Chem/Phys I
0.0(0)
Card Sorting
1/154
There's no tags or description
Looks like no tags are added yet.
Last updated 4:56 AM on 7/21/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
155 Terms
1
New cards
1 μm
10^-6 m
2
New cards
1000g
1kg
3
New cards
100cm
1m
4
New cards
10dm
1m
5
New cards
1000mm
1m
6
New cards
1 nm (nanometer)
10^-9 m
7
New cards
Bose-Einstein principle
collection of atoms cooled to absolute zero will form into a single quantum state.
8
New cards
Heisenberg Uncertainty Principle
one cannot know both the momentum and position of an object with absolute certainty
9
New cards
Le Chateliers Principle
in a reversible process, when stress is applied to a system it will react back by relieving that stress
10
New cards
Pauli Exclusion Principle
two or more identical electrons cannot occupy the same quantum state
11
New cards
protease inhibitors
* a class of drugs that inhibit the activity of proteases, enzymes responsible for breaking down proteins.
* work by blocking the protease enzyme, preventing the virus from replicating and spreading.
* They are commonly used in the treatment of viral infections, particularly HIV/AIDS.
* Examples include ritonavir, saquinavir, and lopinavir.
* work by blocking the protease enzyme, preventing the virus from replicating and spreading.
* They are commonly used in the treatment of viral infections, particularly HIV/AIDS.
* Examples include ritonavir, saquinavir, and lopinavir.
12
New cards
dissociation constant
* denoted as Kd, is a measure of the strength of a chemical bond or the tendency of a compound to dissociate into its constituent parts.
* It is commonly used in chemistry to describe the equilibrium between a compound and its dissociated ions or molecules.
* such as acid-base interactions & protein-ligand interactions
* The Kd value represents the concentration of the dissociated species divided by the concentration of the undissociated species at equilibrium.
* It is typically expressed as a ratio or in logarithmic form.
* It is commonly used in chemistry to describe the equilibrium between a compound and its dissociated ions or molecules.
* such as acid-base interactions & protein-ligand interactions
* The Kd value represents the concentration of the dissociated species divided by the concentration of the undissociated species at equilibrium.
* It is typically expressed as a ratio or in logarithmic form.
13
New cards
enzyme-bound inhibitor
* refers to a molecule that binds to an enzyme, inhibiting its activity.
* occurs in interactions such as *competitive inhibition*, where the inhibitor competes with the substrate for the enzyme's active site, or *non-competitive inhibition*, where the inhibitor binds to a different site on the enzyme, altering its conformation and reducing its activity.
* occurs in interactions such as *competitive inhibition*, where the inhibitor competes with the substrate for the enzyme's active site, or *non-competitive inhibition*, where the inhibitor binds to a different site on the enzyme, altering its conformation and reducing its activity.
14
New cards
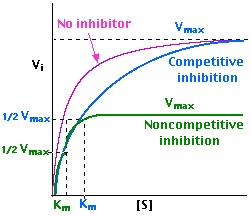
Michaelis Menten Kinetics
* a mathematical model used to describe the rate of enzymatic reactions. It is based on the assumption that the enzyme-substrate complex forms reversibly and that the rate-limiting step is the breakdown of this complex to form products.
* consists of the Michaelis constant (Km), which represents the substrate concentration at which the reaction rate is half of its maximum, and the maximum reaction rate (Vmax).
* consists of the Michaelis constant (Km), which represents the substrate concentration at which the reaction rate is half of its maximum, and the maximum reaction rate (Vmax).
15
New cards
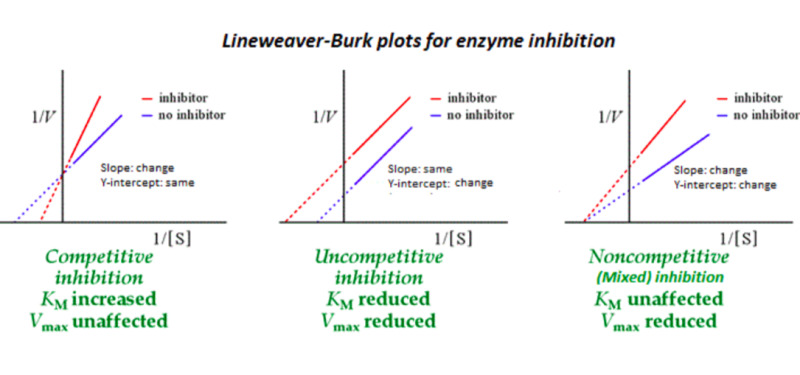
Lineweaver Burk Plot
* known as a double reciprocal plot, is a graphical representation used in enzyme kinetics.
* It is created by plotting the reciprocal of the initial reaction rate against the reciprocal of the substrate concentration.
* The plot can be used to determine the kinetic parameters of an enzyme, such as the maximum reaction rate (Vmax) and the Michaelis-Menten constant (Km).
* The slope of the plot represents the Km/Vmax ratio, while the y-intercept represents 1/Vmax.
* It is created by plotting the reciprocal of the initial reaction rate against the reciprocal of the substrate concentration.
* The plot can be used to determine the kinetic parameters of an enzyme, such as the maximum reaction rate (Vmax) and the Michaelis-Menten constant (Km).
* The slope of the plot represents the Km/Vmax ratio, while the y-intercept represents 1/Vmax.
16
New cards
hydrogen bond
* a type of intermolecular bond that occurs between a hydrogen atom and an electronegative atom, such as oxygen, nitrogen, or fluorine.
* play a crucial role in various biological processes, such as DNA structure, protein folding, and water's unique properties.
* They contribute to the high boiling point and surface tension of water, as well as its ability to dissolve many substances.
* play a crucial role in various biological processes, such as DNA structure, protein folding, and water's unique properties.
* They contribute to the high boiling point and surface tension of water, as well as its ability to dissolve many substances.
17
New cards
hydrogen bond donor
* a molecule or functional group that can donate a hydrogen atom to form a hydrogen bond with an acceptor.
* Common examples include hydroxyl groups (-OH), amino groups (-NH2), and carboxylic acid groups (-COOH)
* Common examples include hydroxyl groups (-OH), amino groups (-NH2), and carboxylic acid groups (-COOH)
18
New cards
hydrogen bond acceptor
* a molecule or atom that can accept a hydrogen bond.
* typically has a lone pair of electrons available for bonding with a hydrogen atom that is already bonded to another electronegative atom.
* Examples include oxygen, nitrogen, and fluorine atoms in molecules such as water (H2O) and ammonia (NH3).
* typically has a lone pair of electrons available for bonding with a hydrogen atom that is already bonded to another electronegative atom.
* Examples include oxygen, nitrogen, and fluorine atoms in molecules such as water (H2O) and ammonia (NH3).
19
New cards
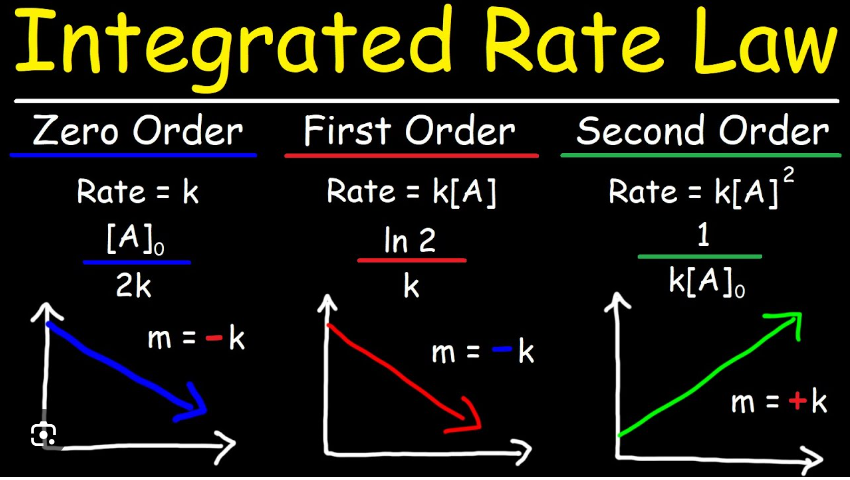
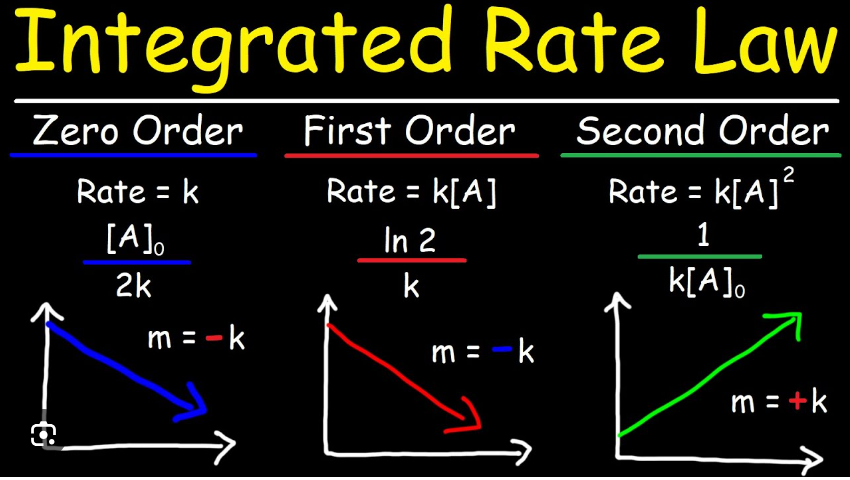
zero order rate constant
* The rate of the reaction is independent of the concentration of the reactant(s).
* The rate equation: Rate = k
* The units: mole L^-1 s^-1
* The rate equation: Rate = k
* The units: mole L^-1 s^-1
20
New cards
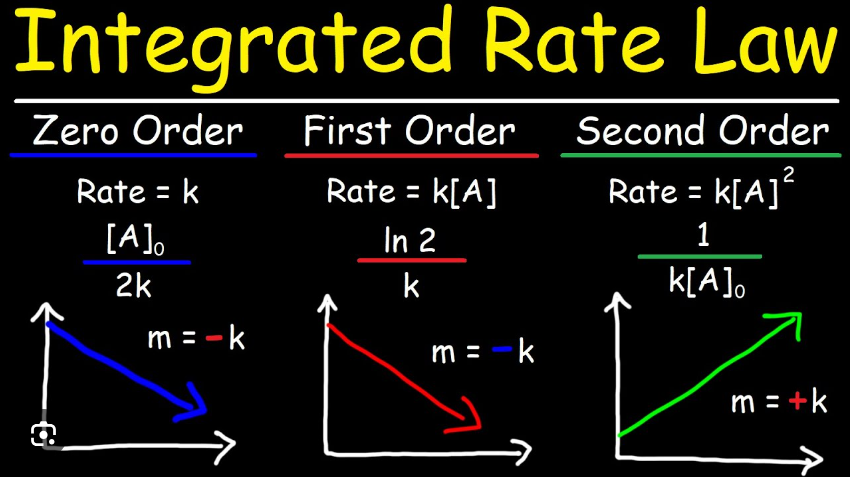
First-order rate constant (k)
* Rate of reaction for a reactant concentration decreasing exponentially.
* units: s^-1
* units: s^-1
21
New cards
second order rate constant
* a measure of the rate reaction occurs.
* It is expressed in units of L mole^-1 s^-1.
* The value of k can be determined experimentally by measuring the rate of reaction at different concentrations of reactants and using the rate equation.
* It is expressed in units of L mole^-1 s^-1.
* The value of k can be determined experimentally by measuring the rate of reaction at different concentrations of reactants and using the rate equation.
22
New cards
Kcat (catalytic rate of an enzyme)
Speed at which an enzyme catalyzes a reaction, measured in substrate molecules converted per enzyme molecule per unit of time.
23
New cards
oxidoreductase
* It is an enzyme that catalyzes oxidation-reduction reactions in biological systems.
* It transfers electrons from one molecule to another, often involving the transfer of hydrogen atoms.
* It transfers electrons from one molecule to another, often involving the transfer of hydrogen atoms.
24
New cards
reductase
It is a type of enzyme that catalyzes reduction reactions, which involve the gain of electrons or the removal of oxygen atoms from a molecule.
25
New cards
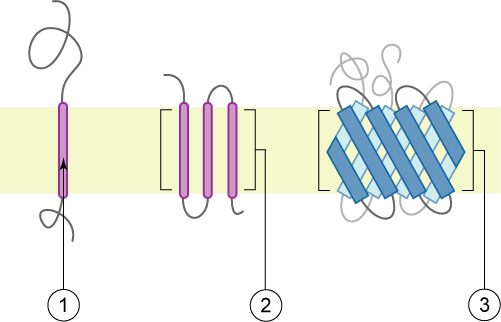
transmembrane protein
* a type of protein that spans across the cell membrane, with portions of it located on both the inside and outside of the cell.
