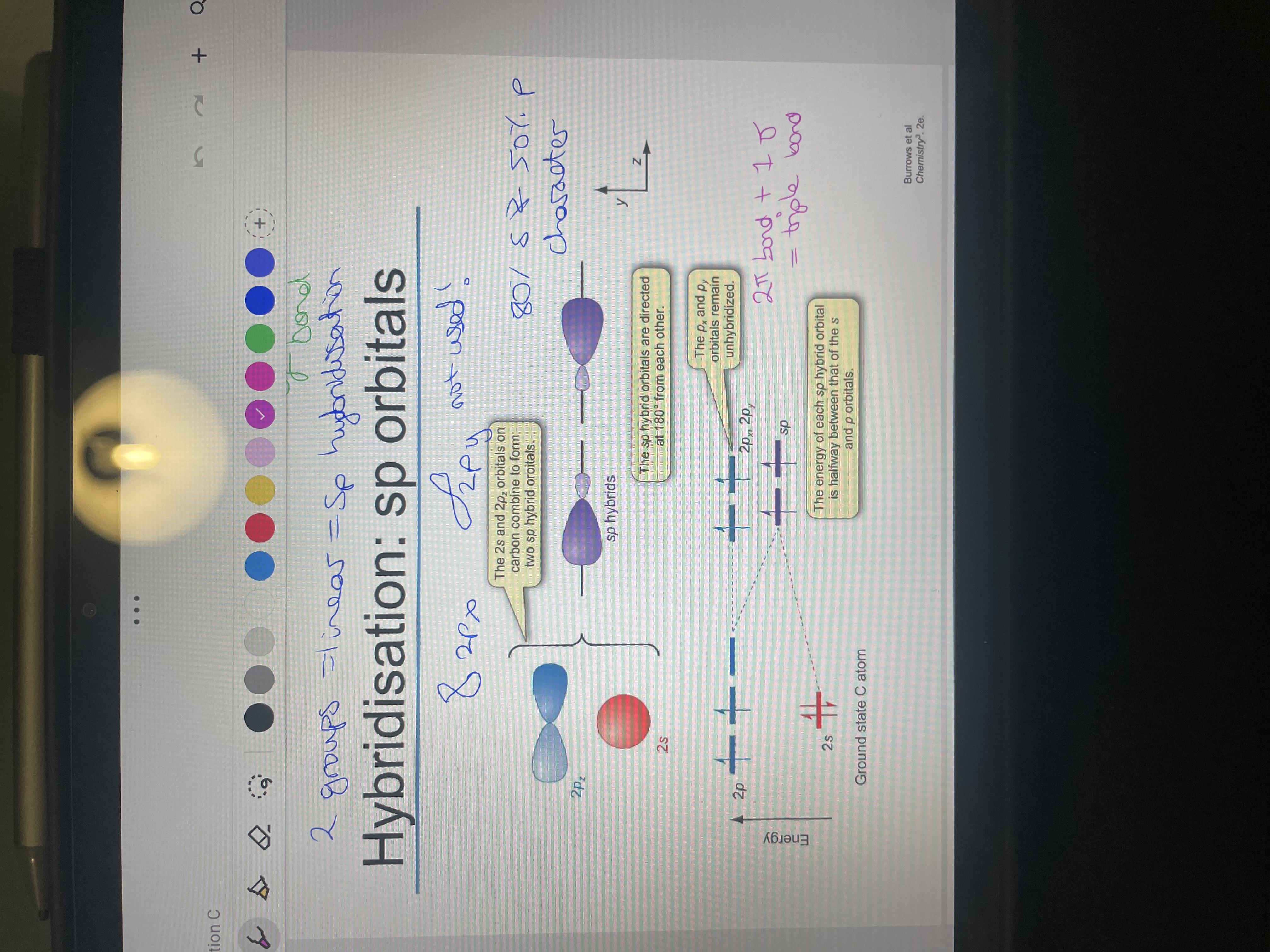
For sp hybridisation what is the orbitals used? How many groups is it for?
What is the ratio of s:p orbitals and bond angle?
what is the energy if an sp orbital? What else would be on the energy diagram?
