5.2- Excretion
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
what is excretion
The process of removing metabolic waste from cells
functions of the liver
control of:
blood glucose levels
amino acid levels
lipids levels
synthesis of:
red blood cells in the foetus
bile
plasma proteins
cholesterol
storage of:
iron
vitamins
glycogen
breakdown of:
hormones
red blood cells
breakdown of excess proteins
body cannot store excess amino acids/proteins that aren’t used in metabolism
excess amino acids are broken down:
deamination
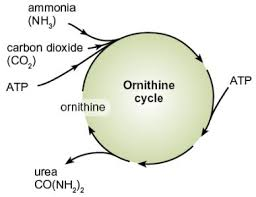
ornithine cycle
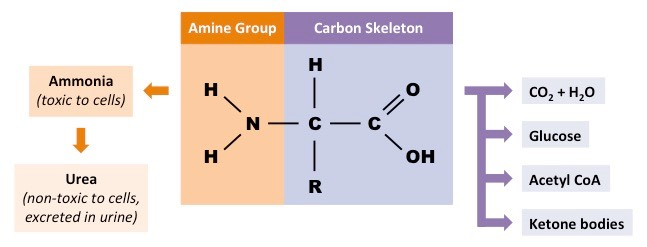
deamination
Amine group is converted to ammonia→ goes through ornithine cycle to be converted to urea
rest of amino acid forms keto acid:
enter krebs cycle to be respired
converted to lipids or cholesterol
ornithine cycle
ornithine is an amino acid used to make urea
process requires input of energy as ATP
detoxification
liver breaks down substances e.g. alcohol, paracetamol, hydrogen peroxide, insulin
occurs in SER of hepatocytes
detoxification of ethanol