* It plays a crucial role in various cellular processes such as cell signaling, transport of molecules across the membrane, and cell adhesion.
* has hydrophobic regions that anchor them within the lipid bilayer of the cell membrane, while their hydrophilic regions interact with the aqueous environments on both sides of the membrane.
* Examples include ion channels, receptors, and transporters.
* It plays a crucial role in various cellular processes such as cell signaling, transport of molecules across the membrane, and cell adhesion.
* has hydrophobic regions that anchor them within the lipid bilayer of the cell membrane, while their hydrophilic regions interact with the aqueous environments on both sides of the membrane.
* Examples include ion channels, receptors, and transporters.
26
New cards
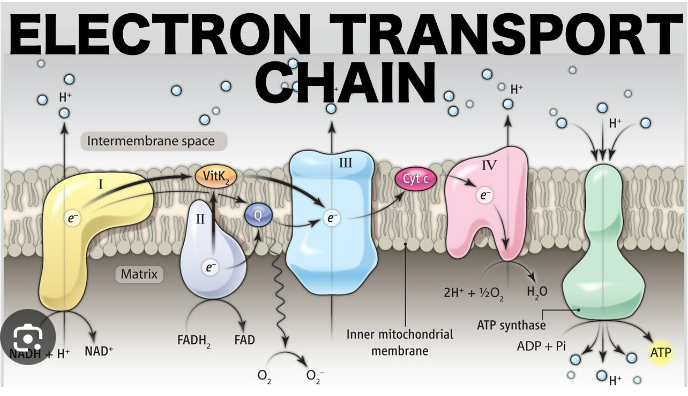
electron transport pathway
* a series of protein complexes and molecules located in the inner mitochondrial membrane.
* It plays a crucial role in cellular respiration, specifically in the process of oxidative phosphorylation.
* During this pathway, electrons are transferred from electron donors, such as NADH and FADH2, to electron acceptors, such as oxygen.
* This transfer of electrons generates a proton gradient across the inner mitochondrial membrane, which is then used to produce ATP, the energy currency of the cell.
* It plays a crucial role in cellular respiration, specifically in the process of oxidative phosphorylation.
* During this pathway, electrons are transferred from electron donors, such as NADH and FADH2, to electron acceptors, such as oxygen.
* This transfer of electrons generates a proton gradient across the inner mitochondrial membrane, which is then used to produce ATP, the energy currency of the cell.
27
New cards
spectroelectrochemistry
* a field that combines spectroscopy and electrochemistry.
* It involves studying the interaction between light and matter in electrochemical systems.
* By applying an electric potential to a sample, spectroscopic techniques can be used to monitor changes in the sample's electronic structure and properties.
* It involves studying the interaction between light and matter in electrochemical systems.
* By applying an electric potential to a sample, spectroscopic techniques can be used to monitor changes in the sample's electronic structure and properties.
28
New cards
midpoint potentials
* redox potentials of a half-reaction when the reactants and products are present in equal concentrations (equilibrium)
* equal amounts of oxidized and reduced species
* They are often used to determine the thermodynamic feasibility of a redox reaction.
* typically measured in volts (V)
* equal amounts of oxidized and reduced species
* They are often used to determine the thermodynamic feasibility of a redox reaction.
* typically measured in volts (V)
29
New cards
cofactors
enzymes that help to stabilize a reaction
30
New cards
phosphatase
* an enzyme that catalyzes the hydrolysis of phosphate ester bonds.
* removes phosphate groups from molecules, such as proteins, nucleotides, and sugars
* removes phosphate groups from molecules, such as proteins, nucleotides, and sugars
31
New cards
amidase
* an enzyme that catalyzes the hydrolysis of amide bonds in molecules.
* involved in the breakdown of molecules into their respective acids and amines.
* play a crucial role in various biological processes, including metabolism and the recycling of nitrogen-containing compounds.
* involved in the breakdown of molecules into their respective acids and amines.
* play a crucial role in various biological processes, including metabolism and the recycling of nitrogen-containing compounds.
32
New cards
protease
* a type of enzyme that helps in the breakdown of proteins into smaller peptides or amino acids.
* plays a crucial role in various biological processes, including digestion, cellular signaling, and protein regulation.
* plays a crucial role in various biological processes, including digestion, cellular signaling, and protein regulation.
33
New cards
esterase
* an enzyme that catalyzes the hydrolysis of ester bonds.
* They are involved in various biological processes, including the metabolism of drugs, toxins, and neurotransmitters.
* play a crucial role in the breakdown of ester-containing compounds, such as triglycerides and ester *prodrugs - biologically inactive compound which can be metabolized in the body to produce a drug.*
* They are involved in various biological processes, including the metabolism of drugs, toxins, and neurotransmitters.
* play a crucial role in the breakdown of ester-containing compounds, such as triglycerides and ester *prodrugs - biologically inactive compound which can be metabolized in the body to produce a drug.*
34
New cards
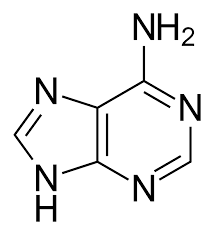
Adenine
* one of the four nitrogenous bases found in DNA and RNA.
* a purine base, along with guanine, and is paired with thymine in DNA or uracil in RNA
* fundamental component of genetic material
* found only in FAD
* a purine base, along with guanine, and is paired with thymine in DNA or uracil in RNA
* fundamental component of genetic material
* found only in FAD
35
New cards
Flavin
* a class of organic compounds that contain a flavin ring system.
* important coenzymes involved in various biological processes, such as redox reactions and energy metabolism.
* They play a crucial role in enzymatic reactions, acting as electron carriers and participating in oxidation-reduction reactions.
* important coenzymes involved in various biological processes, such as redox reactions and energy metabolism.
* They play a crucial role in enzymatic reactions, acting as electron carriers and participating in oxidation-reduction reactions.
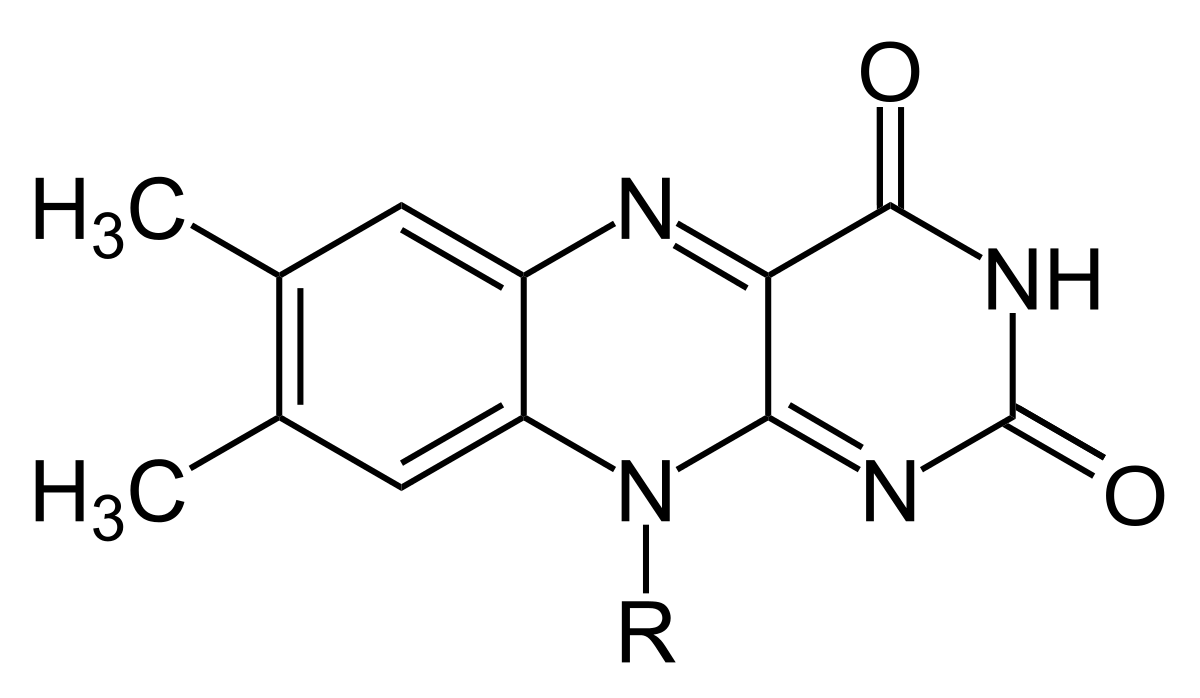
36
New cards
ubiquinone
* known as coenzyme Q10, is a lipid-soluble molecule found in the mitochondria of cells.
* It plays a crucial role in the electron transport chain, which is involved in cellular respiration and energy production.
* acts as an electron carrier, shuttling electrons between different protein complexes in the mitochondria.
* It is also a potent antioxidant, protecting cells from oxidative damage.
* It plays a crucial role in the electron transport chain, which is involved in cellular respiration and energy production.
* acts as an electron carrier, shuttling electrons between different protein complexes in the mitochondria.
* It is also a potent antioxidant, protecting cells from oxidative damage.
37
New cards
amino acid
* organic compounds that serve as the building blocks of proteins. They contain an amino group (-NH2), a carboxyl group (-COOH), and a side chain (R group) attached to a central carbon atom.
* phrases: ‘L49S’ - means Leucine was replaced with Serine at position 49
* phrases: ‘L49S’ - means Leucine was replaced with Serine at position 49
38
New cards
histidine
* abbreviations: His, H
* class: basic & hydrophilic
* involved in methylation
* precursor for the synthesis of histamine, which is involved in immune responses and neurotransmission.
* pKa = 6
* class: basic & hydrophilic
* involved in methylation
* precursor for the synthesis of histamine, which is involved in immune responses and neurotransmission.
