W3L1: Study design
Study Design vs. Research Methods
Study Design
A plan to ensure that the evidence obtained enables us to answer the initial question unambiguously.
This involves specifying the population, sampling techniques, data collection methods, and analytical strategies used throughout the research process.
Research Methods
Processes, procedures, and tools used to collect and analyze data.
Includes sampling mode, data collection methods (e.g., questionnaire, observation), design of questions, and statistical analysis plans, which always confirm with study design.
Comparison of Study Designs
Research Question/Problem: Determines the most appropriate
Study Design: Implements specific types of study plans to determine expected results.
Research Method: Affects the selection of suitable methods to collect and analyze evidence.
Study Designs
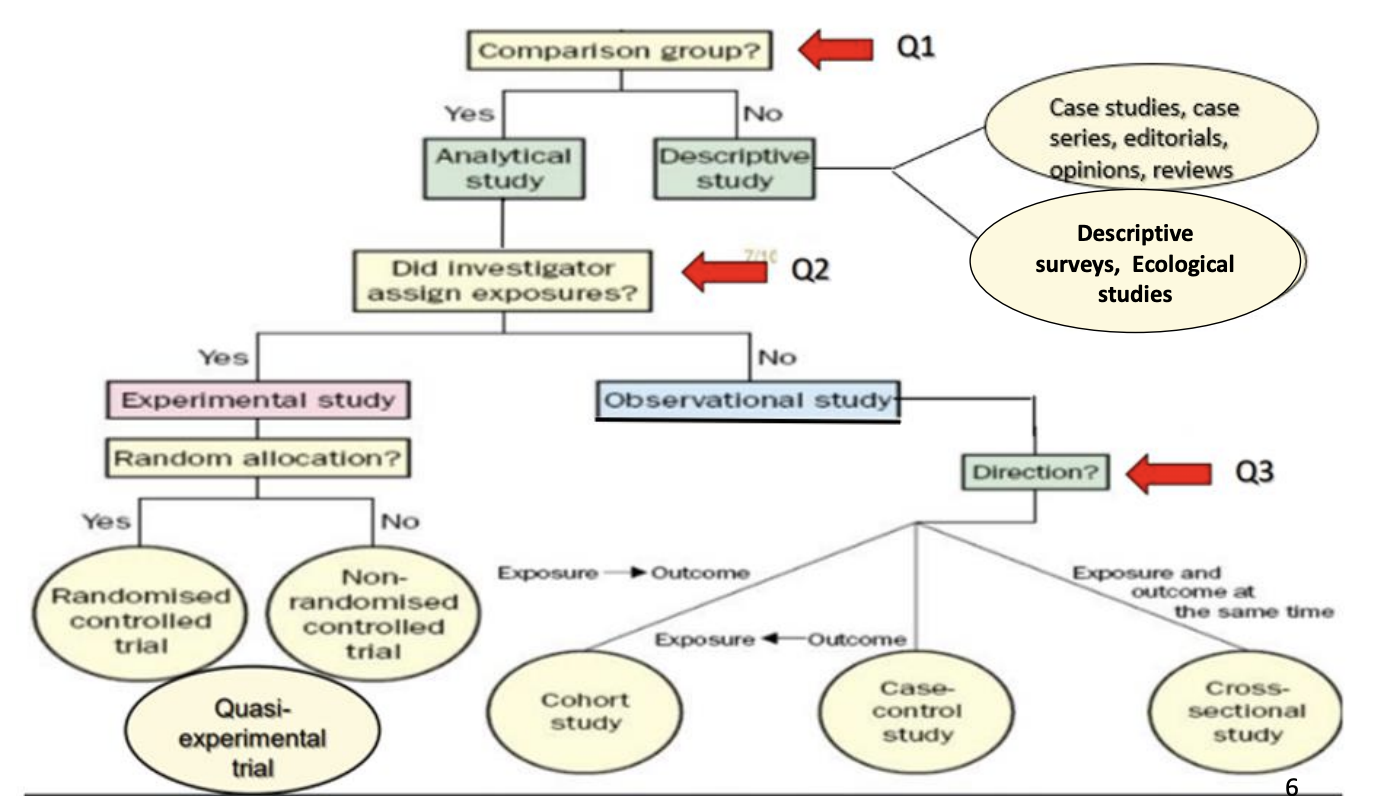
Types of Study Designs
Descriptive Studies
Hypothesis generating without a comparator.
