MMSC 420 Unit 7 - Platelets and Plasma
1/79
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
80 Terms
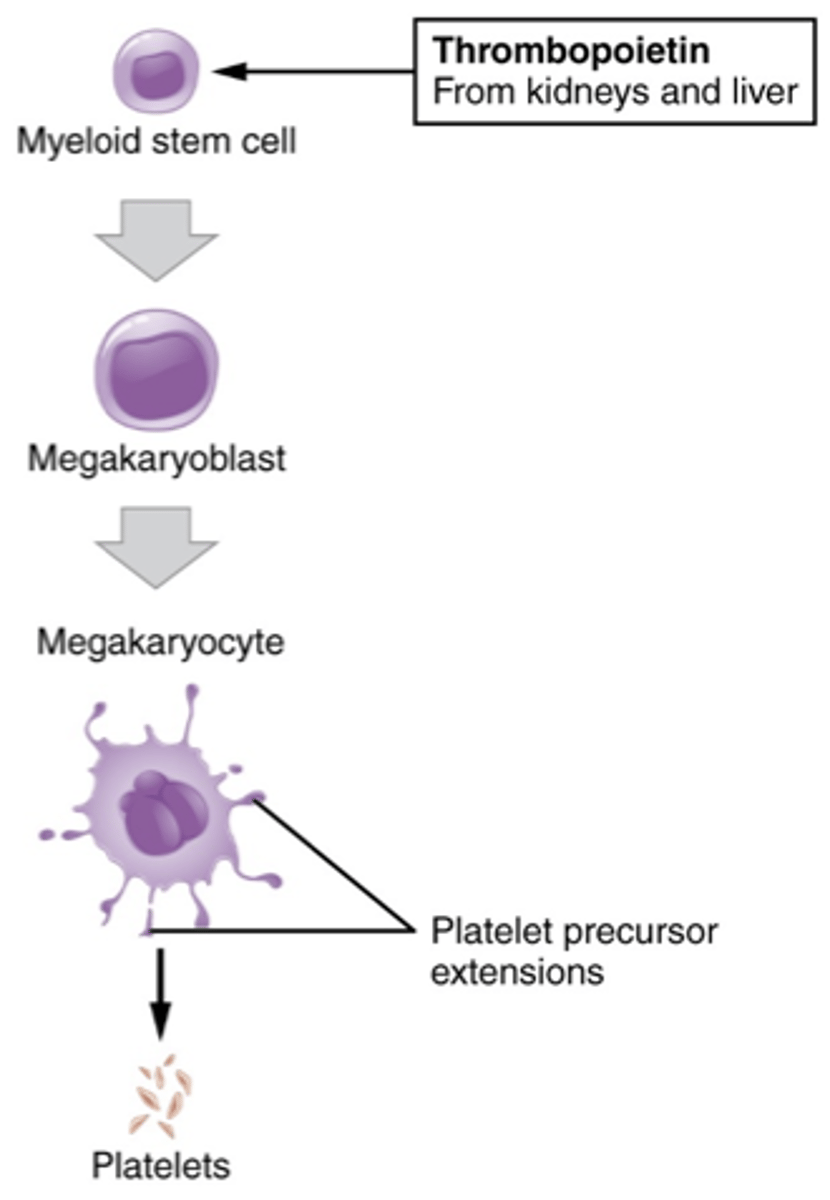
Platelet maturation series
- myeloid stem cell
- megakaryoblast
- megakaryocyte
- platelet
definition of a blood component
A specific portion of blood derived from whole blood designed and manufactured for the purpose of generating a specific therapeutic effect upon transfusion
how many blood components can be generated from one unit of whole blood?
- up to a total of four:
- platelets
- fresh frozen plasma
- cryoprecipitate
- red blood cells
what is the key to component preparation?
centrifugation
Platelets - Random donor (RDP)
- maintain collected WB at 20-24C until platelets are removed
- light spin: approx. 3,200 RPM's for 2-3 minutes
- express off plasma (leave enough for approx. 70-80% HCT for RBC's)
- spin plasma hard: spin at approx. 3,600 RPM's for 5 minutes
- express off plasma (FFP or eventual CRYO) leaving approx. 50 mL of RDP
- platelet incubator 20-24C with agitation
- remember "light spin followed by a hard spin"
Random donor platelets
- can be prepared from WB within 8 hours of collection
- stored at 20 - 24C
- shelf life = 5 days with constant agitation
- avg. vol ~ 55 mL; should contain 5.5 x 10^10 PLC per unit
- typically pool 4 - 6 adult dose = approx 3 x 10^11 PLC
- once pooled, expiration is 4 hours
- therefore, expected increment increase should be 20,000 to 60,000/uL (pool of 4 - 6 transfused)
Platelets - apheresed
- single donor plateletpheresis: most widely used
- must contain 3 x 10^11 PLC (min)
- given as: random plateletpheresis, crossmatched plateletpheresis, HLA matched plateletpheresis (irradiate), and HLA/crossmatched plateletpheresis (irradiate)
Platelet storage
- require storage temperature of 20 to 24 C
- this raises the concern of possible bacterial growth
- whenever venipuncture is performed, there is a chance that skin flora could be introduced into whatever product is being collected from the donor
What product can be used to prevent bacterial replication within blood products?