* pKa = 6
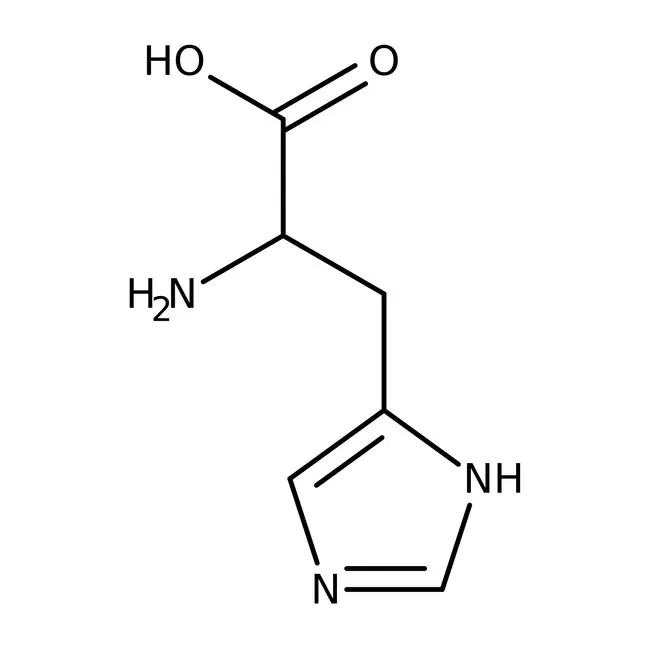
39
New cards
Arginine
* abbreviations: Arg, R
* class: basic & hydrophilic
* pKa = 12.5
* class: basic & hydrophilic
* pKa = 12.5
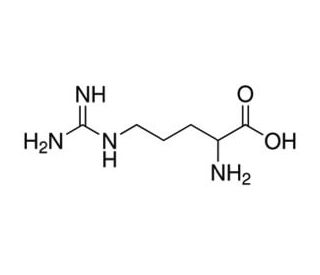
40
New cards
lysine
* abbreviations: Lys, K
* class: basic & hydrophilic
* involved in acetylation
* pKa = 10.4
* class: basic & hydrophilic
* involved in acetylation
* pKa = 10.4
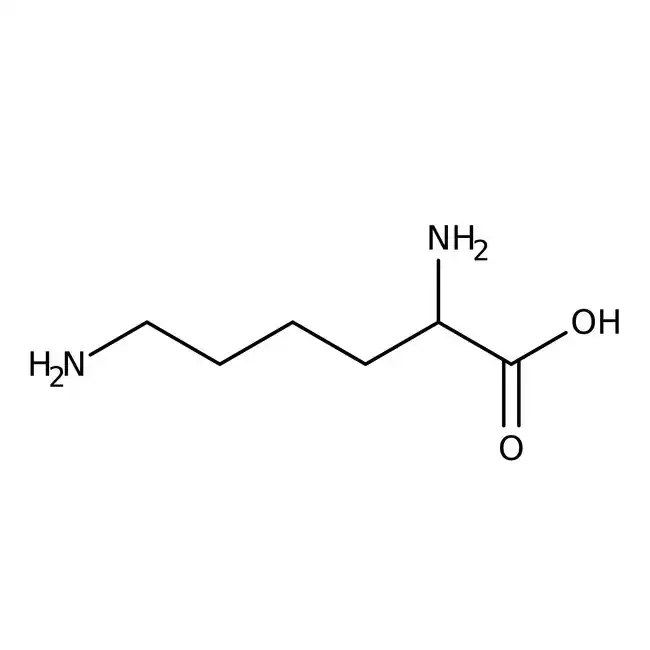
41
New cards
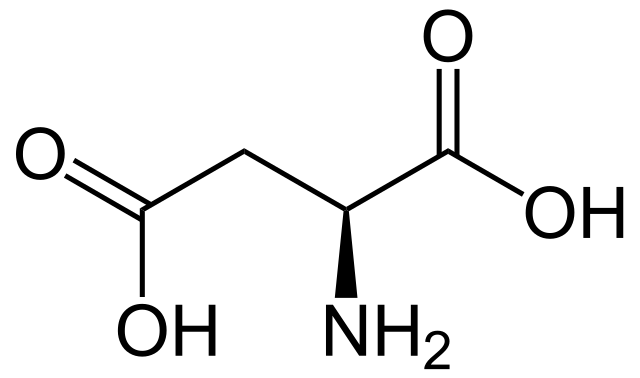
Glutamic Acid
* abbreviations: Glu, E
* class: acidic & hydrophilic
* negatively charged \~ glutamate
* involved in carboxylation
* pKa = 4.25
* class: acidic & hydrophilic
* negatively charged \~ glutamate
* involved in carboxylation
* pKa = 4.25
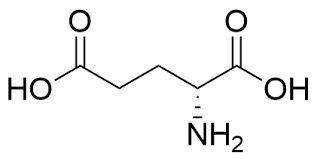
42
New cards
Aspartic acid
* abbreviations: Asp, D
* class: acidic & hydrophilic
* negatively charged \~ aspartate
* pKa = 3.86
* class: acidic & hydrophilic
* negatively charged \~ aspartate
* pKa = 3.86
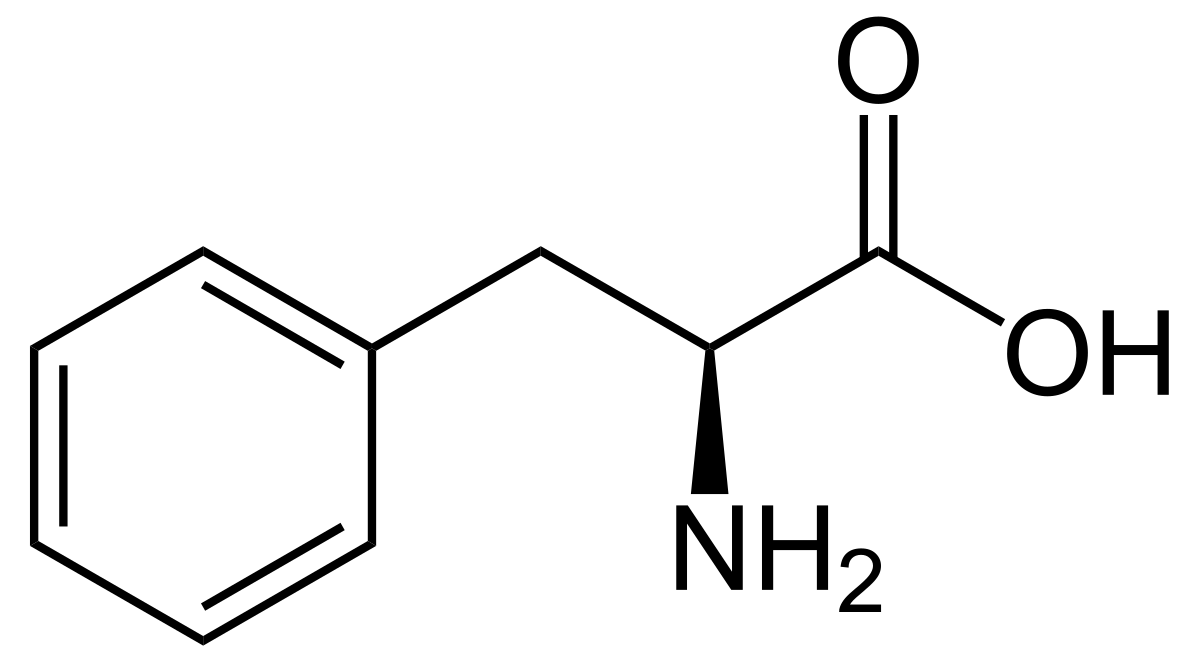
43
New cards
phenylalanine
* abbreviations: Phe, F
* class: neutral & hydrophobic
* aromatic
* class: neutral & hydrophobic
* aromatic
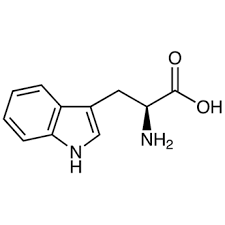
44
New cards
tryptophan
* abbreviations: Trp, W
* class: neutral & hydrophobic
* dipolar ion at neutral pH
* aromatic
* class: neutral & hydrophobic
* dipolar ion at neutral pH
* aromatic
45
New cards
tyrosine
* abbreviations: Tyr, Y
* class: neutral & hydrophilic
* dipolar ion at neutral pH
* aromatic
* involved in phosphorylation & hydrogen-bonding
* pKa = 10
* class: neutral & hydrophilic
* dipolar ion at neutral pH
* aromatic
* involved in phosphorylation & hydrogen-bonding
* pKa = 10
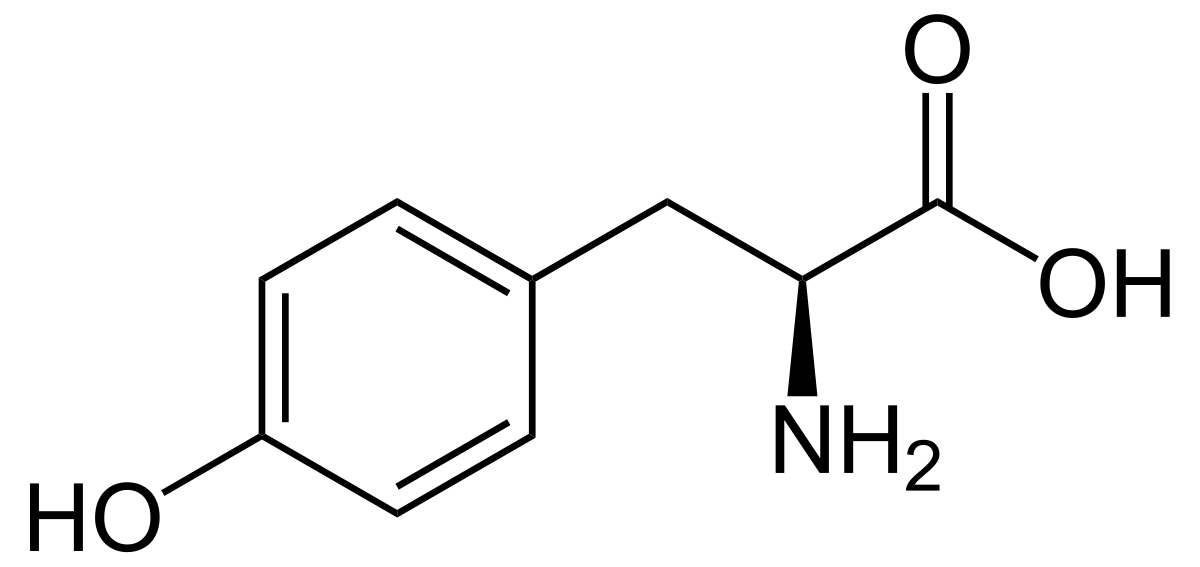
46
New cards
proline
* abbreviations: Pro, P
* class: neutral & hydrophobic
* involved in hydroxylation
* It is unique among amino acids because it has a secondary amino group instead of a primary amino group.
* plays a crucial role in protein structure and stability, as it can form a rigid kink in the protein chain due to its cyclic structure.
* involved in collagen synthesis - important for structure & function of connective tissues in the body.
* uses Vitamin C as a cofactor
* class: neutral & hydrophobic
* involved in hydroxylation
* It is unique among amino acids because it has a secondary amino group instead of a primary amino group.
* plays a crucial role in protein structure and stability, as it can form a rigid kink in the protein chain due to its cyclic structure.
* involved in collagen synthesis - important for structure & function of connective tissues in the body.
* uses Vitamin C as a cofactor
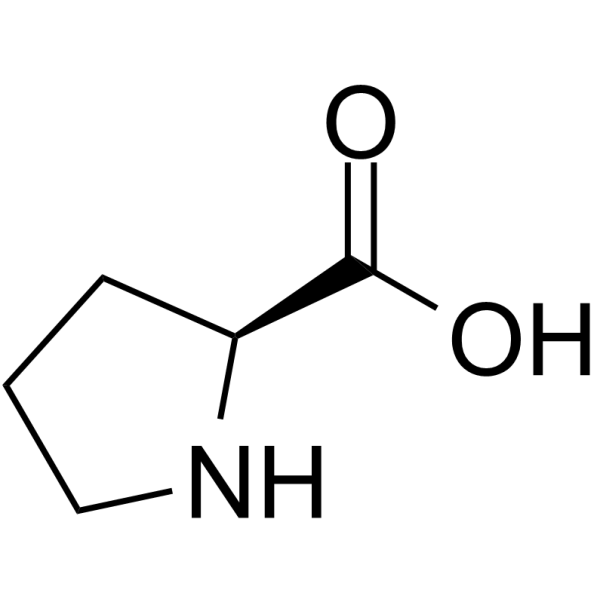
47
New cards
glycine
* abbreviations: Gly, G
* class: neutral & hydrophobic
* smallest amino acid and is achiral
* class: neutral & hydrophobic
* smallest amino acid and is achiral
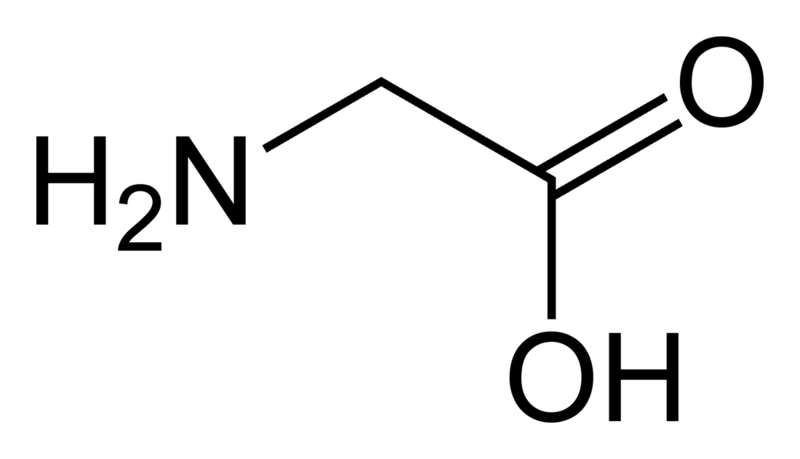
48
New cards
alanine
* abbreviations: Ala, A
* class: neutral & hydrophobic
* plays a crucial role in the metabolism of glucose and helps in the production of energy.
* important for the synthesis of proteins and the regulation of blood sugar levels.
* class: neutral & hydrophobic
* plays a crucial role in the metabolism of glucose and helps in the production of energy.
* important for the synthesis of proteins and the regulation of blood sugar levels.
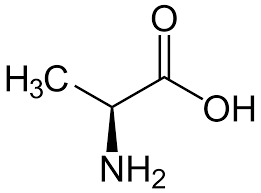
49
New cards
Valine
* abbreviations: Val, V
* class: neutral & hydrophobic
* dipolar ion at neutral pH
* plays a crucial role in protein synthesis and muscle metabolism.
* class: neutral & hydrophobic
* dipolar ion at neutral pH
* plays a crucial role in protein synthesis and muscle metabolism.
