carboxylic acids- reactions
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
why are carboxylic acids called weak acids?
in aqueous solution they are only slightly ionised, to give low concentrations of hydronium ions and alkanoate ions (often called carboxylate ions)
This partial ionisation in solution means that carboxylic acids are weak acids
This means that the position of the equilibrium lies to the left and that the concentration of H+ is much smaller than the concentration of the carboxylic acid
However, the concentration of hydrogen ions is sufficient to react with an aqueous solution of sodium carbonate or sodium hydrogen carbonate to produce carbon dioxide
how aqueous solutions of sodium carbonate or sodium hydrogen carbonate can be used as a test for presence of a carboxylic acid
Sodium carbonate: 2RCOOH + Na2CO3 → 2RCOO-Na+ + CO2 + H2O
Ionic equation with carbonates: 2RCOOH + CO32- → 2RCOO- + CO2 + H2O
Sodium hydrogen carbonate: RCOOH + NaHCO3 → RCOO-Na+ + CO2 + H2O
Ionic equation with hydrogen carbonates: RCOOH + HCO3- → RCOO- + CO2 + H2O
reactions of carboxylic acid with LiAlH4 in dry ether
Carboxylic acids can undergo reduction when they react with a reducing agent such as lithium tetrahydridoaluminate, LiAlH4, suspended in dry ether at room temperature
A carboxylic acid will be reduced to a primary alcohol, for example
CH3CH2COOH (l) + 4[H] → CH3CH2CH2OH (l) + H2O (l)
Addition of water at the end will destroy any excess lithium tetrahydridoaluminate
reduction reaction takes place
reaction of carboxylic aid with bases
a neutralisation reaction occurs
Carboxylate/carboxylic acid salt formed
Carboxylic acids can form salts with metals, alkalis and carbonates.
In the reaction with metal oxides a metal salt and water are produced
2CH3COOH (aq) + MgO (s) → (CH3COO)2Mg (aq) + H2O (l)
In the reaction with alkalis a salt and water are formed in a neutralisation reaction
For example in reaction with potassium hydroxide the salt potassium ethanoate is formed:
CH3COOH (aq) + KOH (aq) → CH3COOK (aq) + H2O (l)
In the reaction with carbonates a metal salt, water and carbon dioxide gas are produced
For example in reaction with potassium carbonate the salt potassium ethanoate is formed:
2CH3COOH (aq) + K2CO3 (s) → 2CH3COOK (aq) + H2O (l) + CO2 (g)
reaction of carboxylic acid with PCl5
Carboxylic acids react with solid phosphorus(V) chloride to form an acyl chloride
For example, propanoic acid will react with phosphorus(V) chloride to form propanoyl chloride, phosphorus trichloride oxide and hydrogen chloride
CH3CH2COOH (l) + PCl5 (s) → CH3CH2COCl (l) + POCl3 (l) + HCl (g)
In this reaction, steamy fumes of HCl are produced
The Acyl chlorideand POCl3 liquid products can be separated by fractional distillation
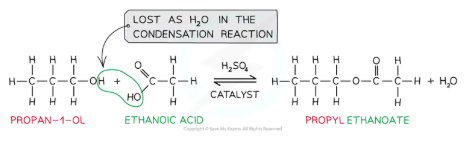
reaction of carboxylic acid with alcohols
When carboxylic acids react with alcohols an ester is formed
Conditions: Warm/acid catalyst, H+ eg concentrated H2SO4 as catalyst
Esters are compounds with an -COOR functional group and are characterised by their sweet and fruity smells
They are prepared from the condensation reaction called esterification between a carboxylic acid and alcohol
The first part of the ester’s name comes from the alcohol and the second part of the name comes from the carboxylic acid
E.g. Propanol and ethanoic acid will give the ester propyl ethanoate
what are acyl chlorides
acyl chlorides are derivatives of carboxylic acids by substitution of the -OH group by a chlorine atom
They are named by identifying the parent hydrocarbon chain and adding the suffix -oyl chloride. They can also be named by removing the -oic acid from the carboxylic acid and adding -oyl chloride
what are acyl anhydrides?
Acid anhydrides are also derivatives of carboxylic acids formed by substitution of the -OH group by an alkanoate
Acid anhydrides are named by identifying the parent hydrocarbon chain and adding the suffix -oic anhydride
They can also be named by removing the -oic acid from the carboxylic acid and adding -oic anyhydride
reactivity of acyl chlorides
the electronegativity of the O and Cl make acyl chlorides very reactive
the S+ carbon is open to nucleophilic attack
In nucleophilic addition-elimination reactions, the nucleophilic addition of a small molecule across the C=O bond takes place followed by elimination of a small molecule
all reactions take place at room temperature. The reactions are vigorous and do not need a catalyst