MC- Molecular Shape Hybridization
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
21 Terms
Why do molecules adopt specific shapes
VSEPR theory - electrons repel each other
-in ammonia and water there are different bonds,lone pairs and bond angles ( due to the lone pairs repel more than bonded pairs so bonds are close together )
Example : methane
-Methane is tetrahedral
-the four pairs of electrons ,one in each bond are repelling each other
-109 degrees bond angle
-there are 4 identical sigma bonds ( from s orbitals) BUT there isn’t four s orbitals there is only one
HOW - Hybridization
What is hybridization ?
atomic orbitals 2s ,2p combine with each other to give four hybridized orbitals
each hybridized orbital contains one electron
the 2 phases can overlap in phase or out of phase
it is used to explain THEORY what we observe vs wha t we expect to happen
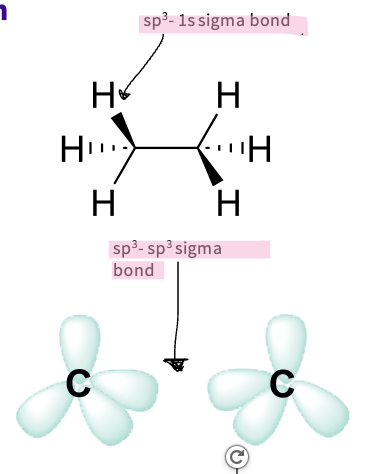
Describe the type of hybridization in alkanes ( ethane)?
ethane-
carbons are sp3 hybridized
Each forms a sigma (σ) bond with:
three hydrogen 1s orbitals
one carbon sp3 hybridised orbital
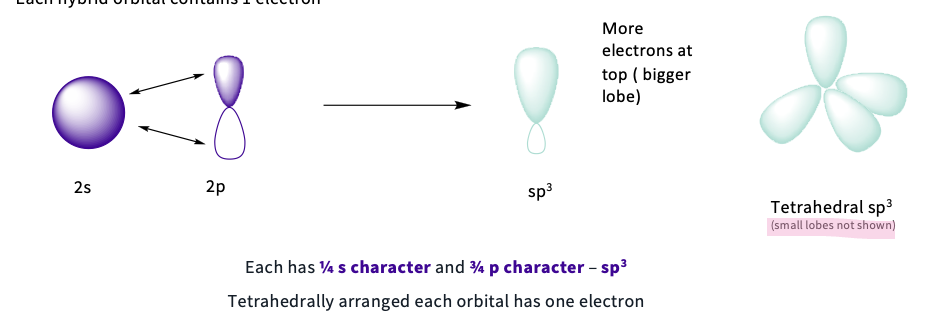
What is sp3 hybridization?
when the 2s orbital combines with 3 x2p orbitals to give 4 hybridized orbitals
note- each has ¼ s character and ¾ p character - sp3 = tetrahedrally arranged
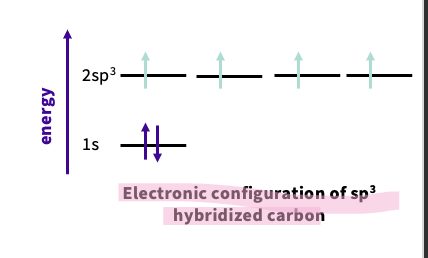
What is the electron configuration of an sp3 hybridized carbon ?
overall energy is the same
-draw sp3 orbital between s and the p
Describe the type of hybridization of carbon in alkenes( Ethene)
carbon has sp2 hybridization
Three sigma (σ) bonds with:
-two hydrogen 1s orbitals
-one carbon – carbon hybridised orbital
-requires 3 hybridised orbitals:
Uses 2px, 2py orbitals
Each has 1/3 s character and 2/3 p character – sp2
Trigonally arranged - all in one plane because of x,y
note - 2pz left unchanged as a p orbital forms a Pi bond ( double bond)
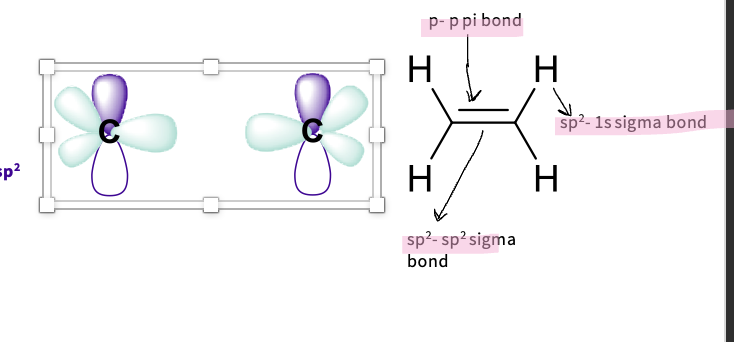
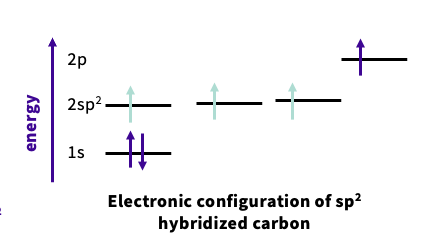
What is sp2 hybridization ?