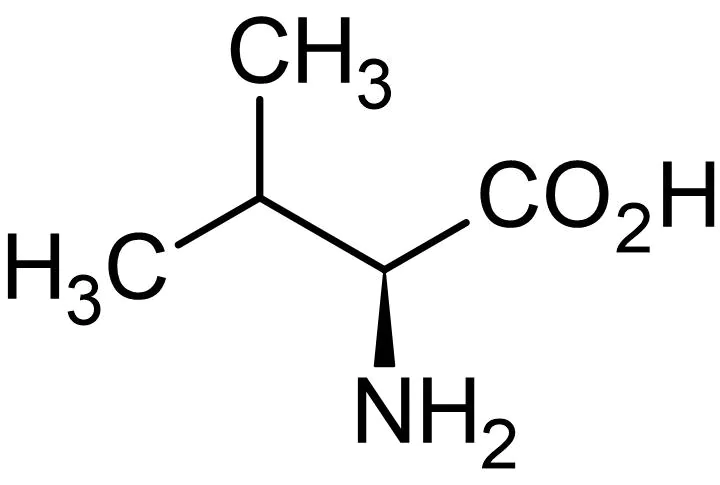
50
New cards
leucine
* abbreviations: Leu, L
* class: neutral & hydrophobic
* plays a crucial role in protein synthesis and muscle growth.
* class: neutral & hydrophobic
* plays a crucial role in protein synthesis and muscle growth.
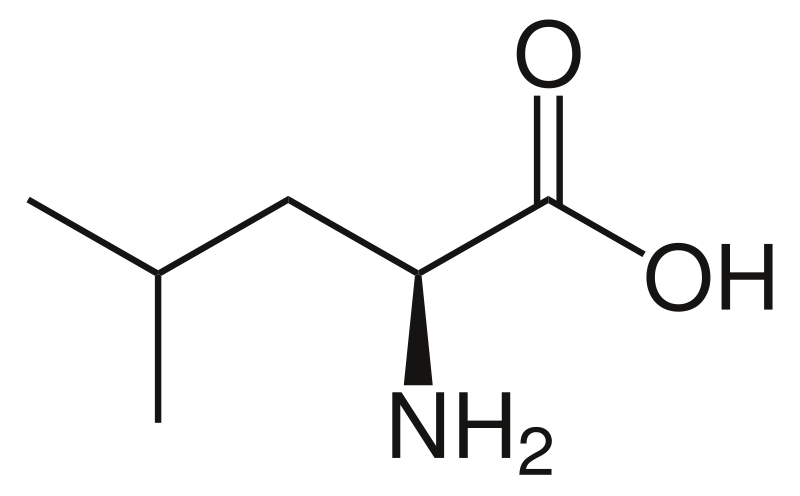
51
New cards
methionine
* abbreviations: Met, M
* class: neutral & hydrophobic
* important for protein synthesis and various metabolic processes in the body.
* class: neutral & hydrophobic
* important for protein synthesis and various metabolic processes in the body.
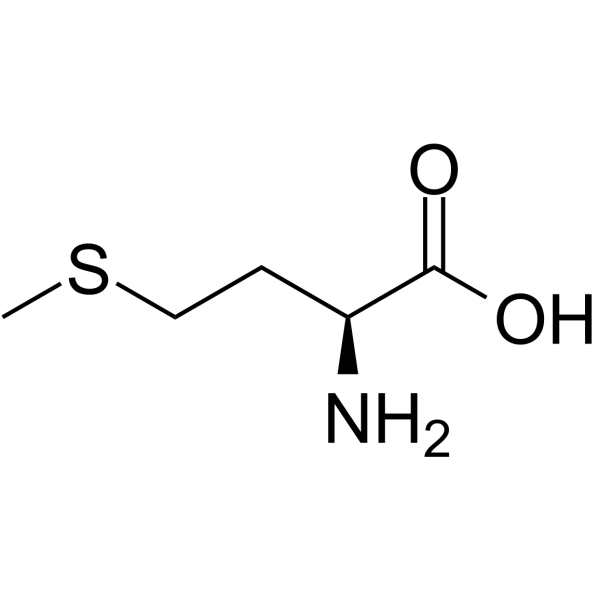
52
New cards
isoleucine
* abbreviations: Ile, I
* class: neutral & hydrophobic
* dipolar ion at neutral pH
* an essential amino acid that is used by the body for protein synthesis.
* important for muscle growth, tissue repair, and the production of hemoglobin.
* class: neutral & hydrophobic
* dipolar ion at neutral pH
* an essential amino acid that is used by the body for protein synthesis.
* important for muscle growth, tissue repair, and the production of hemoglobin.
53
New cards
serine
* abbreviations: Ser, S
* class: neutral & hydrophilic
* dipolar ion at neutral pH
* involved in phosphorylation & hydrogen-bonding
* plays a crucial role in various biological processes, including protein synthesis, cell signaling, and the metabolism of fats and fatty acids.
* involved in the production of neurotransmitters and the synthesis of DNA and RNA.
* class: neutral & hydrophilic
* dipolar ion at neutral pH
* involved in phosphorylation & hydrogen-bonding
* plays a crucial role in various biological processes, including protein synthesis, cell signaling, and the metabolism of fats and fatty acids.
* involved in the production of neurotransmitters and the synthesis of DNA and RNA.
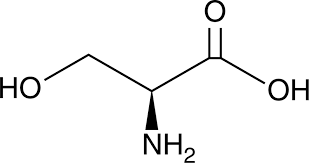
54
New cards
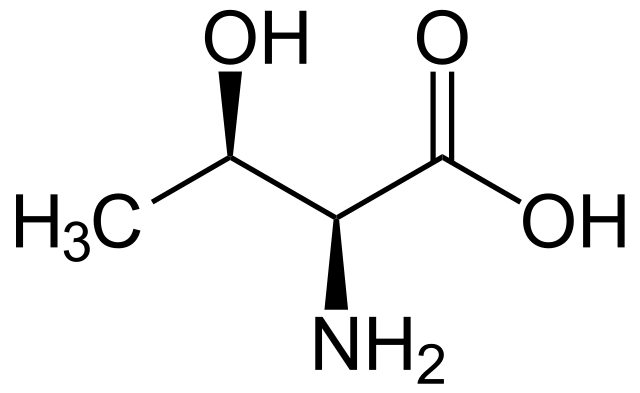
Threonine
* abbreviations: Thr, T
* class: neutral & hydrophilic
* dipolar ion at neutral pH
* involved in phosphorylation & hydrogen-bonding
* plays a crucial role in supporting the growth and maintenance of tissues, as well as in the production of antibodies and enzymes.
* It is also involved in the formation of collagen and elastin, which are important for healthy skin, hair, and nails.
* class: neutral & hydrophilic
* dipolar ion at neutral pH
* involved in phosphorylation & hydrogen-bonding
* plays a crucial role in supporting the growth and maintenance of tissues, as well as in the production of antibodies and enzymes.
* It is also involved in the formation of collagen and elastin, which are important for healthy skin, hair, and nails.
55
New cards
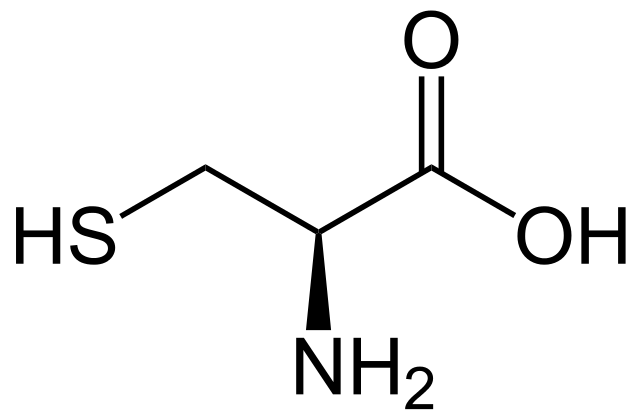
Cysteine
* abbreviations: Cys, C
* class: neutral & hydophilic
* an amino acid that contains a thiol group (-SH) in its side chain.
* plays a crucial role in protein structure and function, as it can form disulfide bridges/bonds with other cysteine residues when oxidized, contributing to protein stability.
* class: neutral & hydophilic
* an amino acid that contains a thiol group (-SH) in its side chain.
* plays a crucial role in protein structure and function, as it can form disulfide bridges/bonds with other cysteine residues when oxidized, contributing to protein stability.
56
New cards
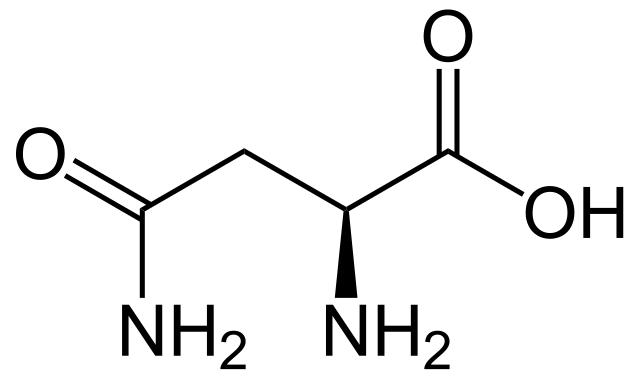
Asparagine
* abbreviations: Asn, N
* class: neutral & hydrophilic
* dipolar ion at neutral pH
* class: neutral & hydrophilic
* dipolar ion at neutral pH
57
New cards
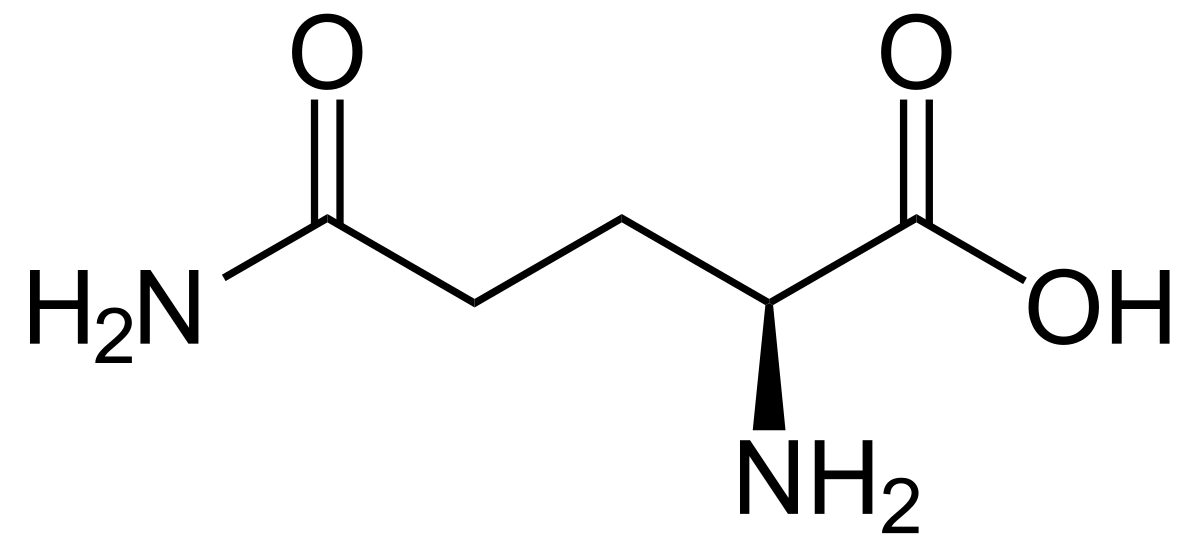
Glutamine
* Abbreviations: Gln, Q
* class: neutral & hydrophilic
* plays a crucial role in various biological processes, including protein synthesis, immune function, and intestinal health.
* also known to be an important fuel source for certain cells, such as immune cells and cells lining the intestines.
* class: neutral & hydrophilic
* plays a crucial role in various biological processes, including protein synthesis, immune function, and intestinal health.
* also known to be an important fuel source for certain cells, such as immune cells and cells lining the intestines.
58
New cards
Strecker synthesis
* a method used in organic chemistry to synthesize amino acids from aldehydes or ketones.
* It involves the reaction of the carbonyl compound with ammonia and cyanide ion, followed by hydrolysis to yield the corresponding amino acid.
* It involves the reaction of the carbonyl compound with ammonia and cyanide ion, followed by hydrolysis to yield the corresponding amino acid.