Analytical Studies
Hypothesis testing with comparator group(s).
Descriptive Studies
Describe characteristics of disease (outcome) or exposure (risk factor) concerning:
Populations: Demographics, socio-economic status, lifestyles.
Geographic Distribution: Place-based variations.
Frequency Over Time: Seasonal variations through time-trend studies.
Research questions in format (PO question).
Generate hypotheses for later testing in analytic studies.
Types of Descriptive Studies
Case Reports and Case Series
Detailed descriptions of one or few patients with:
Unusual diseases or unexpected outcomes.
Speculated exposure leading to the disease (e.g., new syndrome in gay men with PCP pneumonia).
Time Trend Studies: Measure disease occurrence over time to assess fluctuations correlated with community changes.
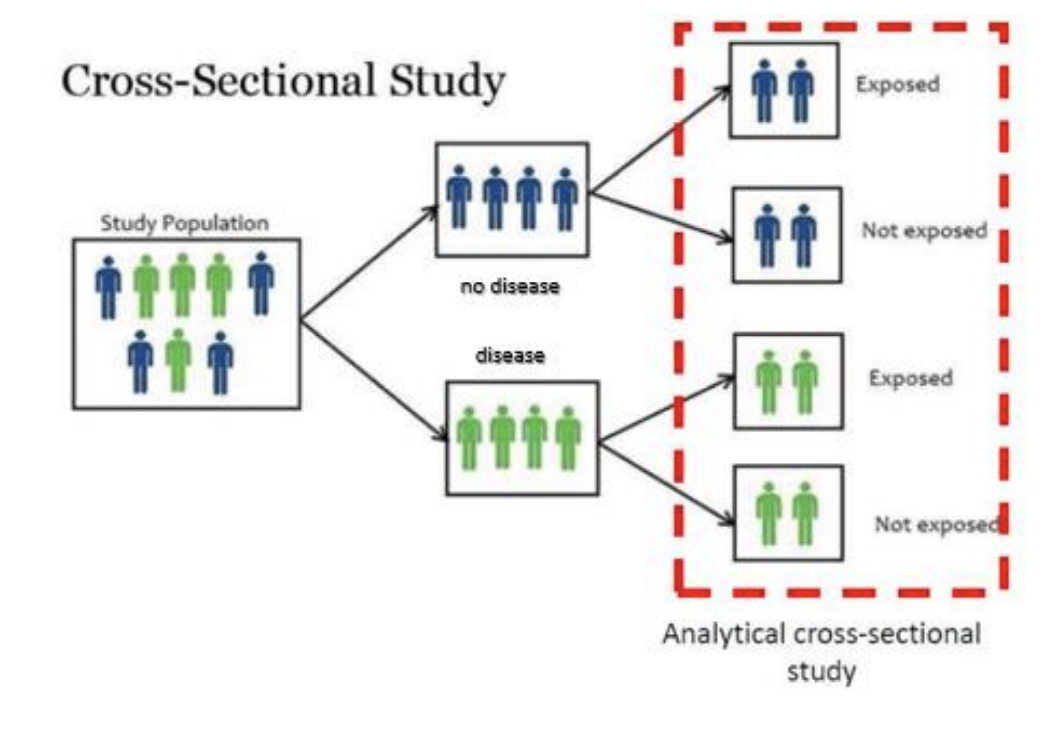
Cross-Sectional Studies (Surveys)
Examine disease prevalence at one point in time by analyzing variables from a population sample.
Gather data on exposure and disease simultaneously
Cross-Sectional Ecological Studies
Population-level analysis rather than individual-level data.
Example Question: Relationship between income and coronary heart disease (CHD) rates across different countries.
Findings suggested richer countries had higher CHD rates, but individual-level data revealed the opposite.