when 1× 2s orbital combine with 2× 2p orbital
you use the 2px,2py orbital
2pz used to from pi ( double bond)
each has 1/3s character and 2/3 p character - sp2
arranged trigonally
note- this is for all carbon carbon double bonds
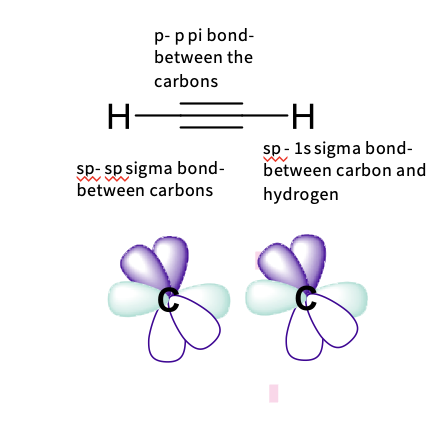
Describe the hybridization in alkyne ( ethyne)
it has sp hybridization
it has a triple bond
Two sigma (σ) bonds with:
-one hydrogen 1s orbitals
-one carbon hybridized orbital
- requires 2 hybridized orbitals
-Uses 2pz orbital
- Each has ½ s character and ½ p character – sp
-Linearly arranged
2px, 2py are left unchanged as p orbitals and form the π bonds
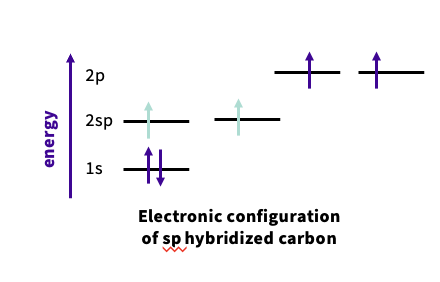
What is sp hybridization ?
-Happens when there is a triple bond
2s orbital combines with a p ( z ) orbital
each has ½ character and ½ character
arranged linearly
What is the electron configuration of sp ?
What are the possible oxygen hybridization?
oxygen has six electrons in its valence shell
it depends on the molecules
types :
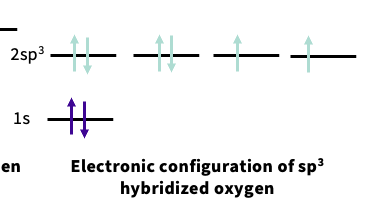
sp3 hybridization- four hybridized orbitals ,two contains 2 electrons
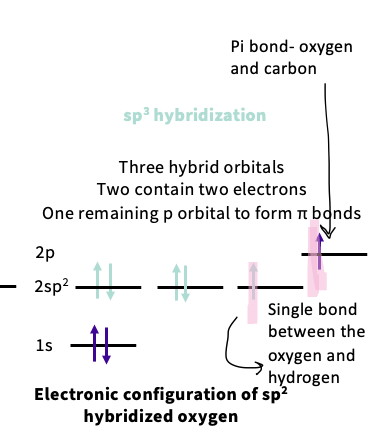
sp2 hybridization - three hybridized orbitals ,two contain 2 electrons,one remaining p orbitals to form Pi orbitals
note- the lone pairs are in the hybridized orbitals at lower energy
electron configuration of sp3 hybridized oxygen
electron configuration of sp2 hybridized oxygen
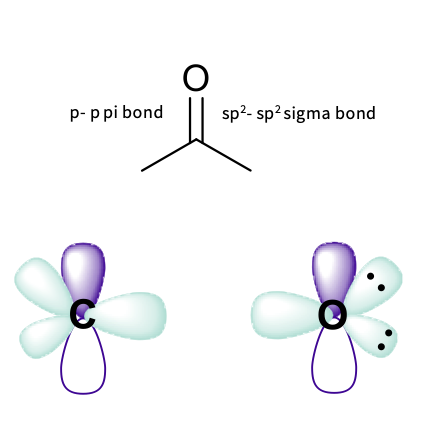
carbonyl groups - hybridized orbitals
the carbon :
-Three sigma bonds
- two hydrogen 1s orbitals
-one oxygen hybridized orbital
-sp2 hybridised
the oxygen :
-one sigma bond – overlap of sp2 hybridized orbitals
-one pi bond – overlap of p orbitals
- lone pairs are in non bonding hybrid orbitals
Describe electrons in nitrogens valence shell ?