INTERCEPT pathogen inactivation (amotosalen)
INTERCEPT pathogen inactivation (amotosalen): step 1
amotosalen targets nucleic acids, and intercalates or "docks" between nucleic acid base pairs
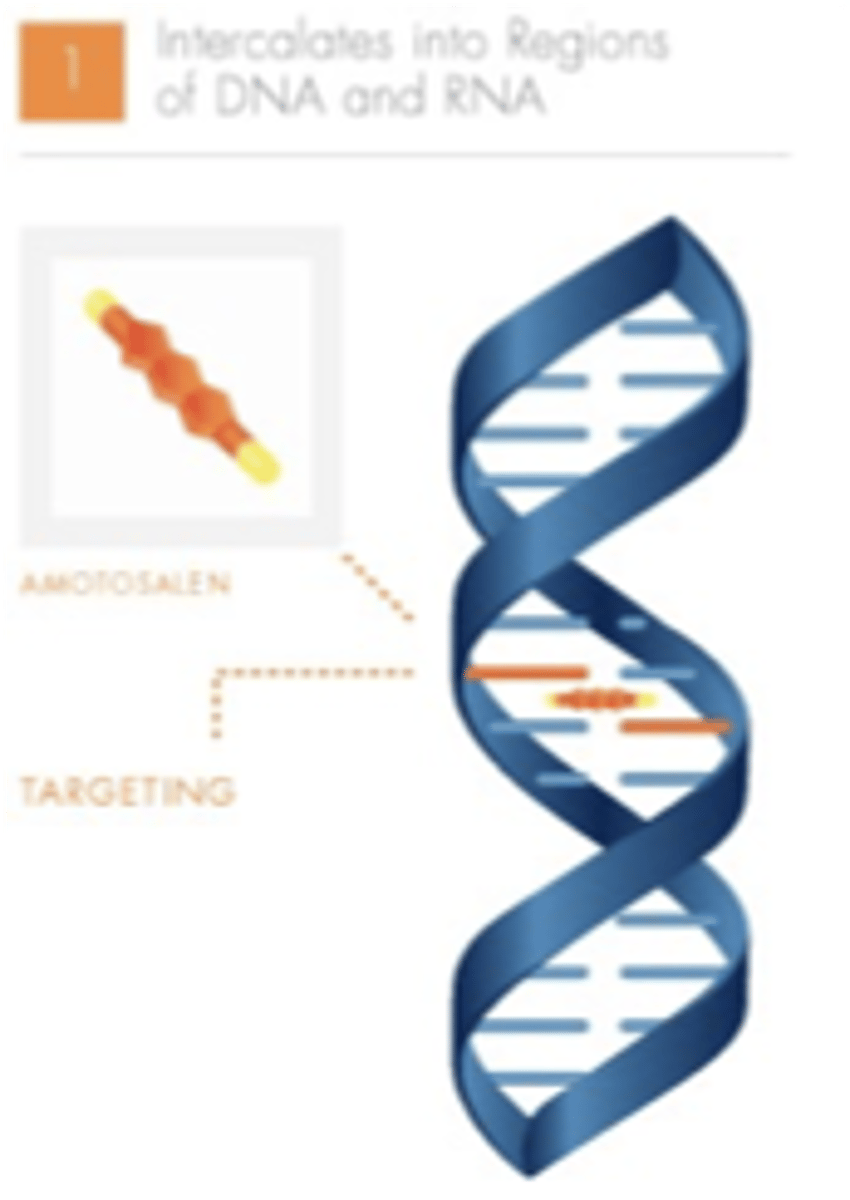
INTERCEPT pathogen inactivation (amotosalen): step 2
UVA illumination activates amotosalen, causing permanent cross-links between the helical strands

INTERCEPT pathogen inactivation (amotosalen): step 3
cross-linking prevents further replication and inactivates the pathogen and/or leukocyte

Indications for platelet transfusion
- treatment of bleeding due to thrombocytopenia
- prophylactic transfusion of PLC is usually indicated in patients with very low PLC counts (under 5,000 - 10,000/µl) to prevent spontaneous cranial hemorrhage (think chemo-induced low PLC count)
- DIC (increased destruction, < 50,000/µl)
- Massive transfusion (PLC dilution, < 50,000/µl)
Thrombocytopenia
- too few platelets: < 150K
- can be caused by chemo, other infection e.g. HIV, HEP C, Epstein-barr, others
- cancer, other conditions
- preeclampsia, enlarged spleen
Platelet transfusion criteria: adult dosage
- adult normal dose = 4 to 6 pooled RDP's or 1 single donor apheresed PLC
Platelet transfusion criteria: children dosage
- children normal dose = 1 unit/10Kg of body weight
- may order XX mL of an apheresed PLC
When making pooled platelets, most of the units used to make the pool were Rh negative, but one was Rh positive... Is this pool considered Rh negative or Rh positive?
If there is even one Rh positive platelet unit in a pool, that entire pool becomes an Rh positive pool.
RDP
Random donor Platelet from one whole blood unit
Platelet transfusion criteria: children dosage (con't.)
