L1-3: Principles of Pharmacology
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
70 Terms
_____________ — drug movement into (absorption), within (distribution) and out of the body (elimination)
pharmacokinetics
____________ — drug actions and their mechanisms
pharmacodynamics
__________ — transfer of drug from site of administration to systemic circulation
absorption
__________ — transfer of drug from systemic circulation to tissues
distribution
______________ — removal of drug from the body
elimination (clearance)
The majority od drug absorption takes place in the ___________
small intestine
metabolism is handled mainly by the _________
liver
excretion takes place __________ and/or ___________
renally; hepatobiliary
A nurse is caring for a client who has ingested a potentially toxic substance. Which rationale best supports the decision to administer the treatment orally rather than intravenously?
Oral administration permits neutralization or removal of the substance before systemic absorption occurs
The ______________ is hepatic metabolism of a drug after oral absorption that reduces the amount of active drug reaching systemic circulation.
first-pass effect
Why should a particular drug not be administered orally due to the first-pass effect?
The drug would be extensively metabolized by the liver before reaching systemic circulation, reducing its therapeutic effectiveness
What is the primar means by which drugs cross membranes?
passive diffusion
Why are many medications formulated with a relatively neutral pH?
Neutral pH allows drugs to be both water-soluble and lipid-soluble, enhancing absorption across cell membranes
A relatively ____________ allows drugs to be both water and lipid soluble, increasing absorption
neutral pH
Why is it important that most medications are formulated as weak acids or weak bases?
Weak acids and bases can exist in both ionized and nonionized forms, allowing drugs to cross cell membranes and be absorbed effectively
_________ — when a drug enters into systemic circulation
bioavailibility
____________ occurs when a drug becomes ionized in a compartment with a different pH, preventing it from crossing membranes and leading to accumulation.
ion trapping
Ion trapping is typically favorable in what types of scenarios?
-ie: why would you want to create a charged environment?
Ingestion of toxic chemicals and/or medications
-consuming milk (base) to counter the stomach (acid) leading to a charged environment and prevent absorption of chemical and/or medication
A pH of ________ is required for a drug to be considered a weak base and/or weak acid and be readily dissolved
6-8
Weak acids __________ their proton resulting in a ______ charge
Weak acids give off their proton resulting in a negative charge
______ is the pH where a drug is 50% charged and 50% uncharged
pKa
pH < pKa → more ______ environment (favors absorption of weak ____)
pH < pKa → more acidic environment (favors absorption of weak acids)
pH > pKa → more ____ environment (favors absorption of weak ____)
pH > pKa → more basic environment (favors absorption of weak bases)
A nurse is reviewing drug absorption principles with a student. A medication is a weak acid with a pKa of 4. Which environment would result in the greatest absorption of this medication?
pH 2
__________________ tells you whether a drug is charged or uncharged.
pH compared to pKa tells you whether a drug is charged or uncharged.
A drug with a charge results in __________
poor absorption
A nurse is preparing a client for suturing of an infected wound. The provider explains that a local anesthetic may be less effective in this area. What is the primary reason inflammation interferes with the effectiveness of local anesthetics?
Inflamed tissue has a lower pH, causing the anesthetic to become ionized and unable to penetrate nerve membranes
something that becomes charged needs to be transported across the membrane by a __________
protein
When drugs bind to a protein, what happens?
The protein changes its shape
Proteins communicate by _________________
changing their shape
Transport mechanisms (i.e. proteins) are usually involved with drugs that are _________________
high polar or charged (i.e. not very lipid soluble)
Charged = __________
ionized
Which takes longer, passive diffusion or carrier-mediated transport?
Carrier-mediated transport
What is the #1 place for drug-drug interaction?
plasma protein binding
Why is plasma protein binding the #1 place for drug-drug interactions?
-When two highly protein-bound drugs are given together:
They compete for the same binding sites
One drug displaces the other from the protein
This causes a sudden increase in free (active) drug
Result → toxicity, even if the dose was correct
-Unbound (free) drug = pharmacologically active, can:
Cross membranes
Bind receptors
Cause effects and side effects
A nurse is reviewing a client’s medication list and notes that two newly prescribed drugs are both highly protein bound. Why does this combination place the client at increased risk for adverse effects?