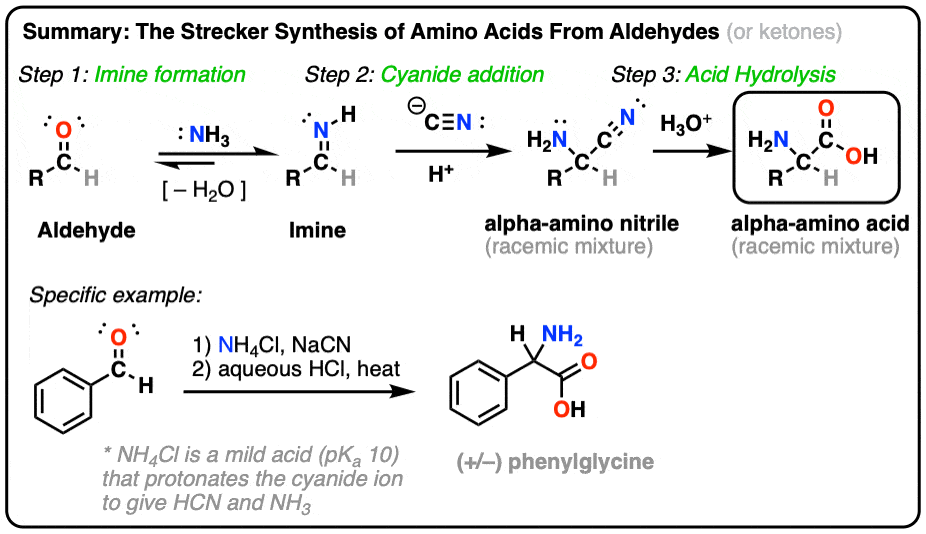
59
New cards
Gabriel synthesis
* a method used in organic chemistry to prepare primary amines from alkyl halides.
* It involves the reaction of an alkyl halide with phthalimide, followed by hydrolysis and decarboxylation.
* demonstrates the conversion of a primary alkyl halide to a primary amine.
* It involves the reaction of an alkyl halide with phthalimide, followed by hydrolysis and decarboxylation.
* demonstrates the conversion of a primary alkyl halide to a primary amine.
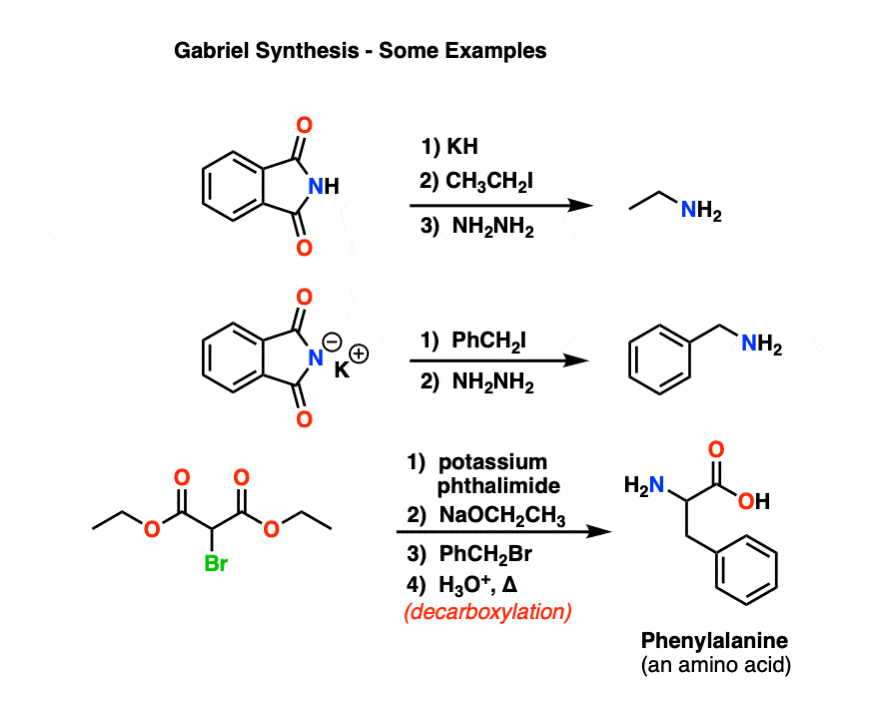
60
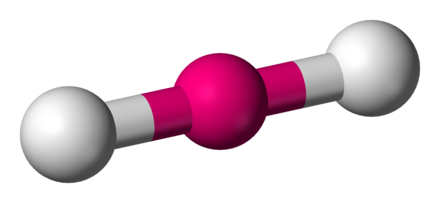
New cards
molecular geometry: linear
2 electron domains & no lone pairs in central molecule
180 degrees
ex. CO2
180 degrees
ex. CO2
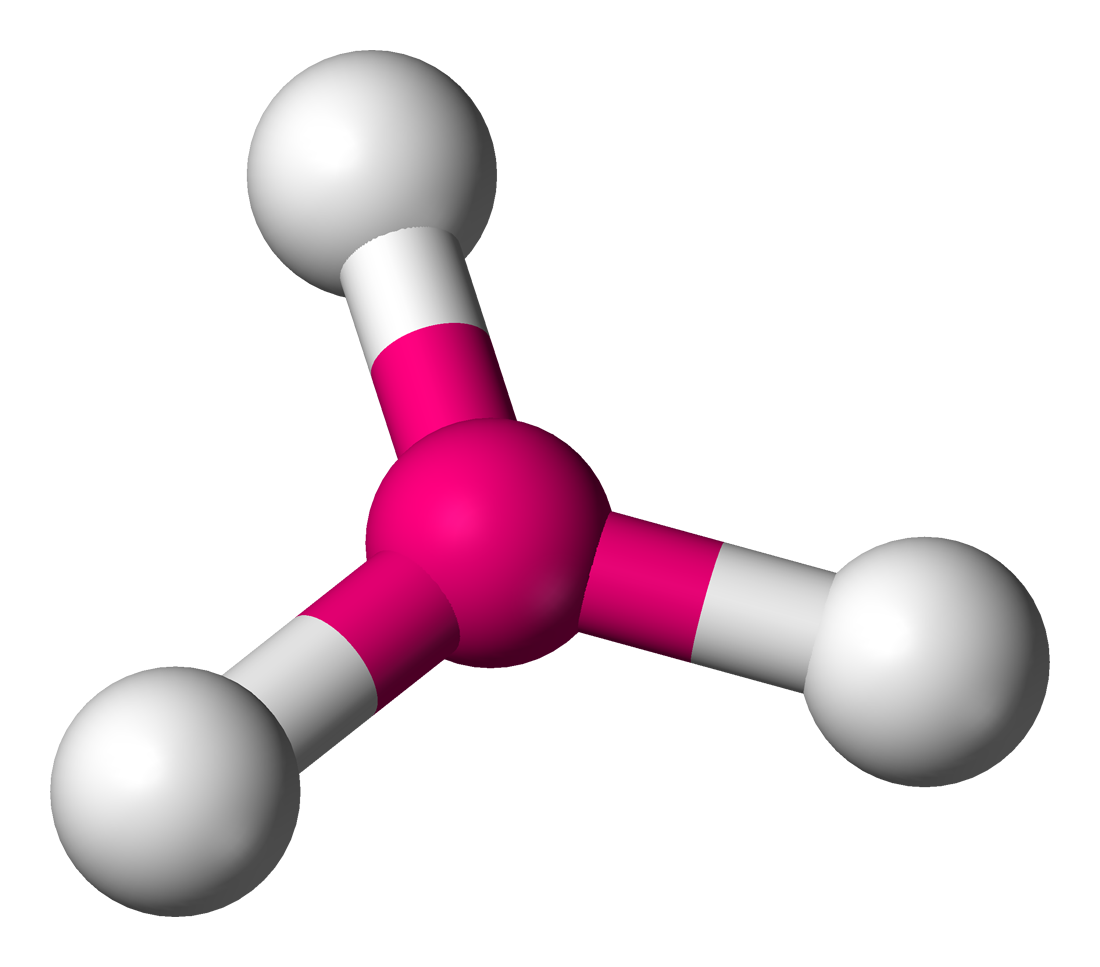
61
New cards
molecular geometry: trigonal planar
3 electron domains & no lone pairs in central molecule
120 degrees
ex. BH3
120 degrees
ex. BH3
62
New cards
molecular geometry: bent
3 electron domains & 1 lone pair in central molecule
< 120 degrees
ex. SO2
< 120 degrees
ex. SO2
63
New cards
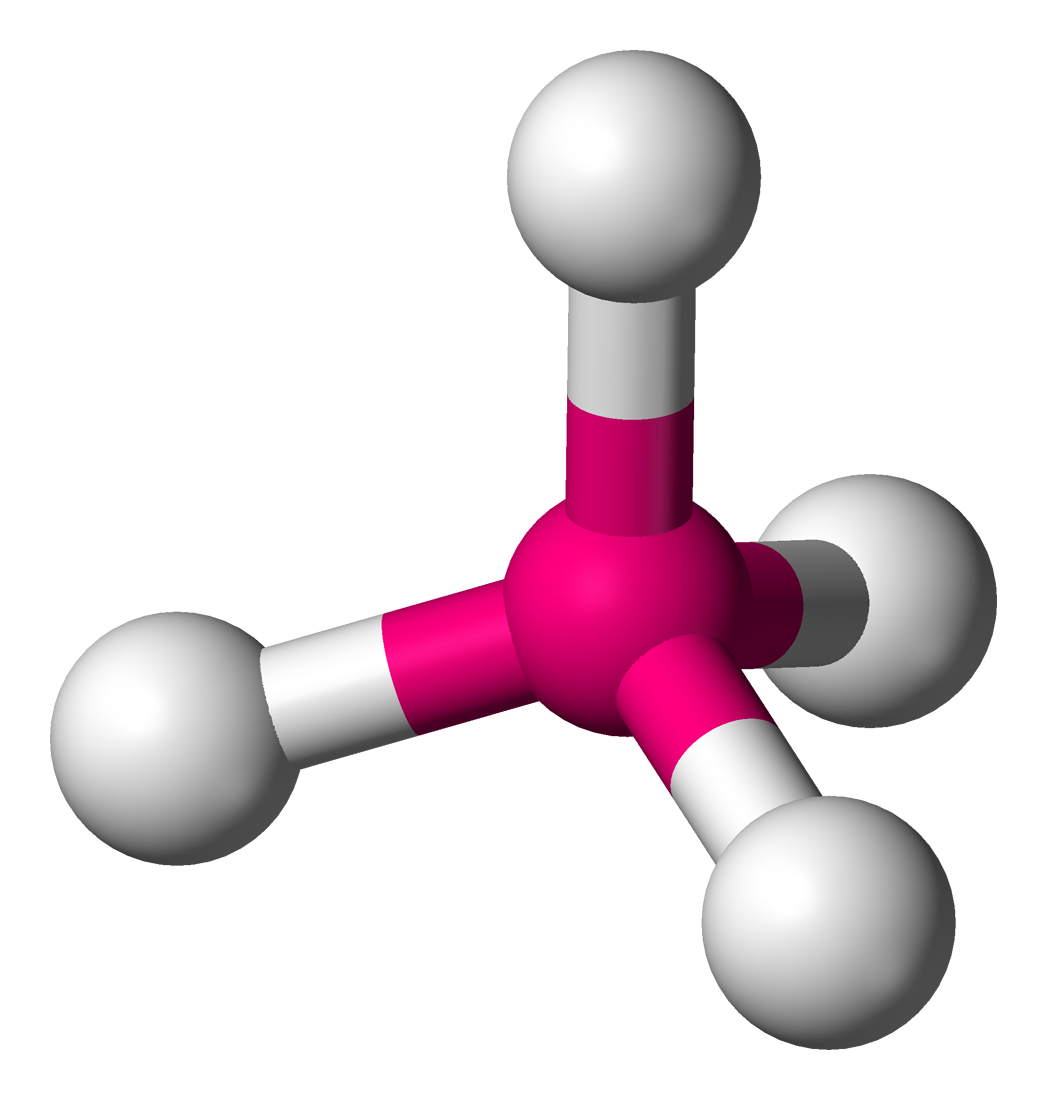
molecular geometry: tetrahedral
4 electron domains & no lone pairs in central molecule
109\.5 degrees
ex. CH4
109\.5 degrees
ex. CH4
64
New cards
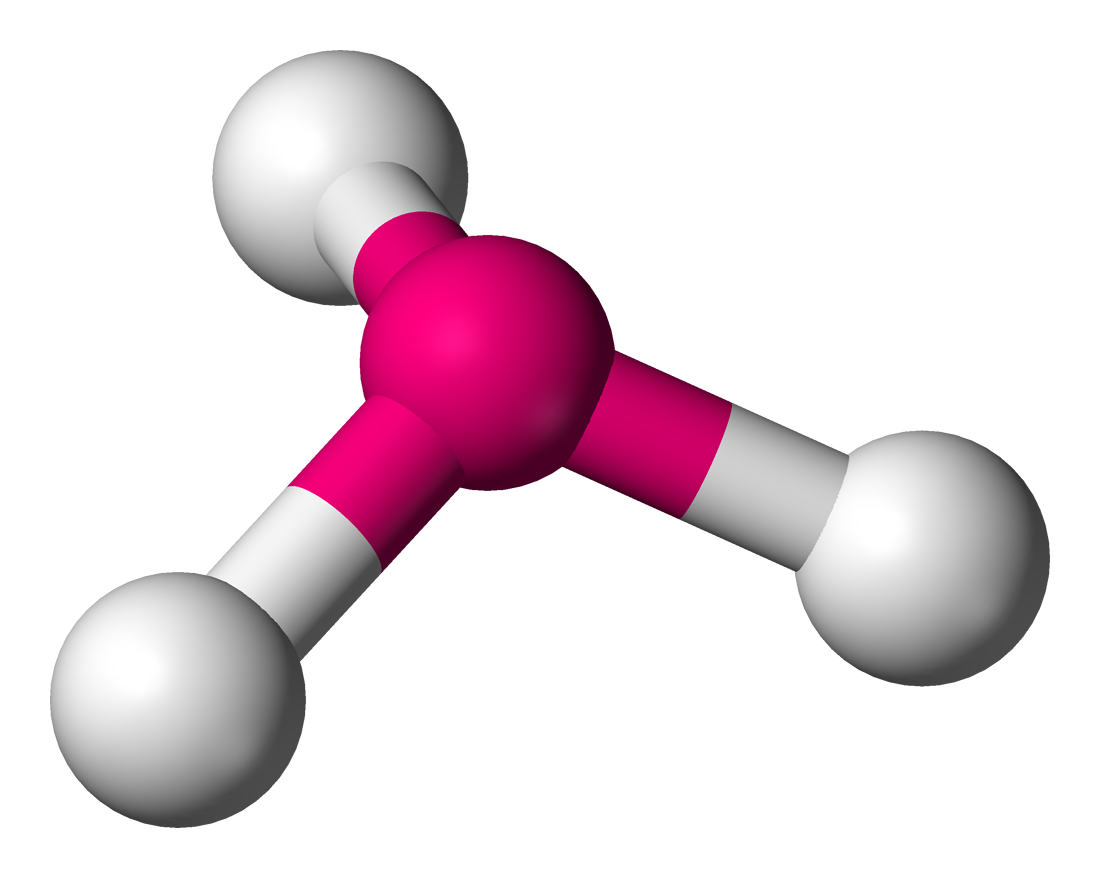
molecular geometry: trigonal pyramidal
4 electron domains & 1 lone pair in central molecule
< 109.5 degrees
ex. NF3
< 109.5 degrees
ex. NF3
65
New cards
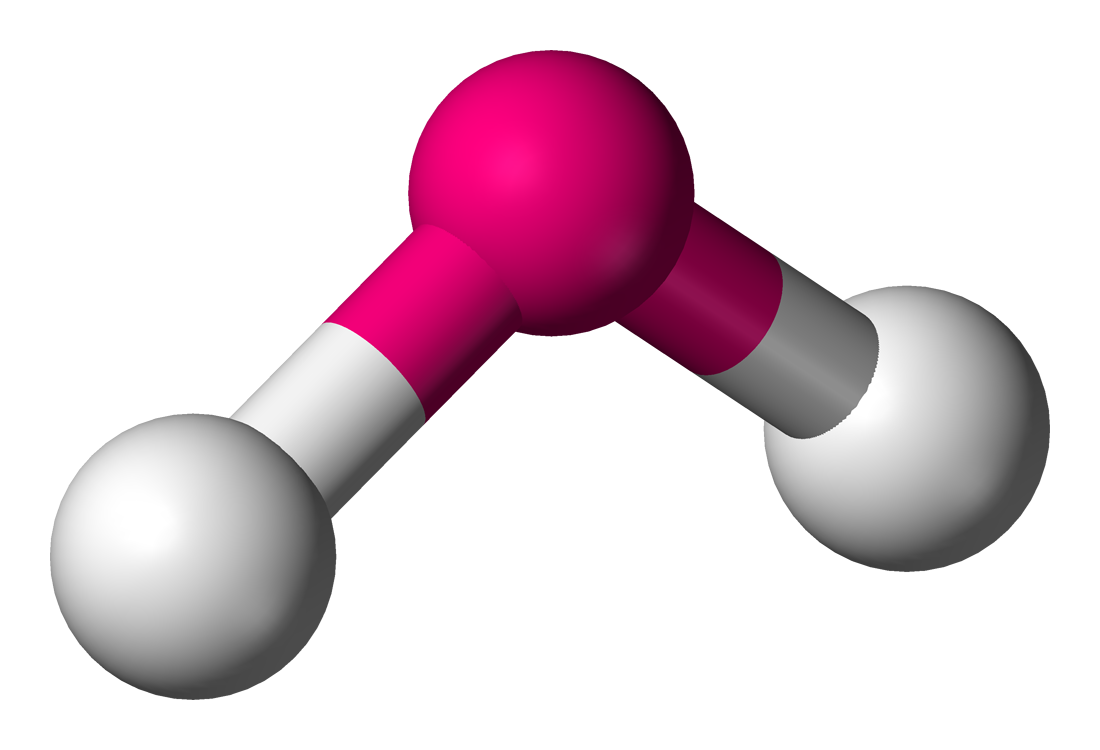
molecular geometry: bent
4 electron domains & 2 lone pairs in central molecule
ex. H2O
ex. H2O
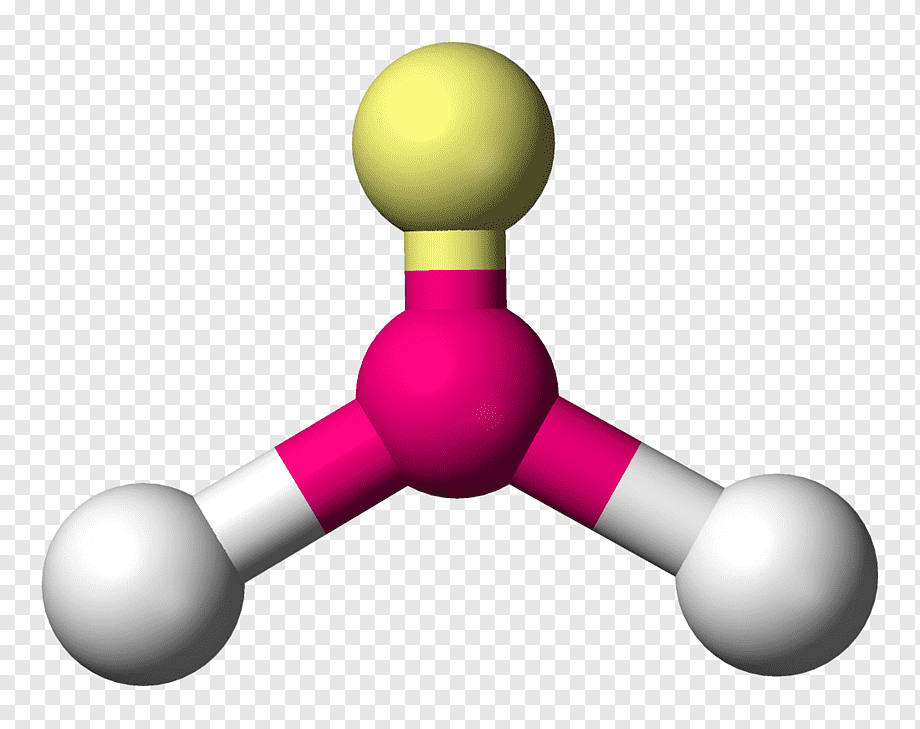
66
New cards
redox reaction
* known as an oxidation-reduction reaction, involves the transfer of electrons between species.
* one species undergoes oxidation (loses electrons) while another species undergoes reduction (gains electrons).
* transfer of electrons is accompanied by a change in oxidation states of the species involved.
* fundamental in various chemical and biological processes, such as combustion, corrosion, and cellular respiration. They are represented using balanced chemical equations, with oxidation numbers indicating the change in electron distribution.
* one species undergoes oxidation (loses electrons) while another species undergoes reduction (gains electrons).
* transfer of electrons is accompanied by a change in oxidation states of the species involved.
* fundamental in various chemical and biological processes, such as combustion, corrosion, and cellular respiration. They are represented using balanced chemical equations, with oxidation numbers indicating the change in electron distribution.
67
New cards
Boyles Law
* pressure of a gas is inversely proportional to its volume, assuming the temperature and amount of gas remain constant.
* mathematically expressed as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
* mathematically expressed as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