- children > 10 kg would get 1 RDP / 10 kg
- children < 10 kg (if getting apheresed platelets): would specify XX mL to give based upon weight
If an infants is given 5 - 10 mL of RDP, how much should their PLC count increase?
the PLC count should increase by 50,000 to 100,000/uL
how many lbs are in a kg?
approx. 2.2 lbs
Expected platelet count increase after transfusion (adult)
- 20,000 to 60,000/uL
- corrected count increment calculation should be performed if we don't think we're seeing the platelet count increase that we should be seeing, post transfusion
Platelet refractoriness
- potential side effect of platelet transfusion the may develop associated with:
- formation of antibodies to HLA and/or platelet specific antigens
- massive splenomegaly
- DIC
- sepsis
- high fever
When might you suspect platelet refractoriness?
- 10 minutes to 1 hour post transfusion, the PLC count is less than 50% of that expected on two consecutive occasions (meaning platelet transfusions)
- ex: less than a 10,000/uL increase on two consecutive platelet transfusions
Corrected count increment (CCI) for platelets
- calculate CCI using the 10 minute to 1 hour post transfusion platelet count
- corrects for body size differences
- more reliable estimate of expected platelet count increment upon transfusion
- expected corrected PLC increment is > 10,000 uL per m^2
Formula for CCI
(absolute PLC increment per uL x body surface in square meters) / number of PLC transfused (10^11)
(on the exam, you will be given the body surface area in square meters)
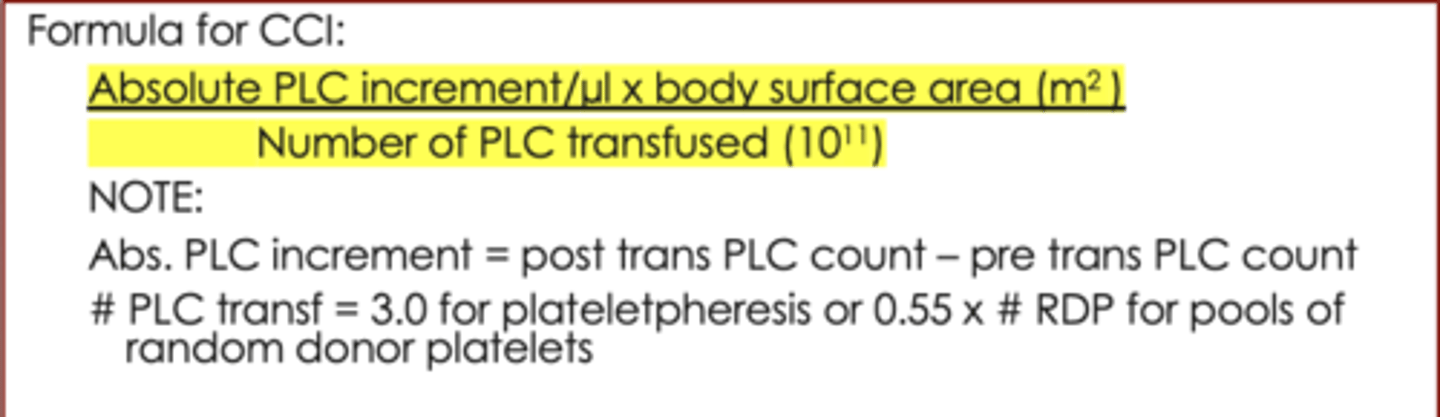
When calculating the CCI, what is the absolute PLC increment?
Abs. PLC increment = post transfusion PLC count - pre transfusion PLC count
When calculating the CCI, what is the number of PLC transfused?
- 3.0 for plateletpheresis
- 0.55 x number of RDP for pools of random donor platelets
Corrected count increment (CCI) example
- patient pre-transf. PLC count = 10,000/uL
- body surface area = 1.3 m^2
- 6 RDP are transfused
- post-transf. PLC count = 50,000/uL
- [(50,000/uL - 10,000/uL) x 1.3] / (6 units x .55 per unit) = 15,758/uL
- 15,758/uL is a good increment, so the patient is not refractory
What complications can arise from transfusion of non-group specific (ABO) platelets?
development of POS DAT for group A, B, AB recipients of several non-ABO matched PLC
What complications can arise from transfusion of Rh Pos PLC to an Rh Neg recipient?
- small amount of RBC's in PLC concentrates possible
- can immunize recipient to Rh (D) antigens
- Rh immune globulin can be given to female recipients of childbearing age to prevent Rh (D) sensitization
- one 300 ug dose adequate for 30 RDP or 3 plateletphereses
Other complications associated with platelet transfusions
- refractoriness
- transfusion transmitted diseases
- FNAIT
fetal/neonatal alloimmune thrombocytopenia
- similar to HDFN: maternal antibodies typically directed against HPA-1a
- we must give the baby platelet specific antigen negative units
In the event of FNAIT, and you can't get platelets from your supplier quickly, who could you get platelet specific antigen negative units from?