ethanol converted to ethanal (acetyl aldehyde)→ catalysed by alcohol dehydrogenase:
oxidised NAD→NADH
Ethanal converted to ethanoate (acetate)→ catalysed by aldehyde dehydrogenase
acetate enters krebs cycle
oxidised NAD→ NADH
structure of the liver lobules
several lobules form liver
hepatic artery supplies oxygenated blood
hepatic vein carried deox. blood to heart
hepatic portal vein brings nutrient rich blood from intestines
bile duct transports bile to gallbladder
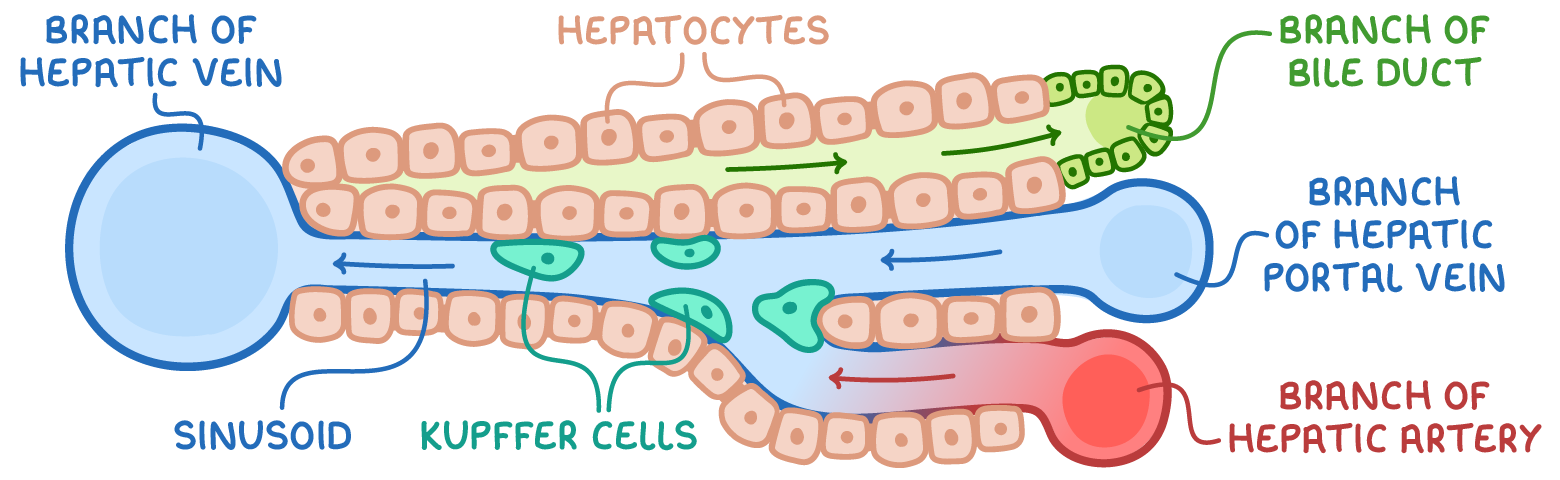
sinusoids
receive blood from hepatic portal vein and hepatic artery
leaky→ allows small and medium sized proteins to enter and leave blood
contain Kupffer cells→
specialised macrophages- engulf microbes
involved in breakdown + recycling of old rbc
lined by hepatoctyes that have microvilli→ increase SA
bile canniculus
hepatocytes separated by bile canniculus
hepatocytes that are close to bile cannuli are rich in golgi vessels→ transport of bile into channels
cannuli join to form bile duct→ delivers bile to duodenum, diverting through gall bladder
hepatocytes
in contact with blood in sinusoids