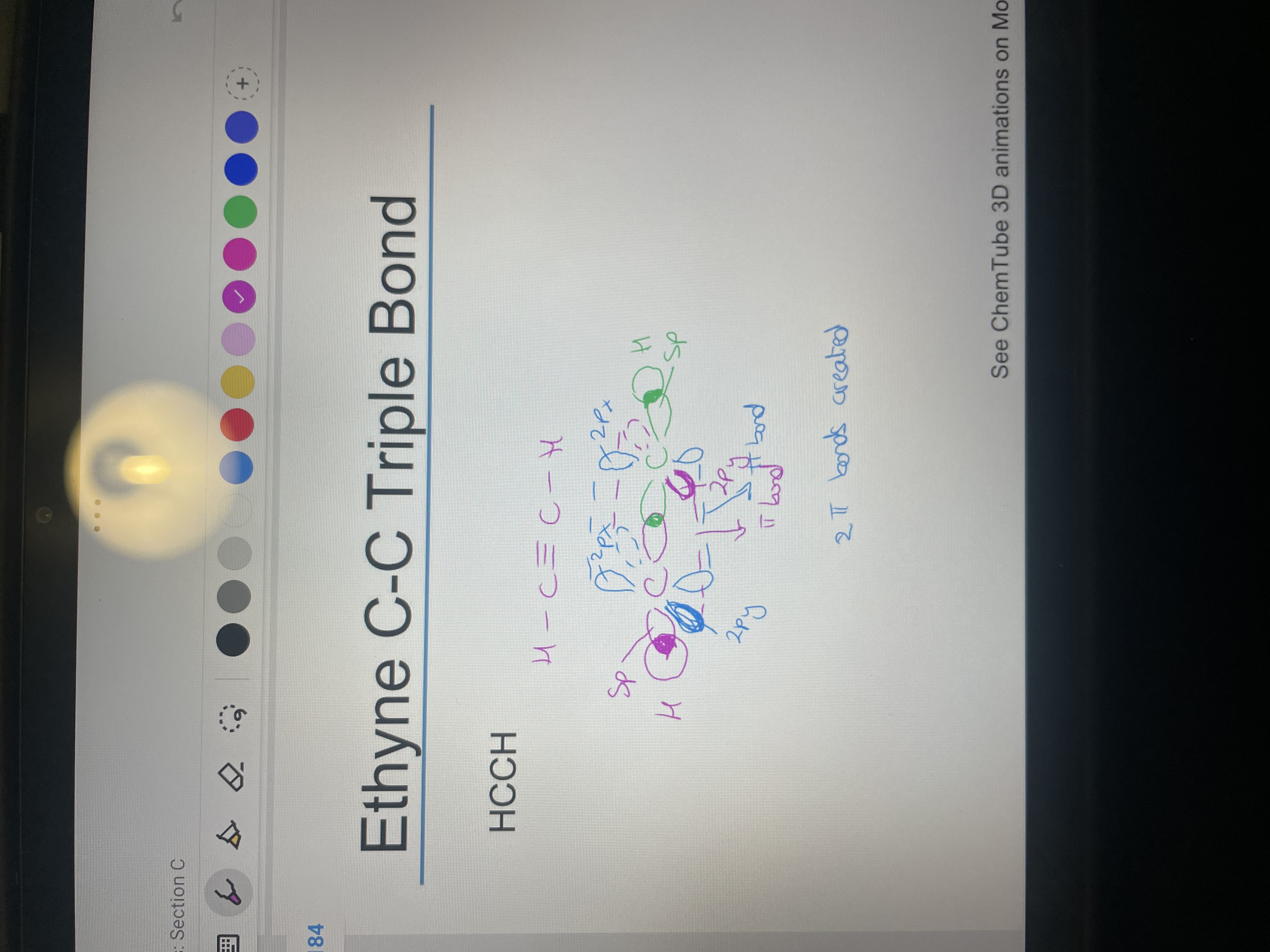
Draw HCCH with showing 2p hybridisation: Also explain how the triple bond forms.
one 2p and one 2s (only the horizontal p orbital) that form sp hybrid. 2 groups so linear
50% s and 50% p, 180
exactly half way between that of s and p orbitals.
the other 2p orbitals at normal energy as unhybridised.
2 PI BONDS form and one sigma to make a triple bond and the pi bonds form with each carbons has two p orbitals not hybridised so they form a bond with the same p orbital on the other carbon ie 2pz is unhybridised on both carbons so forms a pi bond as they line up whereas 2pz and 2py on different carbons can't as don't line up as 90 degrees from each other.
How do you quickly tell with a molecule that has only single, one double or one triple bond what hybridisation to use?