- the mother
- the antibodies would be washed out of the platelet product during production
- the mother does not possess the antigen that is being targeted by the antibody
Development of FNAIT
- during delivery, the HPA-1a negative mother is exposed to the fetus's HPA-1a platelets
- after delivery, the mother's immune system produces anti-HPA-1a antibodies
- upon a subsequent pregnancy, anti-HPA-1a antibodies from the mother's immune system attack and destroy HPA-1a platelets in the subsequent fetus
- the subsequent fetus suffers from FNAIT as a result
Prophylaxis of FNAIT
- during delivery, the HPA-1a negative mother is exposed to the fetus's HPA-1a platelets
- after delivery, the mother is injected with anti-HPA-1a antibodies that attack the fetus' HPA-1a platelets... as a result, the fetus' HPA-1a platelets are destroyed before an allo-immune response is initiated
- upon a subsequent pregnancy, the mother's blood stream is cleared of anti-HPA-1a antibodies and HPA-1a platelets from the fetus (from the previous pregnancy)
- the subsequent fetus remains healthy as a result, and is not at risk of FNAIT
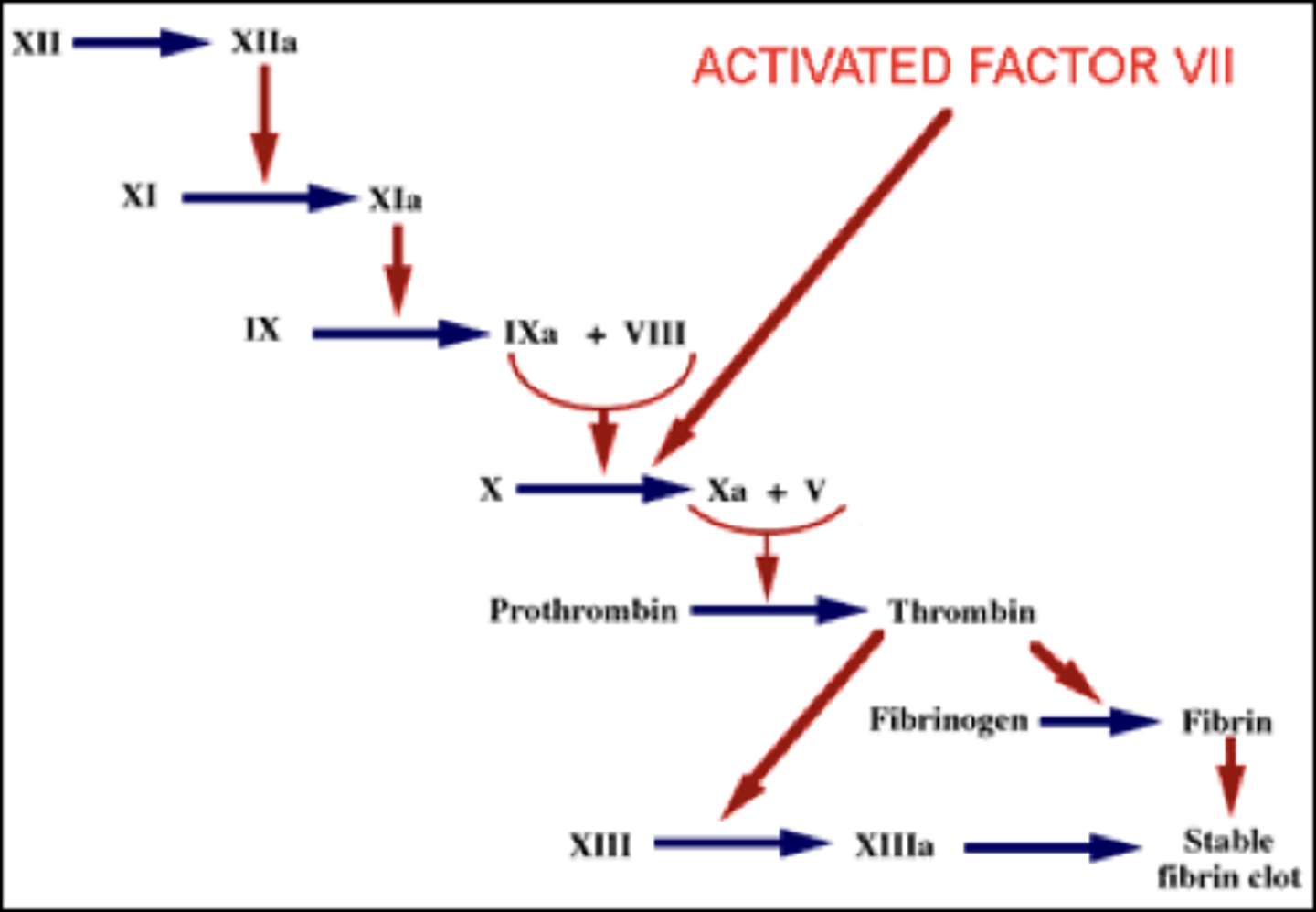
Flowchart of the coagulation cascade
What symptoms are caused by an overdose of coumadin?
- hemorrhage
- headache
- bruising
- back pain
What is coumadin used for?
coumadin is used to prevent clot formation with DVT, PE, A-fib with embolism, TIA and coronary occlusive problems
Fresh frozen plasma - made from WB
- referred to as the "liquid" portion of WB donation; primarily water, 7% protein, 2% lipids
- FFP must be separated and frozen within 8 hours of WB collection to maintain levels of all clotting factors; volume approx. 200 - 250 mL
- shelf life *frozen = 1 year stored at less than or equal to 18 C
- thawed at 37 C with agitation
- thawed FFP is stored at 1 to 6 C until issued; shelf life 24 hours
What is fresh frozen plasma (FFP) a rich source of?