Competition for protein-binding sites can increase the amount of free, active drug in the bloodstream
A nurse is evaluating the risk for drug–drug interactions in a client prescribed multiple medications. Which statement best explains how drug affinity influences binding at plasma protein sites?
Drugs with higher affinity preferentially occupy protein-binding sites and can displace other bound drugs
How can the first pass effect be minimized?
By using other routes of administration besides oral (i.e. sublingual, subcutaneous, IM)
True/False: sublingual administration is a systemic route?
True; sublingual skips GI tract
True/False: there is no difference in rate of absorption between a liquid and solid dosage form
true
Does plasma protein binding affect bioavailability?
No; plasma protein binding occurs after bioavailibility
Protein bound drugs cannot distribute to tissues or be eliminated, therefore bound drugs are pharmacologically _______________
inactive
Only the ___________ form of a drug is available for distribution to sites of action and elimination
free/unbound
What factors affect distribution?
-blood flow
-pH differences between plasma and intracellular space
-specialized barriers (i.e. blood-brain barrier)
-tissue factors affecting accumulation or binding of drug
Drugs are eliminated by the liver and kidneys as they move from the bloodstream to these organs by following their ________________________
concentration gradient
How are drugs eliminated from the body?
Via metabolism, which makes the drug more water soluble, and therefore easier to be eliminated (Kidneys eliminate drugs through urine, which is mostly water)
True/False: Metabolism determines effectiveness and/or toxicity of a drug
False; metabolism only makes a drug easier to eliminate
____________ is how the body chemically changes a drug so it can be eliminated.
Biotransformation
Where does biotransformation happen?
liver
Biotransformation occurs by a group of liver enzymes called the ______________
Cytochrome P450 (CYP) system
Biotransformation phase 1 reactions are responsible for _______________
modifying the drug
Biotransformation phase 1 reactions include __________, which is carried out by __________________
oxidation and reduction; oxygenases and reductases
Biotransformation phase 2 reactions are responsible for _____________
making the drug excretable
Biotransformation phase 2 reactions include the formation of __________ by _____________
conjugates; transferases
During biotransformation phase 2 conjugate reactions, the drug attaches to:
-___________
-___________
-___________
-a sugar
-an amino acid
-sulfafe
Drug metabolism (biotransofmration) produces ____________ that are usually more water-soluble but may be less active, more active, inactive, or even more toxic than the parent drug.
metabolites
True/False: All drugs have to go through both phases of biotransformation
False; some drugs just go through one, and others go through both
__________ → drug conjugate secreted into the bile and reconverted to parent compound by intestinal bacteria can be reabsorbed from the small intestine back into the bloodstream
Enterohepatic recycling
What is the significance of enterohepatic recycling?
prolongs drug action
To increase the elimination of weak acids renally, __________ the urine by administering _________
alkalinize; acetazolamide
To increase the elimination of weak bases renally, ______ the urine by administering _____________
acidify ; ammonium chloride
_________ elimination is constant percentage of drug eliminated per unit time
first order
What is half-life?
time it takes to eliminate 50% of the drug
Most drugs are dosed based on their __________
half-life
Most drugs are eliminated during ____________
first order
A nurse is administering a medication that follows first-order elimination and has a half-life of 4 hours. If the dose of the medication is doubled, which outcome should the nurse expect?
A. The medication will take twice as long to be eliminated
B. The peak plasma concentration will increase, but the half-life will remain the same
C. The drug will be eliminated at a constant amount per hour
D. The medication will reach steady state more quickly
B. The peak plasma concentration will increase, but the half-life will remain the same
How long does it take to reach steady-state blood levels when taking a drug orally?