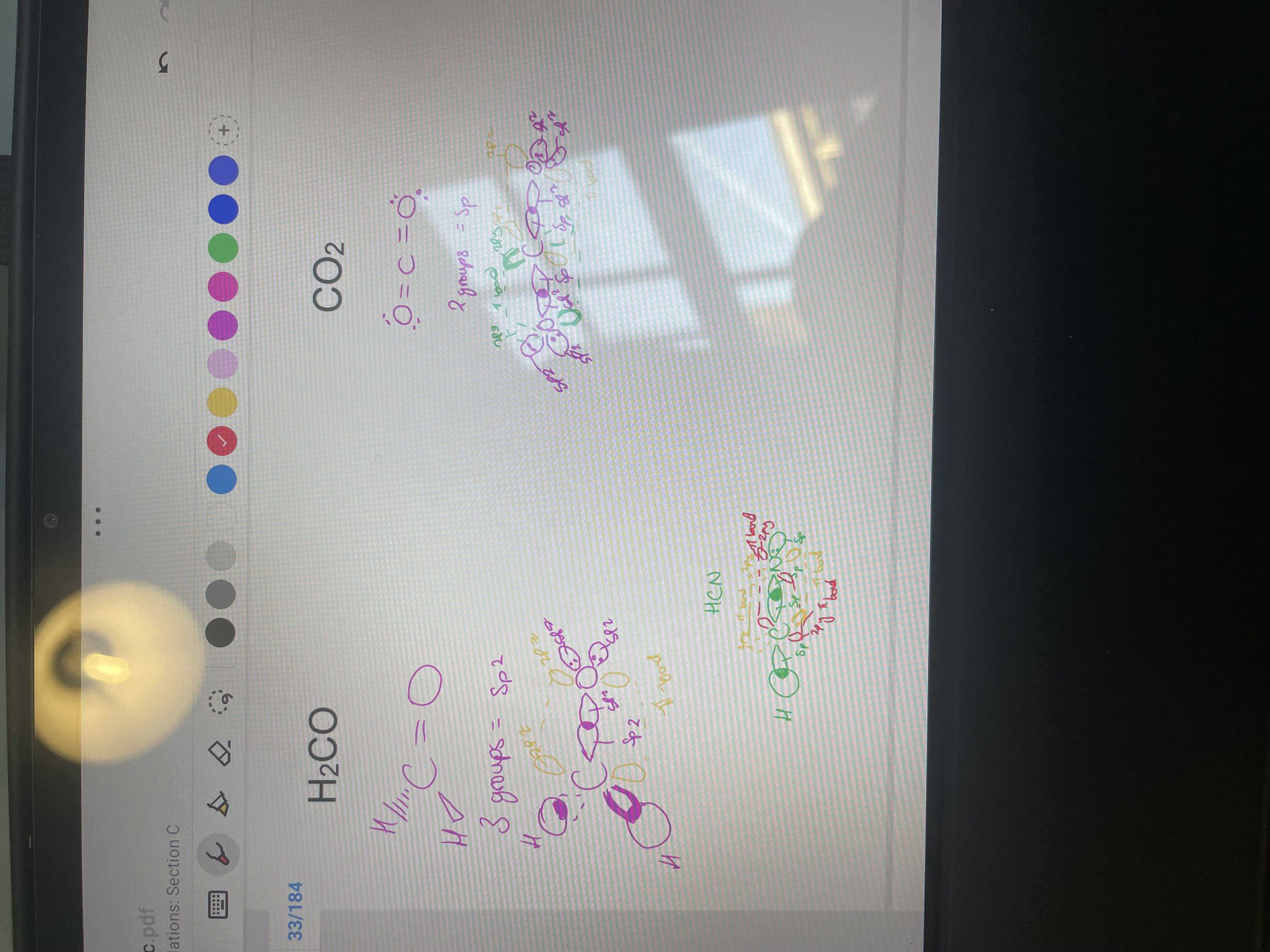
for example what is the hybridisation on HCN, H2CO, CO2?
Draw H2CO and CO2 and HCN showing hybridisation and pi bond formation:
if all single bonds = sp3, if double bond - sp2, if triple bond - sp
HCN, C and N are both sp
H2CO, C and O are both SP2.
CO2 has C as sp and Os as SP2
Draw the hybridisation structure of O3:
1) draw the Lewis structure
2) now try draw the hybridisation structure
A