- all coagulation factors
- especially labile factors: factor V and factor VIII
- FFP has the highest concentration of all of these coagulation factors
Frozen plasma (not the same thing as fresh frozen plasma)
- AKA "liquid plasma" or "plasma frozen within 24 hours (PF24"
- separated and frozen after 8 hours but within 24 hours after collection
- contain variable amounts of factors V and VIII (they don't last as long as the other factors last)
- shelf life/storage same as FFP
- indications: treatment of stable coag factor deficiencies; plasma exchange recipients; sent for further manufacturing of plasma derivatives
Cryo-reduced plasma
- separated and frozen within 24 hours after collection - cryo made first
- contain variable amounts of factor V, and reduced to no: factor VIII, vWF, factor XIII, cryoglobulin, fibronectin (still has albumin, factors II, V, VII, IX, X, XI and ADAMTS13)
- shelf life/storage same as FFP
- indications: treatment of stable coag factor deficiencies; plasma exchange recipients; sent for further manufacturing of plasma derivatives
Plasma - apheresed (FFP or plasma)
- same as others - collection different
- volume may be as high as 400 to 600 mL
- can have as many as 3 to 4 units from one donation
Thawed FFP expiration
- thawed FFP expires within 24 hours of thawing (start of thaw) - e.g., placed into plasma thawer at 1345 on 10/28/2024, expires at 1345 on 10/29/2024
Thawed plasma expiration
- thawed plasma expires 5 days from date of thaw - e.g., thawed on 10/28/2024, expires on 11/2/2024 (midnight = 2359 or 2400)
If babies need FFP, what type of FFP do we give them, and how much?
- we usually give AB FFP
- babies usually only need around 20 or 30 mL of FFP
- frozen aliquots of FFP products are made specially for babies because they only need small amounts of FFP
Indications for thawed Fresh frozen plasma (TFFP) transfusion
- preop or bleeding - needs several coag factors
- massive transfusion
- need for brief/temporary warfarin reversal (vit K n/a)
- TTP - transfusion or exchange
- coag factor deficiencies/no factor concentrates available
Indications for thawed plasma (TP) transfusion
- same as TFFP, except:
- not indicated for treatment of deficiencies of labile coagulation factors including factors V, VIII and protein C
Indications for cryo reduced plasma transfusion
- TTP thrombotic thrombocytopenic purpura - used for transfusion or exchange
- patient has a need for clotting factors except fibrinogen, factor VIII, factor XIII, and vWF
When should you not transfuse FFP or thawed plasma?
- FFP and TP should not be used as simple plasma expanders (use albumin, other nonprotein expanders e.g. dextran - shock, severe dehyrdation such as burns)
- FFP and TP should not be used if vit K or prothrombin complex concentrates are available for warfarin reversal
- FFP and TP should not be used if specific concentrate is available
- FFP and TP should not be used if cryoprecipitated AHF is indicated
FFP: dosage
- depends on the clinical situation and underlying disease and the patient's PT, aPTT, fibrinogen and INR
When to order FFP?
- patients experiencing multiple coag factor deficiencies from:
- liver failure
- DIC
- vit K deficiency
- warfarin OD
- massive transfusion
- TTP - therapeutic exchange transfusion
- more
FFP: dosage (con't.)
- liver disease of DIC: often need 4 to 6 FFP
- continued hemorrhage: more
- some factors have a short half life, patients will need more frequent transfusions until their deficiency is resolved (e.g., factor XI has an 18 to 24 hr half life; may require daily FFP transfusions)
Constant infusion of FFP
- 1 FFP every 2 hours
- 2 FFP every 4 hours
therapeutic plasma pheresis (exchange)
- remove blood, separate out plasma, return RBC's
- replace plasma with FFP
- we can see orders to plasma exchange with FFP replacement daily; volume is determined by patient size and whether need they need 1 x volume replacement or 1.5 x volume replacement... volumes could be as high as 3,500 to 4,000 mL or more
cryoprecipitated AHF (cryos)
- concentrated source of certain plasma proteins: fibrinogen, vWF, factors VIII, XIII, and fibronectin
- make FFP, feeze, thaw in cold (refrigerator), white precipitate forms = cryo
- remove all but 15 to 30 mL of plasma
- refreeze within 1 hour at less than or equal to 18 C
- shelf life = 1 year frozen
cryoprecipitated AHF (cryos) (con't.)