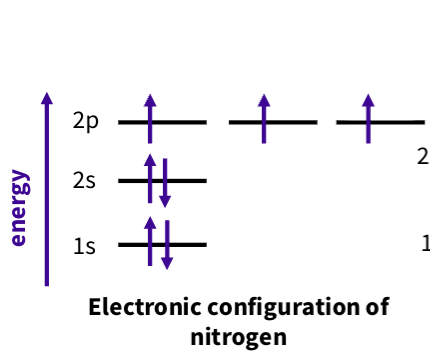
Five electrons in valence shell
two in the 2s, two in the 2px and one each in 2py and 2pz
What is the elctron configuration for nitrogen?
2 electron sin 2s ,one in each 2p
What is the sp3 hybridization of nitrogen ?
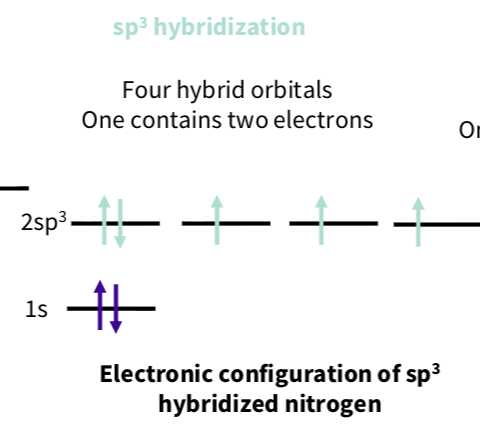
What is sp2 hybridization of nitrogens ?
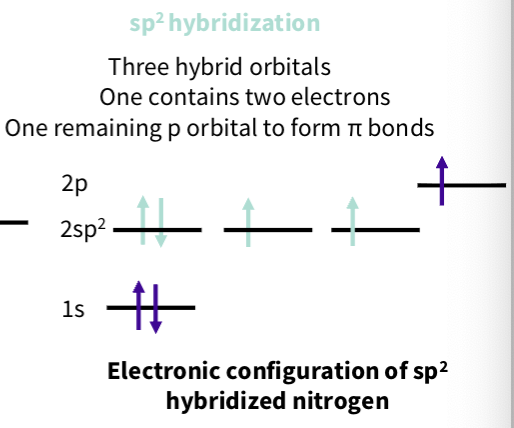
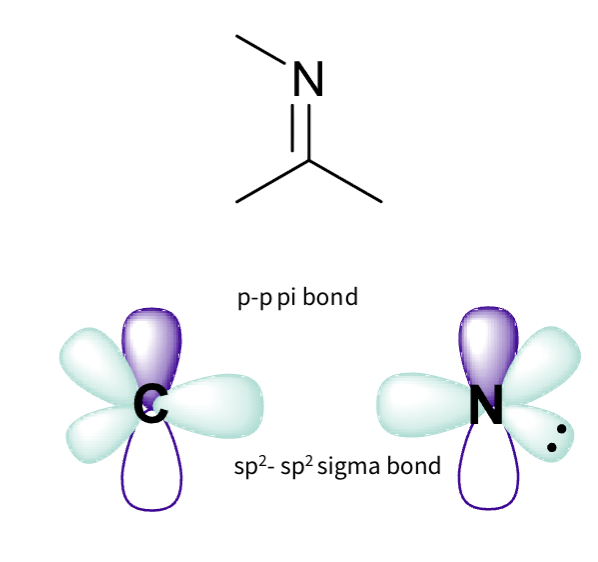
what is an imine group hybridization ?
imine- carbon doble bond nitrogen
Carbon :
Three sigma bonds
two hydrogen 1s orbitals
one oxygen hybridised orbital
sp2 hybridised
Nitrogen:
two sigma bonds – overlap of sp2 hybridised orbitals
one pi bond – overlap of p orbitals
lone pair is in non bonding hybrid orbitals
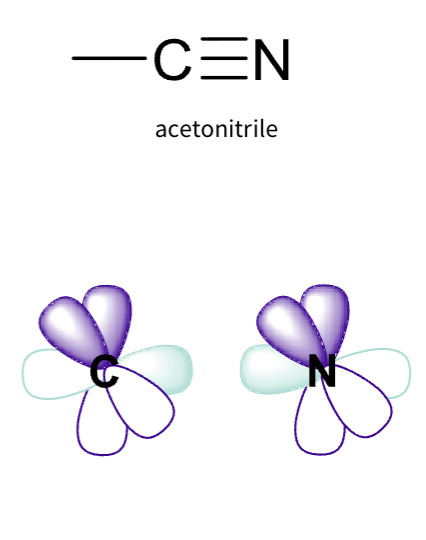
what is acetonitrile hybridization ?
sp hybridisation
Two hybrid orbitals
One contains two electrons – the lone pair
Two remaining p orbitals to form π bonds