4-5 half-lives
_______ elimination is the constant amount of drug eliminated per unit time
zero order (capacity limited)
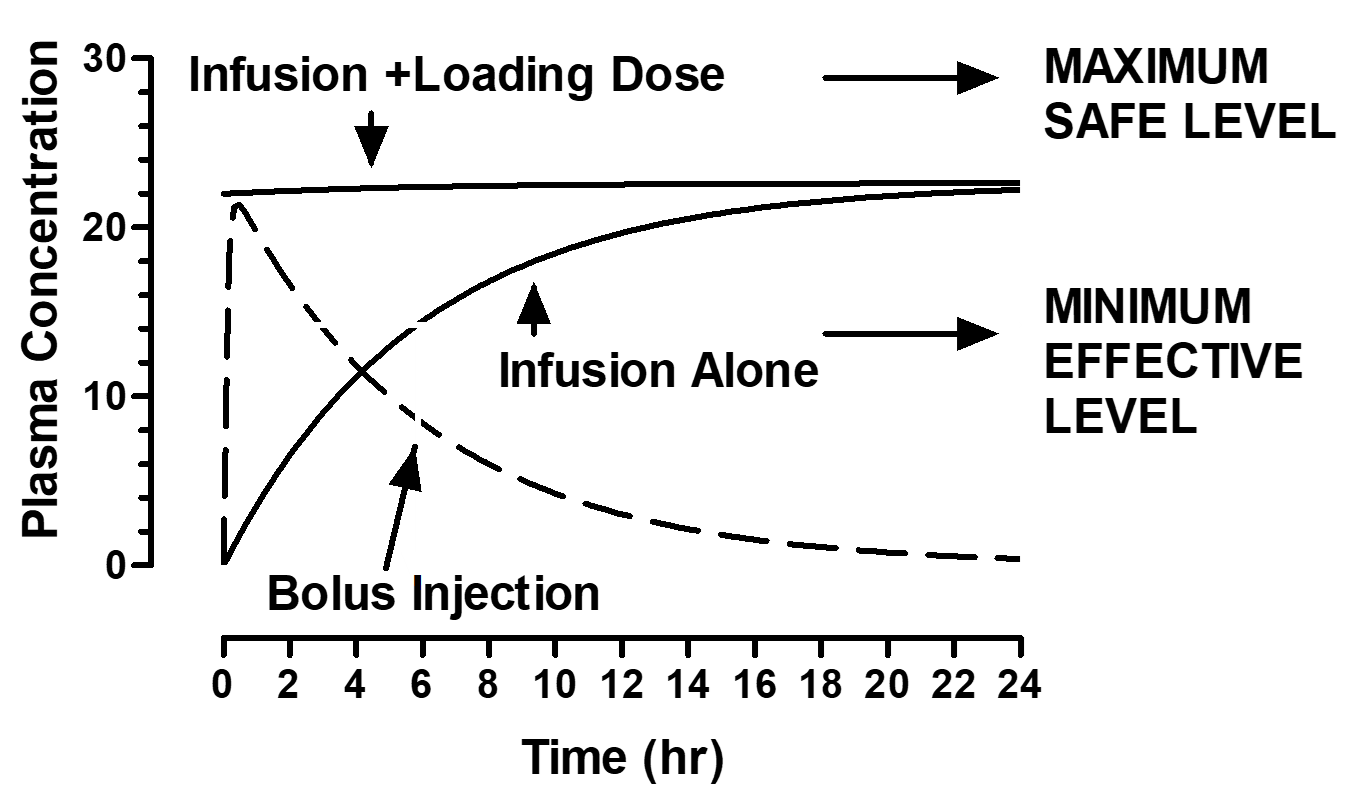
A nurse is administering a medication by continuous IV infusion. The provider prescribes an initial loading dose followed by a maintenance infusion. What is the primary purpose of giving the loading dose?
A. To prevent the drug from reaching toxic levels
B. To maintain a constant drug concentration over time
C. To rapidly achieve a therapeutic plasma concentration
D. To shorten the drug’s half-life
C. To rapidly achieve a therapeutic plasma concentration
How can steady-state levels be accomplished rapdily (i.e. stroke patient)?
combining slow drip infusion and bolus injection (loading dose)