- thaw at 37 C with agitation
- thawed product stored at 20 to 24 C for up to 6 hours
- multiple cryo thawed and pooled, stored at 20 to 24 C (on rotator, typically) for up to 4 hours
- may also come prepooled, pools of 5
Concentrations of proteins in cryos
- each single unit of cryoprecipitated AHF should contain greater than or equal to 80 IU factor VIII, greater than or equal to 150 mg of fibrinogen in approx. 5 to 20 mL of plasma
- if prepooled, assume these amounts per unit in pool
- example: a pool of 5 cryoprecipitates would be expected to contain: 5 x 80 IU of factor VIII (400 IU) and 5 x 150 mg of fibrinogen (750 mg)
indications for transfusion of cryos
- treatment of hemophilia A or von willebrand's disease
- congenital or acquired fibrinogen deficiencies
- factor XIII deficiency
- fibrin glue for surgical hemostasis
- if concentrated coagulation factors are available, they are preferred for specific coagulation deficiencies in most cases
transfusion criteria - cryo (dosage)
- adult typically 6 - 10 single cryos pooled or 2 cryo pools (already pools of 5 per bag)
- neonates usually get part of 1 cryo (volume requested by MD)
- primary source of fibrinogen (also factors VIII, vWF, XIII, and fibronectin)
- must have at least 150 mg of fibrinogen/cryo
- target pre-surgery fibrinogen level at least 80 mg/dL
- may prefer to transfuse prior to surgery if less than 100 mg/dL
when to order cryo? what is the cut-off or trigger to transfuse?
- liver failure
- DIC
- massive transfusion