68
New cards
Charles Law
* volume of a gas is directly proportional to its temperature, assuming the pressure and amount of gas remain constant.
* expressed mathematically as V1/T1 = V2/T2, where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.
* expressed mathematically as V1/T1 = V2/T2, where V1 and T1 are the initial volume and temperature, and V2 and T2 are the final volume and temperature.
69
New cards
ideal gas law
* a mathematical relationship between the pressure, volume, temperature, and number of moles of a gas. It is expressed as: **PV = nRT**
* P is inversely proportional to V
* where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant (0.08206 (L•atm)/(K•mol)), and T is the temperature.
* P is inversely proportional to V
* where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant (0.08206 (L•atm)/(K•mol)), and T is the temperature.
70
New cards
Avogadro’s Law
* number of gas molecules is proportional to the volume.
* Avogadro’s number = 6.02 x 10^23
* Avogadro’s number = 6.02 x 10^23
71
New cards
cations
positively charged & lose electrons
72
New cards
anions
negatively charged & gain electrons
73
New cards
heterocycles
* organic compounds that contain at least one ring structure with atoms other than carbon.
* These atoms can include nitrogen, oxygen, sulfur, or other elements.
* Examples include pyridine, furan, thiophene, and pyrrole.
* These atoms can include nitrogen, oxygen, sulfur, or other elements.
* Examples include pyridine, furan, thiophene, and pyrrole.
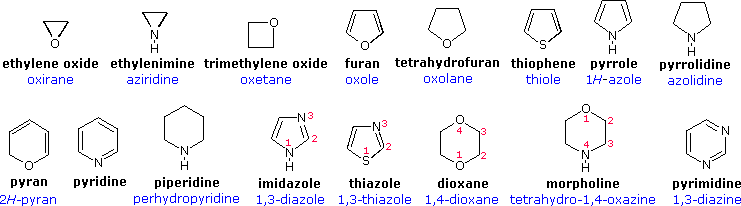
74
New cards
Huckels rule
* known as the 4n+2 rule
* predicts the aromaticity of cyclic conjugated systems.
* a cyclic system is aromatic if it contains 4n+2 π electrons, where n is an integer.
* This rule helps in determining the stability and reactivity of aromatic compounds.
* lone pair of electrons are counted as π electrons
* predicts the aromaticity of cyclic conjugated systems.
* a cyclic system is aromatic if it contains 4n+2 π electrons, where n is an integer.
* This rule helps in determining the stability and reactivity of aromatic compounds.
* lone pair of electrons are counted as π electrons
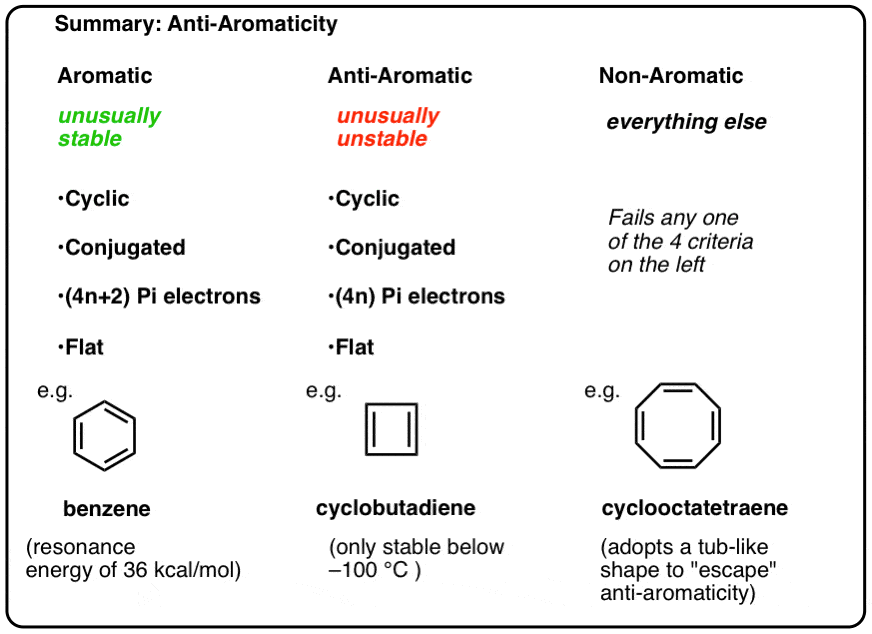
75
New cards
STP (Standard Time & Pressure)
* occurs at 0C and 1 atm
* considered an ideal gas 1 mol of gas = 22.4 L
* gas constant: 8.314 J/mol•K
* use PV = nRT
* considered an ideal gas 1 mol of gas = 22.4 L
* gas constant: 8.314 J/mol•K
* use PV = nRT
76
New cards
1 cm3
1 ml
77
New cards
1000 mL
1 L
78
New cards
energy of wavelength
E = hc/lambda = hf
h - Plancks constant: 6.62607015×10^−34 joule-hertz−1
c - speed of light: 3.00 x 10^8 ms
lambda - wavelength
f - frequency
h - Plancks constant: 6.62607015×10^−34 joule-hertz−1
c - speed of light: 3.00 x 10^8 ms
lambda - wavelength
f - frequency
79
New cards
fluorescence
* the emission of light by a substance that has absorbed light or other electromagnetic radiation.