Ecological fallacy: an assumption that an association observed at the group level applies at the individual level. → major weakness
Descriptive Studies: Advantages and Disadvantages
Advantages
Quick and inexpensive.
Utilize existing databases.
Efficient resource allocation for education and health promotion.
Answers non-clinical research questions
Disadvantages
Cannot establish causality; conclusions can be misleading.
Prone to ecological fallacy.
Analytical Studies
Observational Studies
Primarily hypothesis testing using comparator groups.
Goal: Determine if interventions or exposures affect outcomes.
Advantages and Disadvantages of Observational Studies
Advantages
No intervention or manipulation is required—just observation.
Useful for studying naturally occurring exposures or conditions where randomization is unethical.
Efficient sampling opportunities (e.g., studying rare diseases).
Disadvantages
More prone to bias and confounding than experimental designs.
Axes of Observational Analytical Study Designs
Directionality
Exposure first → Cohort
Outcome first → Case-control
Both together → Cross-sectional
Sample selection
Exposure → Cohort
Outcome → Case-control
Random/all → Cross-sectional
Type of outcome
Incident → Cohort
Prevalent → Case-control/Cross-sectional
Cross-Sectional Studies
Asses the prevalence of a condition at a specific point in time by collecting data from a representative sample.
Shows correlations between exposure and disease
no directionality - do not track changes over time or determine cause-and-effect relationships.
Steps in Analytical Cross-sectional Study
select a sample from population
measure predictor and outcome variable at a single time point
estimate prevalence of outcome
asses correlation between exposure and outcome
Example Question:
Research Question: In patients with Polycystic Ovarian Syndrome, is neuro-endocrine dysfunction associated with irregular cycles? What is the study design?
Cross-sectional analytical study
Case-control Studies
Examine the people with/without disease and find differences in predictor variables that may explain why the cases got the disease and the control didn’t.
Usually retrospective.
look back in time to examine how certain exposure/risk factors may have influenced the occurrence of the outcome
Steps in a Case-control Study
Classifies individuals based on presence/absence of disease
select a sample from a population of people with the diseases (cases)