- congenital fibrinogen deficiency
- fibrin glue: 1-2 cryo mixed with bovine thrombin and placed on tip of atomizer (spray gun)
- acts as sealant or glue (prosthetic vascular grafts)
apheresis methodology
- withdraw, separate and return blood
- intermittent flow centrifugation (one arm method)
- continuous flow centrifugation
- closed systems
- 6 to 8 cycles of reinfusion
- requires anticoagulant: usually ACD
apheresis applications: component collections
- plateletpheresis
- leukopheresis
- erythrocytapheresis
- plasmapheresis
- stem cells
apheresis applications: therapeutic procedures
- cytopheresis
- therapeutic plasmapheresis: plasma exchange
- immunoadsorption
- photopheresis
what is an indication for cytopheresis?
sickle cell disease
what are indications for immunoadsorption?
- systemic lupus erythematosus (SLE)
- pemphigus vulgaris (blisters, mouth, skin, corticosteroids, plasma exchange)
photopheresis
- lymphocytes are separated
- photosensitizing agent added
- exposure to UV light alters lymphs
- patient is reinfused with blood and plasma
- stimulates prevention of GVHD, BMT, transplant, etc.
apheresis adverse effects
- citrate toxicity: numbness and tingling around the mouth, tetany, cardiac arrythmia
- hematoma
- infection
- vasovagal reactions
- hypovolemia
- hemolysis
- air emoli
plasma derivatives
- pooled human plasma: separated to isolate factor for product being manufactured
- purification techniques to help reduce/eliminate risk of transfusion-transmitted diseases
- volunteer and paid donor pools utilized