* When these substances absorb photons of a specific wavelength, they become excited and then emit light of a longer wavelength.
* This emitted light is typically of a different color than the absorbed light, giving rise to the characteristic fluorescence.
* When these substances absorb photons of a specific wavelength, they become excited and then emit light of a longer wavelength.
* This emitted light is typically of a different color than the absorbed light, giving rise to the characteristic fluorescence.
80
New cards
chirality
* refers to the property of a molecule or object that is not superimposable on its mirror image.
* associated with asymmetric carbon atoms, which have four different groups attached to them.
* have two mirror image forms called enantiomers.
* associated with asymmetric carbon atoms, which have four different groups attached to them.
* have two mirror image forms called enantiomers.
81
New cards
enantiomers
* a pair of molecules that are mirror images of each other and cannot be superimposed.
* They have the same chemical formula and connectivity but differ in their spatial arrangement.
* exhibit identical physical and chemical properties, except for their interaction with polarized light.
* They rotate the plane of polarized light in equal but opposite directions.
* They have the same chemical formula and connectivity but differ in their spatial arrangement.
* exhibit identical physical and chemical properties, except for their interaction with polarized light.
* They rotate the plane of polarized light in equal but opposite directions.
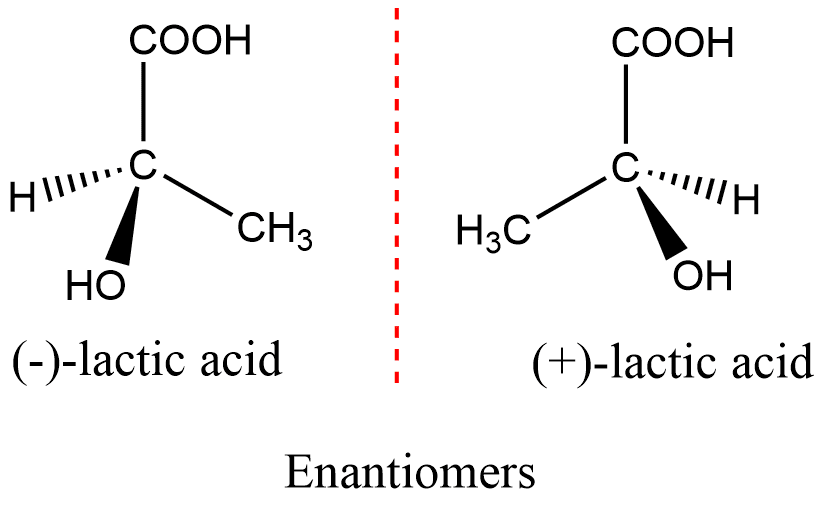
82
New cards
diastereomers
* stereoisomers that are not mirror images of each other and have different physical and chemical properties.
* They arise when a molecule has two or more stereocenters and the relative configuration of some stereocenters is the same while others are different.
* have different arrangements of atoms in space, resulting in different properties such as melting points, boiling points, and reactivity.
* They can be identified by their distinct physical properties and by their different behavior in reactions
* They arise when a molecule has two or more stereocenters and the relative configuration of some stereocenters is the same while others are different.
* have different arrangements of atoms in space, resulting in different properties such as melting points, boiling points, and reactivity.
* They can be identified by their distinct physical properties and by their different behavior in reactions
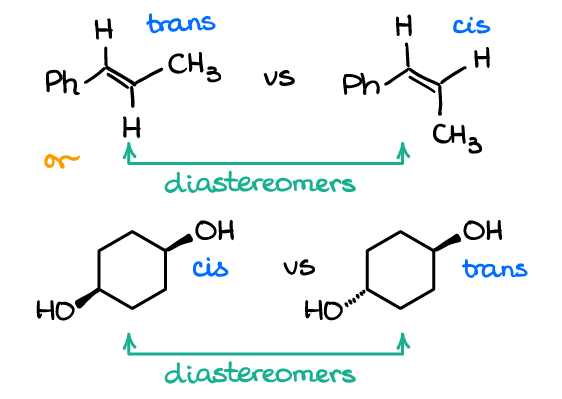
83
New cards
SDS-PAGE (Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis)
a technique used in biochemistry and molecular biology to separate proteins based on their size.
The proteins are denatured and coated with SDS, a detergent that gives them a negative charge.
They are then loaded onto a gel matrix made of polyacrylamide and subjected to an electric field.
The proteins migrate through the gel based on their size, with smaller proteins moving faster. This allows for the separation and analysis of proteins in a sample.
The proteins are denatured and coated with SDS, a detergent that gives them a negative charge.
They are then loaded onto a gel matrix made of polyacrylamide and subjected to an electric field.
The proteins migrate through the gel based on their size, with smaller proteins moving faster. This allows for the separation and analysis of proteins in a sample.
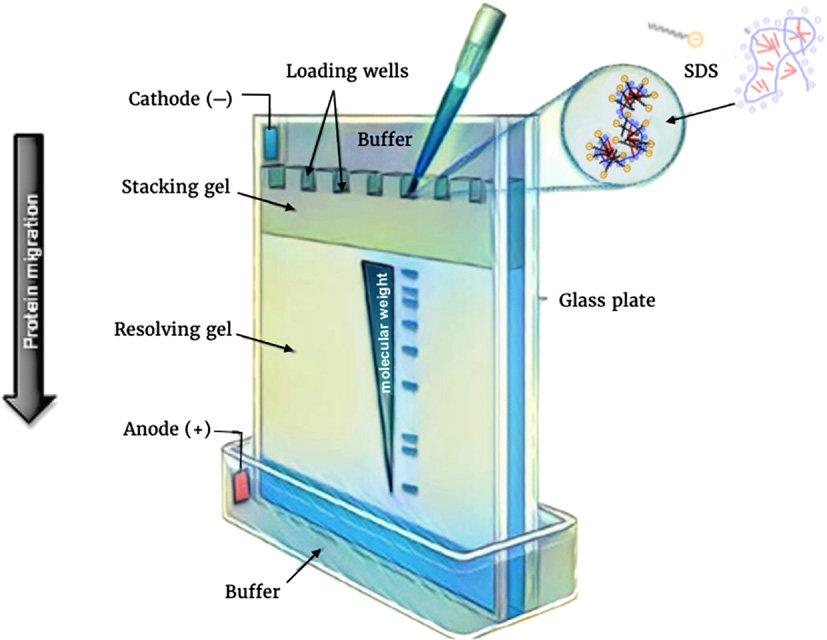
84
New cards
proteolysis
* the process of breaking down proteins into smaller peptides or amino acids.
* primarily carried out by enzymes called proteases, which cleave peptide bonds within proteins.
* primarily carried out by enzymes called proteases, which cleave peptide bonds within proteins.
85
New cards
protein aggregation
the process in which proteins misfold and clump together, forming aggregates or insoluble structures.
86
New cards
polymorphic
refers to the ability of an object or method to take on different forms or behaviors.
87
New cards
ligand
molecule or atoms that binds reversibly to a protein
88
New cards
steroid
* a class of an organic compound that have a specific molecular structure consisting of four fused rings.
* class of lipids that are derived from cholesterol
* class of lipids that are derived from cholesterol
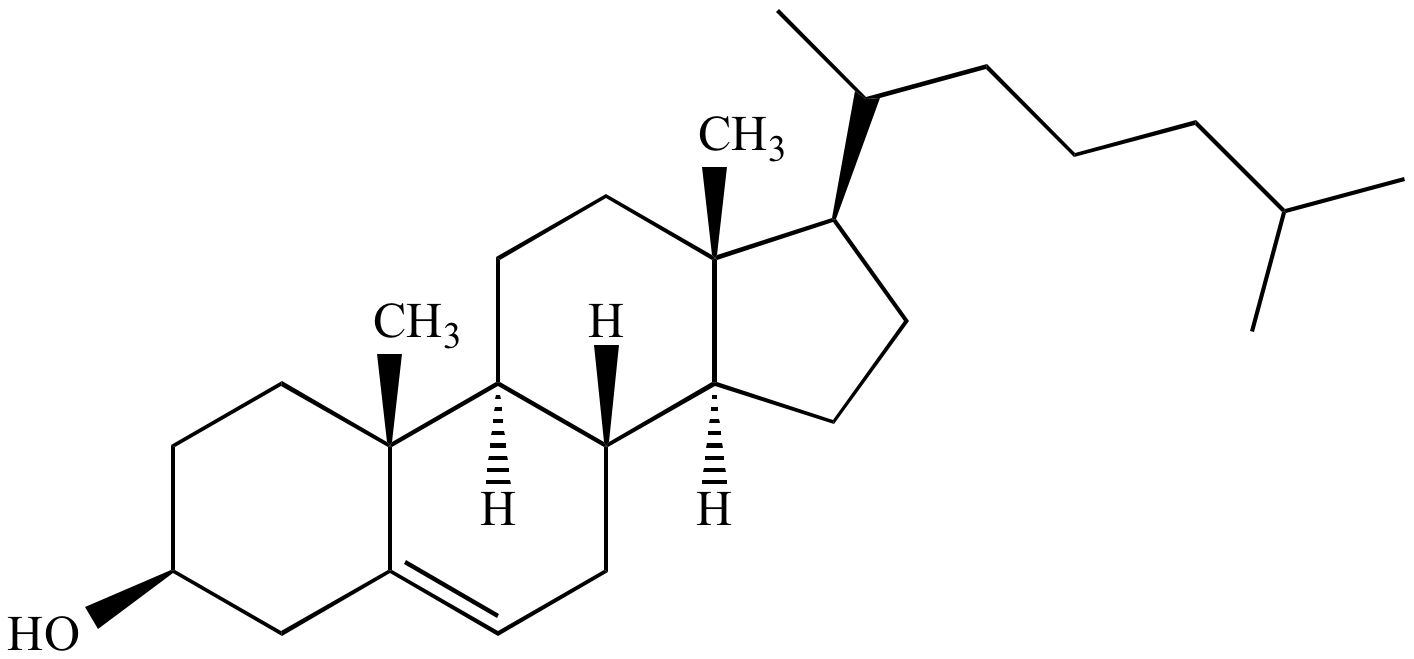
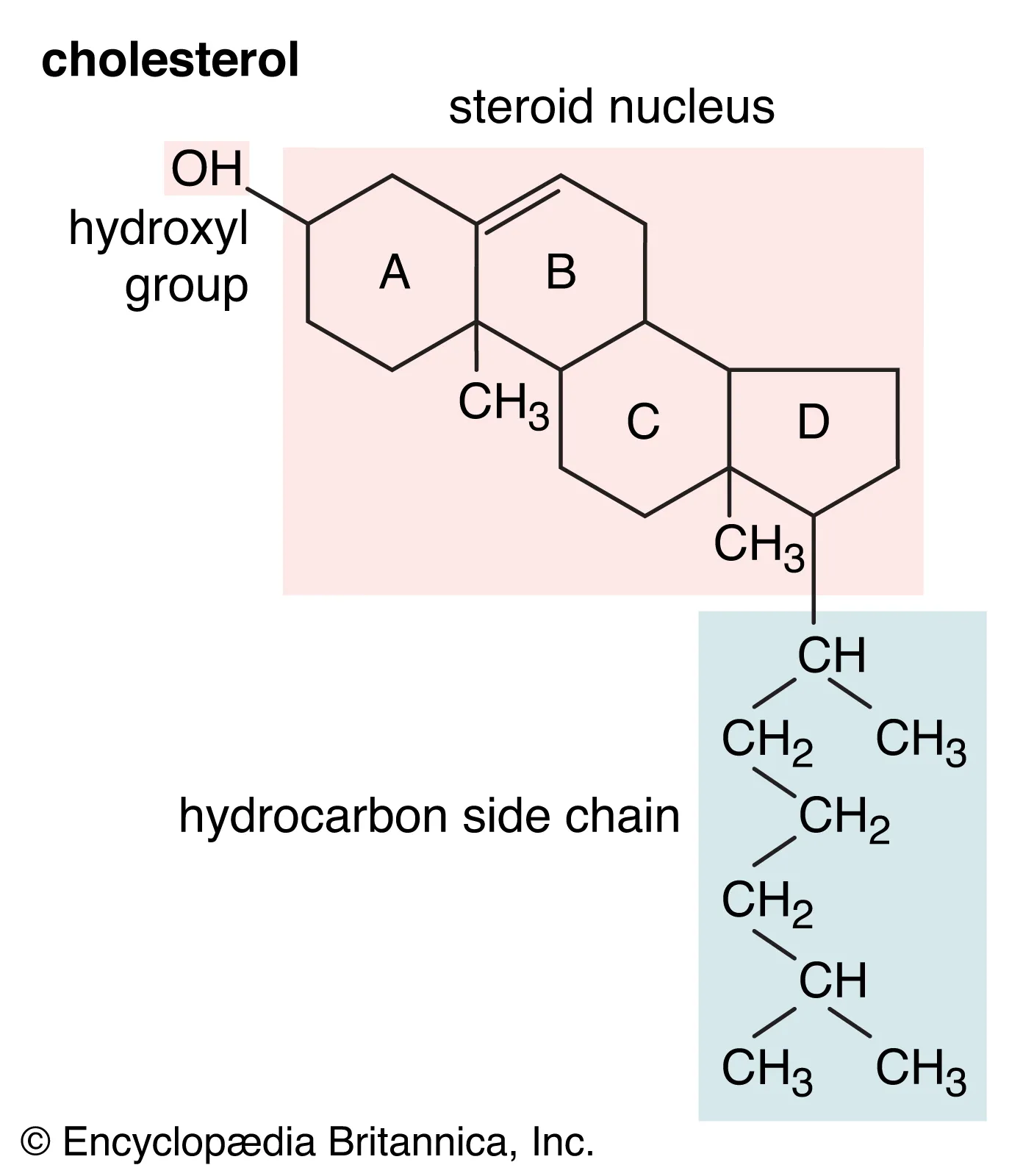
89
New cards
cholesterol
* a type of lipid molecule found in the body.