select a sample from a population at risk that are free of the diseases (controls)
each case is matched with a similar control
Compare exposure history between cases and controls
Study Base
a defined study population
both cases and controls should be selected from the same study base
important to minimize bias
Case-control Example Question
Research Question: In patients with Reye’s syndrome, is there an association between the use of aspirin and the development of Reye’s syndrome?
30 patients with Reye’s syndrome were accessible to the investigator for study
60 patients were drawn from the much larger population of accessible patients who have had minor viral illnesses without Reye’s syndrome.
Subjects in both groups asked about history of aspirin use.
Subjects who had minor viral illness and took aspirin had 9 times the odds of developing Reye’s syndrome.
What is the study design?
Case-control
Odds Ratio
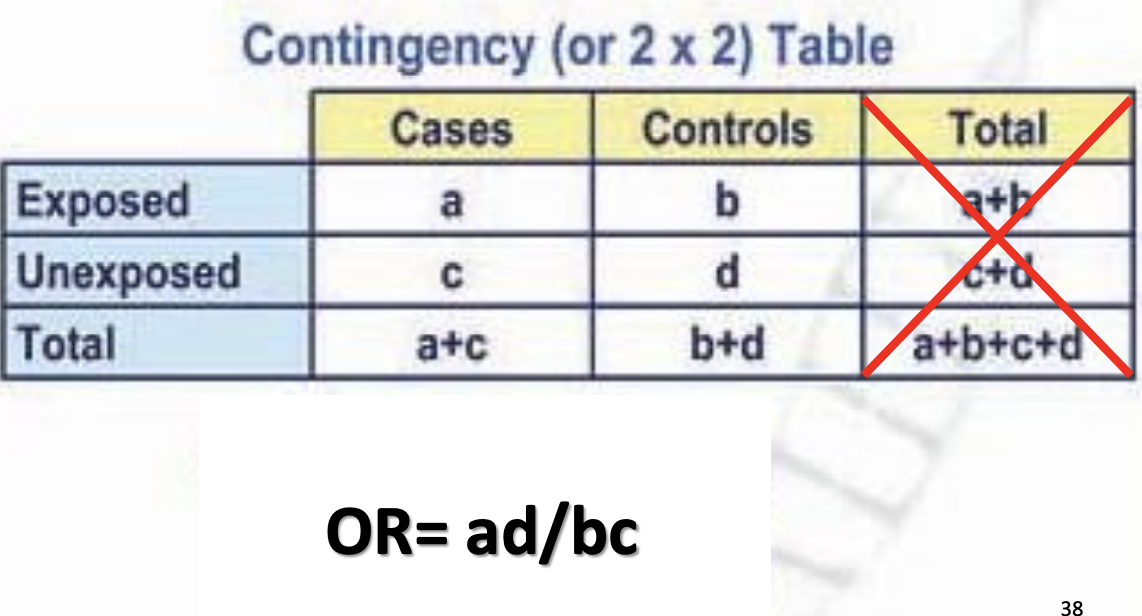
Measure of strength of association
Odds in cases/Odds in controls
a/b / c/d
can be greater than/less than 1
OR > 1 = Positive association
OR = 1 = No association
OR < 1 = Negative association
Advantage
Quick & Inexpensive
only method for studying rare disorders with long lag times between exposure&disease
fewer subjects require
useful for answering clinical questions about etiology and harm
Disadvantages
susceptible to bias
potential for confounding
can only study one outcome
cannot calculate incidence rates for exposed vs unexposed patients
Cohort Studies
Design
Individuals classified by exposure status: can be prospective or retrospective.
Purpose
Examine incidence of disease over time, collecting data on outcomes compared to initial predictors.
prospective → investigator defines the sample and collects data about the predictor before any outcomes have occurred.
retrospective → investigator defines the sample and collects data about the predictor variable after the outcomes have occurred.
Steps in a Prospective Cohort Study
Select a sample from the study cohort
eligible participants need to be free from the disease under study
measure exposure first (risk factor present/absent at the time of study onset)
follow-up the cohort overtime until outcome occurs
measure outcome variable (developed disease or did not develop disease)
Relative Risk
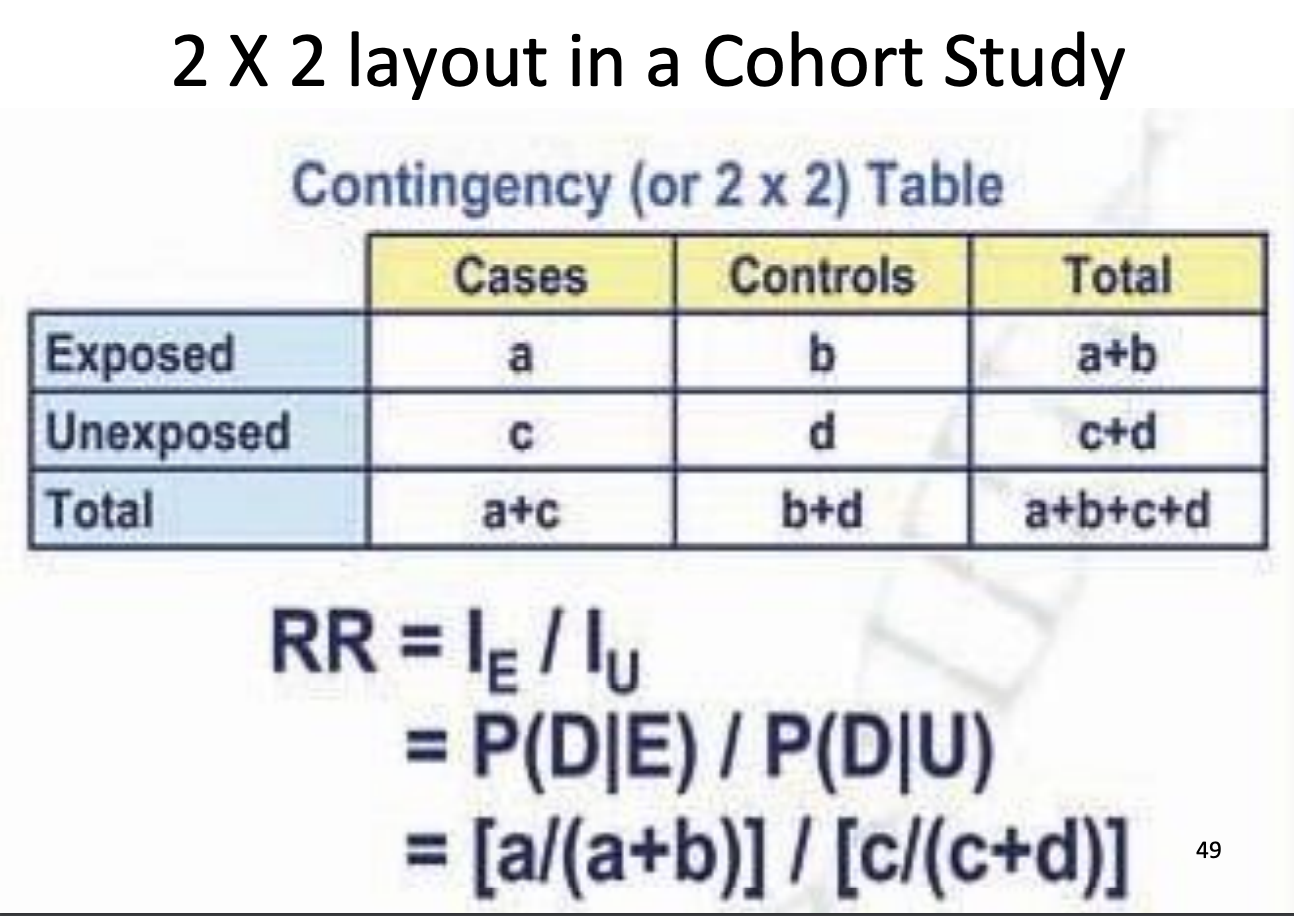
Measure of strength of association
RR>1 = positive association, higher risk