plasma derivatives: fractionation technique
- most common: Cohn fractionation-precipitation technique
- WWII
- five variables used to obtain differential solubility: ETOH concentrations, pH, temperature, ionic strength, protein concentrations
plasma derivatives: purification
- inactivate viral pathogens
- dry heating: heat sealed, lyophilized vial
- pasteurization (wet heating): while still in solution
- vapor heating: expose lyophilized concentrate to 60 C steam for 10 hours, then 1 hour at 80 C
- solvent detergent process: exposed to organic solvent and detergent which dissolves lipid envelope and kills virus within
Plasma derivatives: recombinant products
- using recombinant DNA technology
- produce in laboratory (e.g. Factor VIII)
- ADVATE antihemophilic factor (recombinant)
Plasma derivatives: examples
- albumin
- factor VIII concentrate
- factor IX concentrate
- antithrombin III concentrate
- Rh immune globulin (RhIG)
- intravenous immune globulin (IVIG)
- factor VIIa
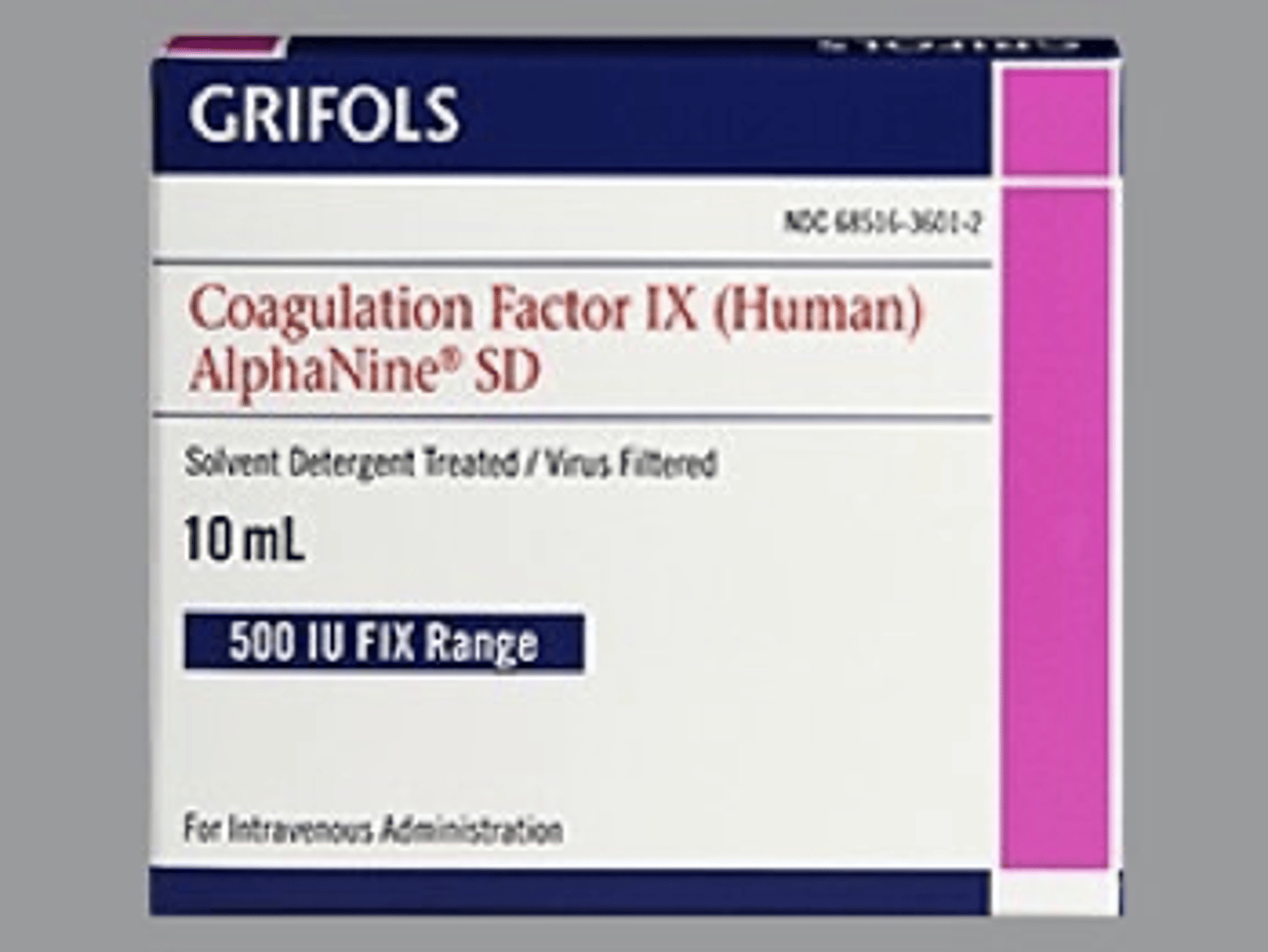
Grifols coagulation factor IX
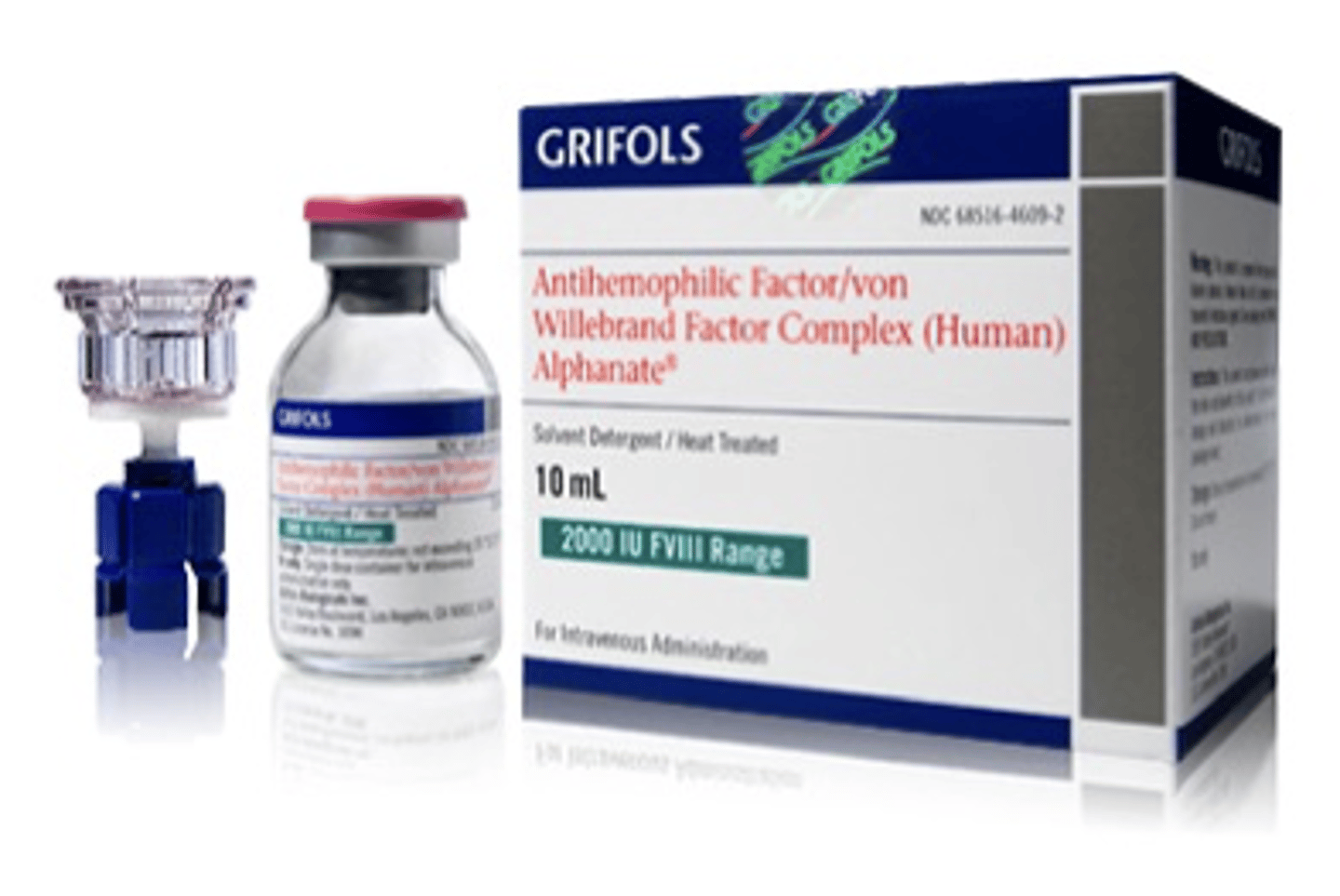
Grifols antihemophilic factor/von Willebrand factor complex
Benefix coagulation factor IX (recombinant) (250 IU range)
- this product is also available in the 3,000 IU range (different box)
panzyga immune globulin intravenous
RhoGAM Rh (D) immune globulin
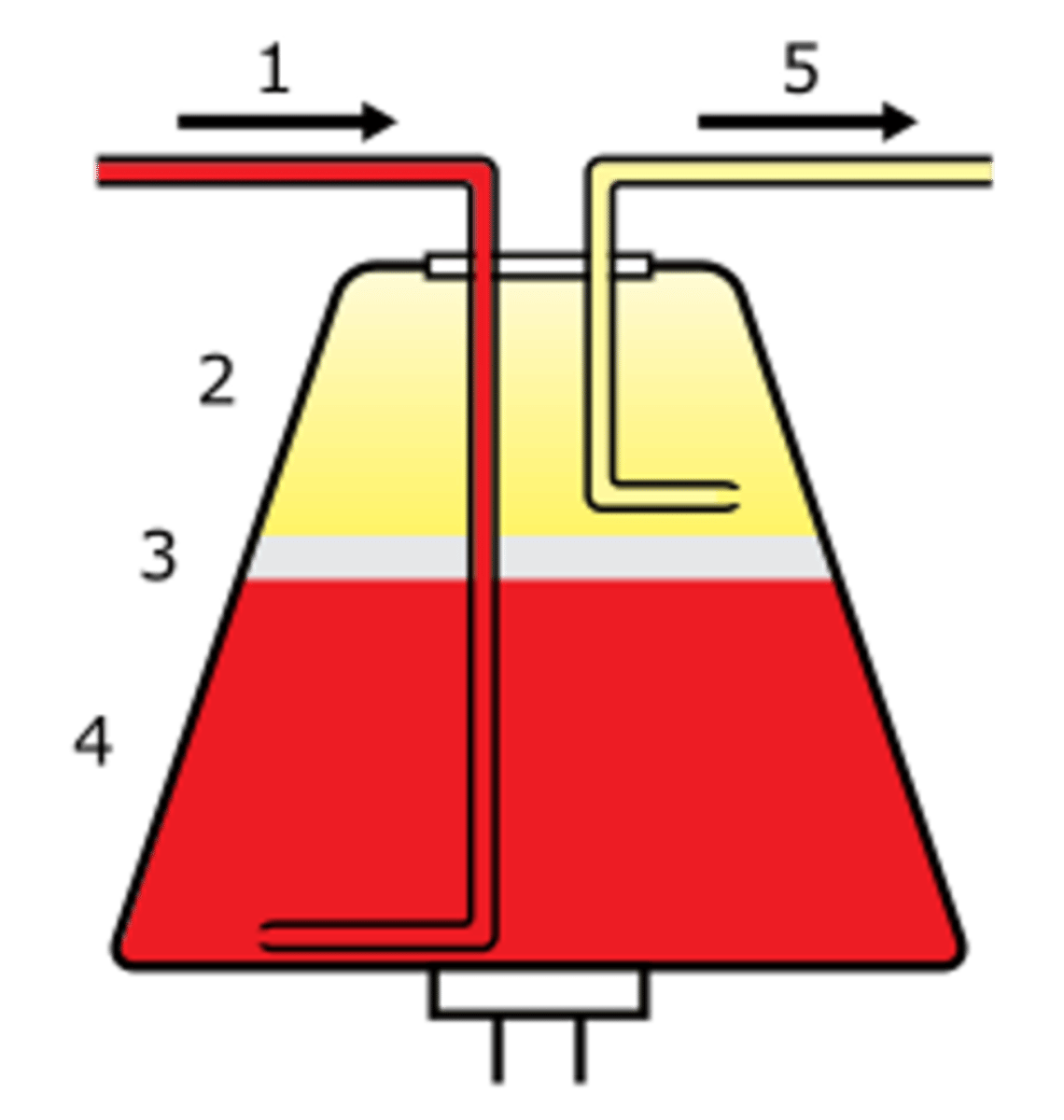
Platelet donation: 2 arm method
- blood out/platelets removed
- blood anticoagulated and returned with most of the plasma