* It is an essential component of cell membranes and is involved in various biological processes
* belongs to the steroid family and has a structure consisting of four fused rings.
* It is primarily synthesized in the liver and can also be obtained from dietary sources.
* plays a crucial role in the production of hormones, vitamin D, and bile acids.
* It is an essential component of cell membranes and is involved in various biological processes
* belongs to the steroid family and has a structure consisting of four fused rings.
* It is primarily synthesized in the liver and can also be obtained from dietary sources.
* plays a crucial role in the production of hormones, vitamin D, and bile acids.
90
New cards
glucose
* a simple sugar and an essential source of energy for living organisms.
* It is a type of carbohydrate that is commonly found in plants and animals.
* produced through the process of photosynthesis in plants and is also obtained from the breakdown of carbohydrates in the body.
* plays a crucial role in cellular respiration, where it is converted into ATP (adenosine triphosphate) to fuel various metabolic processes.
* used as a primary fuel source for the brain.
* It is a type of carbohydrate that is commonly found in plants and animals.
* produced through the process of photosynthesis in plants and is also obtained from the breakdown of carbohydrates in the body.
* plays a crucial role in cellular respiration, where it is converted into ATP (adenosine triphosphate) to fuel various metabolic processes.
* used as a primary fuel source for the brain.
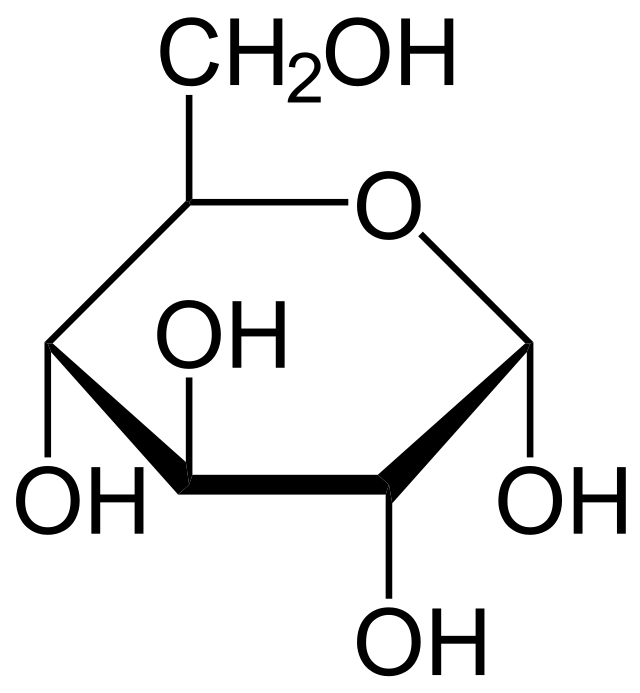
91
New cards
affinity chromotography
* refers to the study of the interactions between molecules or substances based on their attraction towards each other.
* involves understanding how molecules bind to each other, form complexes, or undergo chemical reactions based on their specific properties and affinities.
* involves understanding how molecules bind to each other, form complexes, or undergo chemical reactions based on their specific properties and affinities.
92
New cards
cation exchange chromotography
* a technique used in analytical chemistry to separate and purify cations based on their affinity for a stationary phase containing negatively charged functional groups.
* The cations in the sample are attracted to the stationary phase and can be eluted by adjusting the pH or using a competing cation.
* The cations in the sample are attracted to the stationary phase and can be eluted by adjusting the pH or using a competing cation.
93
New cards
anion exchange chromotography
* a technique used to separate and purify anions, based on their affinity for a positively charged stationary phase.
* The stationary phase contains positively charged groups that attract and bind the negatively charged molecules.
* By adjusting the pH and ionic strength of the mobile phase, the bound molecules can be eluted and collected separately.
* The stationary phase contains positively charged groups that attract and bind the negatively charged molecules.
* By adjusting the pH and ionic strength of the mobile phase, the bound molecules can be eluted and collected separately.
94
New cards
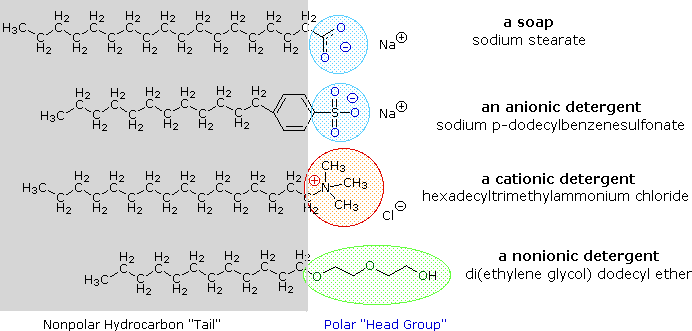
detergents
* They are typically composed of surfactants, which lower the surface tension of water and help to break down and remove dirt.
* hydrophobic & hydrophilic side
* three main types: cationic, anionic, and nonionic
* hydrophobic & hydrophilic side
* three main types: cationic, anionic, and nonionic
95
New cards
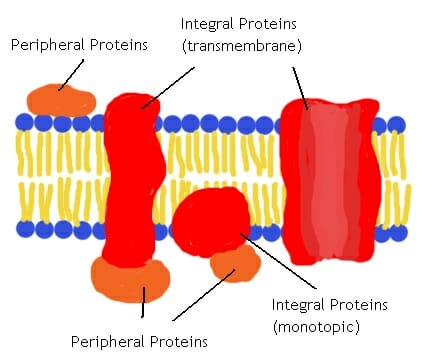
peripheral membrane proteins
* a class of proteins that are associated with the cell membrane but do not penetrate or span across it.
* They are typically attached to the membrane through weak interactions, such as electrostatic interactions or binding to lipid molecules.
* These proteins play important roles in various cellular processes, including signal transduction, cell adhesion, and membrane trafficking.
* can be easily dissociated from the membrane without disrupting its structure.
* They are typically attached to the membrane through weak interactions, such as electrostatic interactions or binding to lipid molecules.
* These proteins play important roles in various cellular processes, including signal transduction, cell adhesion, and membrane trafficking.
* can be easily dissociated from the membrane without disrupting its structure.
96
New cards
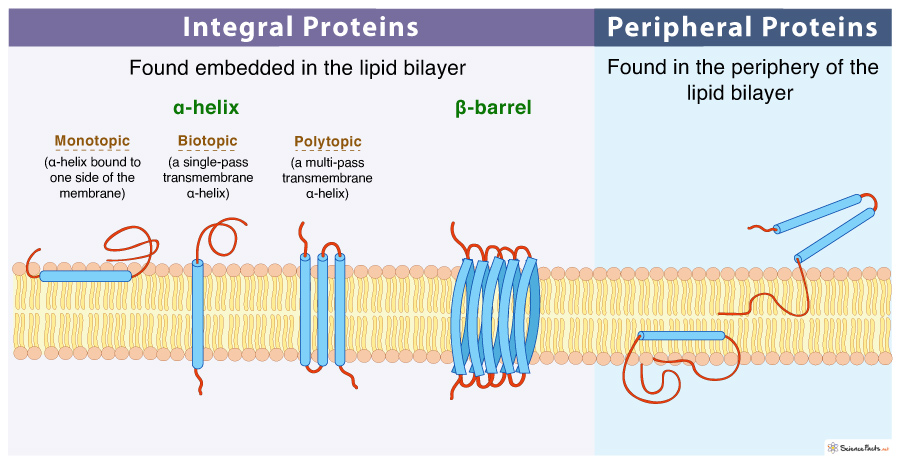
integral membrane proteins
* a type of protein that are embedded within the lipid bilayer of a cell membrane.
* They play crucial roles in various cellular processes, such as cell signaling, transport of molecules across the membrane, and cell adhesion.
* These proteins have hydrophobic regions that interact with the hydrophobic interior of the lipid bilayer, anchoring them in the membrane.
* can have different structures and functions, including ion channels, receptors, and transporters.
* They are essential for the proper functioning of cells and are the target of many drugs and therapies.
* They play crucial roles in various cellular processes, such as cell signaling, transport of molecules across the membrane, and cell adhesion.
* These proteins have hydrophobic regions that interact with the hydrophobic interior of the lipid bilayer, anchoring them in the membrane.
* can have different structures and functions, including ion channels, receptors, and transporters.
* They are essential for the proper functioning of cells and are the target of many drugs and therapies.
97
New cards
hydrolysable linkages
* refer to chemical bonds that can be broken down by hydrolysis, a reaction that involves the addition of water.
* commonly found in organic compounds such as carbohydrates, proteins, and nucleic acids.
* Hydrolysis breaks the bond and releases the individual components of the molecule.
* For example, in carbohydrates, hydrolysis breaks the glycosidic linkages between sugar units, resulting in the release of monosaccharides.
* Similarly, in proteins, hydrolysis breaks the peptide bonds between amino acids, leading to the release of individual amino acids.
* commonly found in organic compounds such as carbohydrates, proteins, and nucleic acids.
* Hydrolysis breaks the bond and releases the individual components of the molecule.
* For example, in carbohydrates, hydrolysis breaks the glycosidic linkages between sugar units, resulting in the release of monosaccharides.
* Similarly, in proteins, hydrolysis breaks the peptide bonds between amino acids, leading to the release of individual amino acids.
98
New cards
binding affinity
* refers to the strength of the interaction between a ligand (molecule) and its target (receptor or enzyme).
* It quantifies how tightly the ligand binds to the target.
* The higher the binding affinity, the stronger the interaction between the two molecules.
* typically measured using techniques such as equilibrium dialysis, surface plasmon resonance, or isothermal titration calorimetry.
* It quantifies how tightly the ligand binds to the target.
* The higher the binding affinity, the stronger the interaction between the two molecules.
* typically measured using techniques such as equilibrium dialysis, surface plasmon resonance, or isothermal titration calorimetry.
99
New cards
confirmational stability
* refers to the ability of a molecule or compound to maintain its specific shape or conformation under certain conditions.
* crucial for the proper functioning of biological molecules such as proteins and nucleic acids.
* is influenced by various factors, including temperature, pH, solvent conditions, and the presence of other molecules.
* Changes in these factors can disrupt the stable conformation of a molecule, leading to functional impairment or loss of activity.
* crucial for the proper functioning of biological molecules such as proteins and nucleic acids.
* is influenced by various factors, including temperature, pH, solvent conditions, and the presence of other molecules.
* Changes in these factors can disrupt the stable conformation of a molecule, leading to functional impairment or loss of activity.
100
New cards
chiral ordant receptors
* a type of molecular receptors that exhibit chirality, meaning they have a non-superimposable mirror image.
* These receptors are capable of selectively binding to chiral molecules, which are molecules that also possess chirality.
* can be designed and synthesized to have specific binding properties, making them valuable tools in asymmetric synthesis and chiral analysis.
* These receptors are capable of selectively binding to chiral molecules, which are molecules that also possess chirality.
* can be designed and synthesized to have specific binding properties, making them valuable tools in asymmetric synthesis and chiral analysis.