RR=1 = no association
RR<1 = negative association, exposure is protective
Steps in a Retrospective Cohort
Identify a cohort that had been assembled in the past (historical cohort)
Eligible participants need to be free from the disease under study at that past period
collect data on predictor variables (exposure measured first, but in the past)
Follow-up the cohort regarding outcome occurrence
Collect data on outcome variables (measured in the past as per medical records or in the present)
Advantages
allows measurement of mortality rates in exposed vs unexposed
subjects can be matched for possible confounders
can study rare exposure
can examine multiple outcomes
easier and cheaper
useful for answering clinical questions about etiology, harm, or prognosis
Disadvantages
expensive and time consuming
lost to follow-up → serious threat to validity
potential for bias and contamination
blinding of subjects and investigators difficult
inefficient for uncommon outcomes
Experimental Studies
Randomized Controlled Trials (RCTs)
Investigates the cause-effect relationship by randomly allocating participants to either intervention or control groups.
Steps in an RCT
Select a sample and
randomly divide it into experimental and control groups.
The experimental group receives an intervention
Measure outcome variables on both groups.
Advantages
minimal bias
gold standard design for examining treatment efficacy
Disadvantages
Expensive
Time consuming
Loss to follow-up
Quasi-Experimental Studies
Description
Lack random assignment; groups are pre-existing, limiting ability to infer cause-effect relationships.
Example
Investigating pain levels in burn patients by comparing treatment across different centers.
Advantages
practical when random assignment is not feasible due to ethical or practical constraints
Disadvantages
Highly [one to bias and confounding due to lack of randomization → provides lower quality evidence
Conclusion
Types of Clinical Questions
Etiology/Harm: Questions regarding negative impacts from interventions.
Therapy: Questions surrounding treatment efficacy.
Prognosis: Questions about disease progression.
Diagnosis: Questions about identifying disorders in patients presenting with symptoms.
Selection of Study Designs
Different study designs optimize answers to varying clinical questions:
Etiology (cohort, case-control),
Therapy (RCT),
Prognosis (cohort),
Harm (cohort, case-control),
Diagnosis (cross-sectional).