nuclei distinctly round:
some binucleate cells
active in protein and lipid synthesis→ lots of RER and SER, golgi membranes
glycogen granules and vesicles contain low density lipoproteins
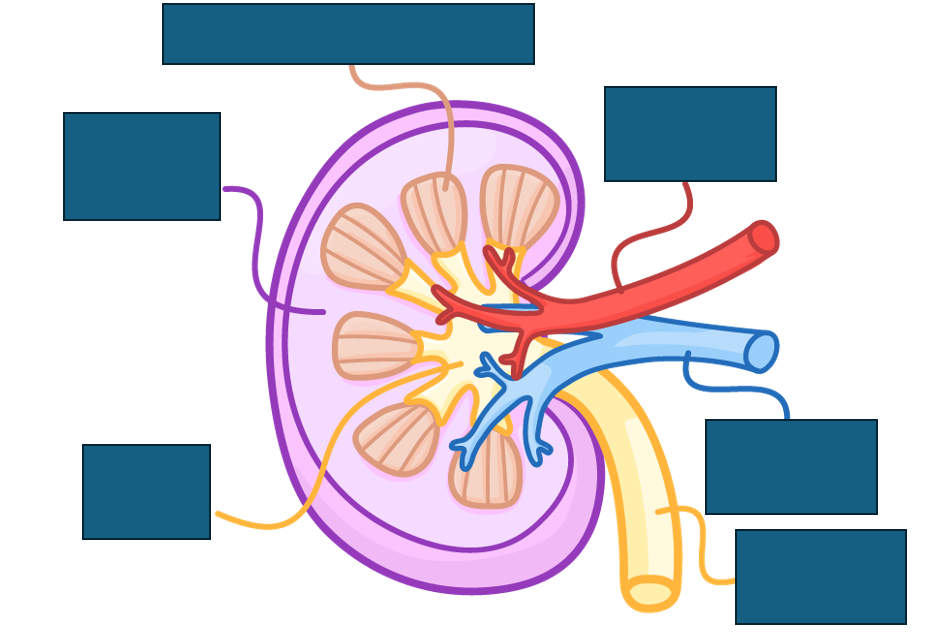
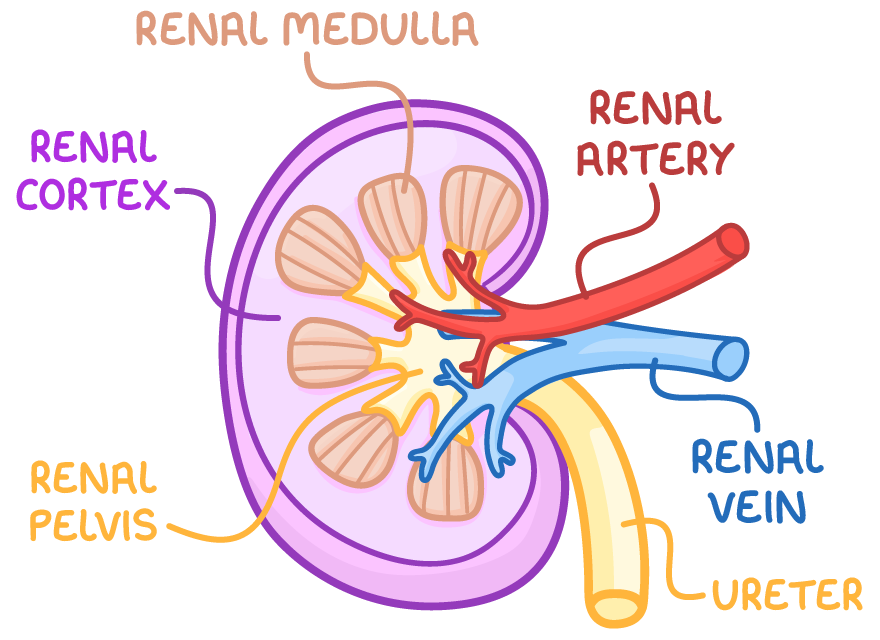
structure of the kidney
renal cortex contains bowman’s capsule, convoluted tubules and blood vessels
renal medulla contains loops of Henle, collecting ducts and blood vessels
renal pelvis collects urine into the ureters
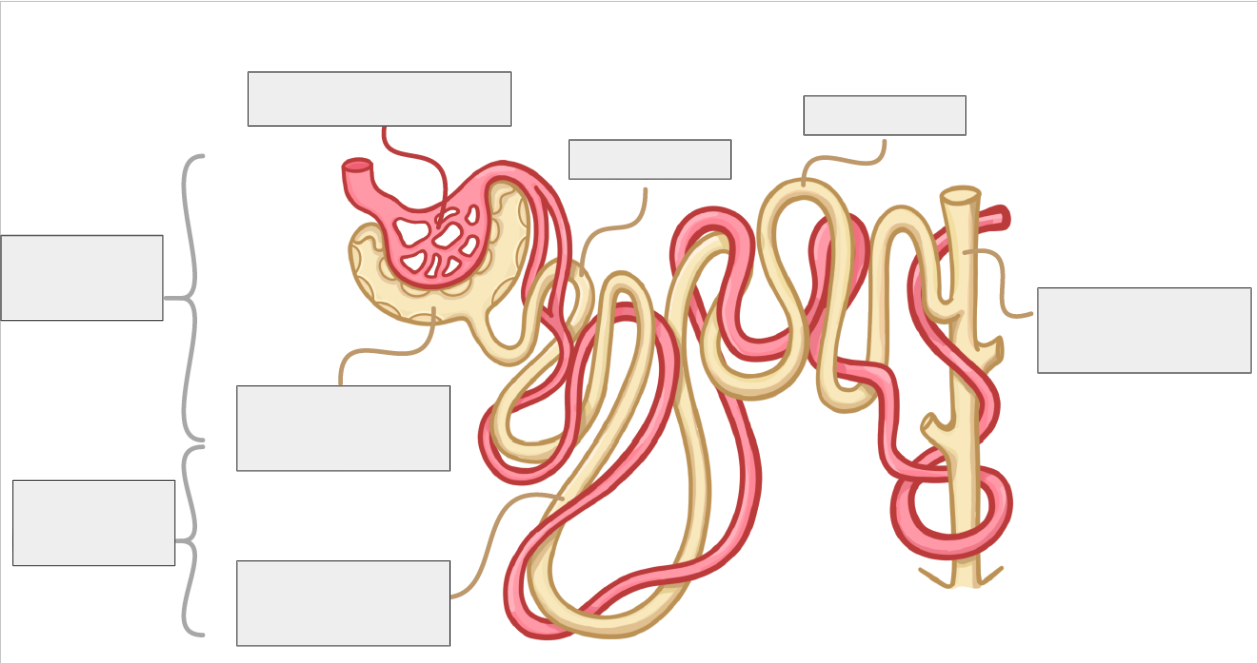
structure of nephrons
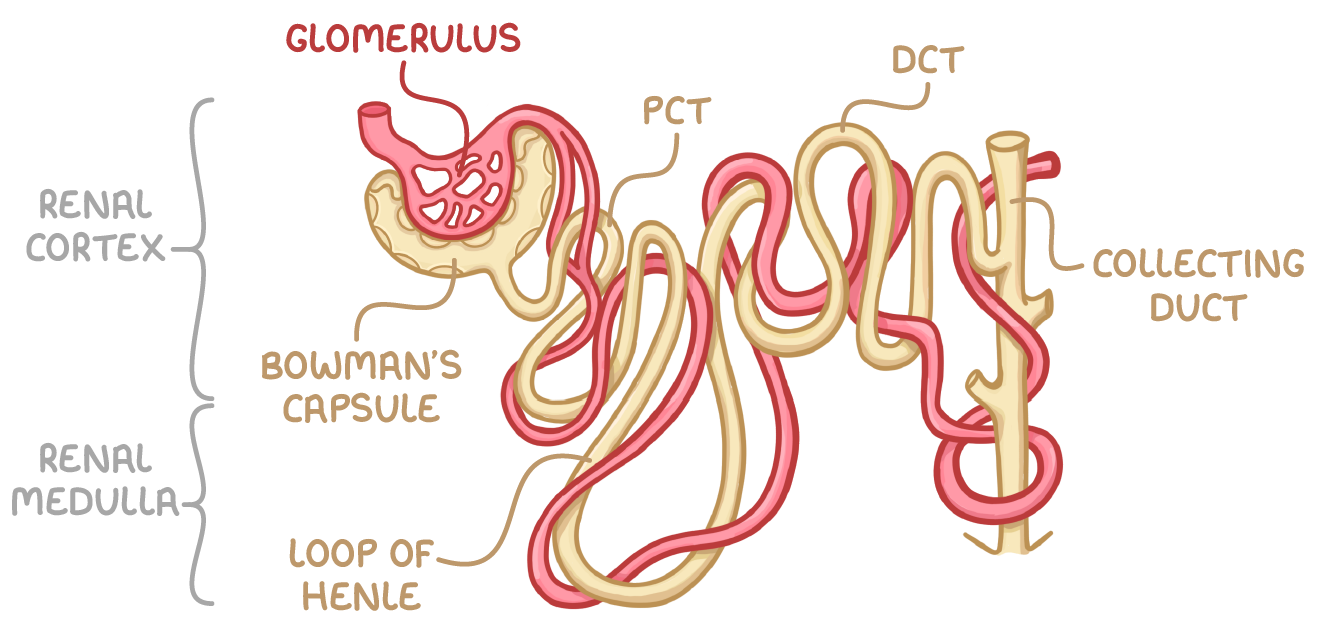
Bowman’s capsule
surrounds capillary ball called glomerulus, from which filtrate is formed
proximal convoluted tubule
reabsorbs useful substances e.g. water, glucose, salts, into surrounding capillaries
Loop of Henle
extends from cortex into medulla and back into cortex and creates high solute gradient in medulla→ helps with reabsorption
Distal convoluted tubule
Fine tunes water balance by reabsorbing water into capillaries
collecting duct
Collects filtrate from multiple nephrons and further fine-tunes water balance
blood vessels in the nephron
afferent arteriole→ supplies glomerulus with blood
glomerulus→ fluid forced out of blood within mass of capillaries into Bowman’s capsule through ultrafiltration
efferent arteriole→ carries blood away from glomerulus
capillaries around P and DCT and Loop of Henle absorbs salts, glucose, water
ultrafiltration
process by which small molecules e.g. water, glucose, mineral ions and urea are filtered out of the blood and into the bowman’s capsule
process of ultrafiltration
blood enters glomerulus through afferent arteriole
blood leaves glomerulus via smaller efferent arteriole→ high HS pressure
High pressure= molecules forced out of the blood through pores in capillary endothelium
molecules move through basement membrane → has collagen fibres that act as selective filter preventing large molecules and blood cells from passing into Bowman’s capsule
Molecules move through Bowman’s capsule epithelium→ has podocytes with extensions called pedicels- wrap around capillaries and help filter blood
filtered fluid collects in Bowman’s capsule
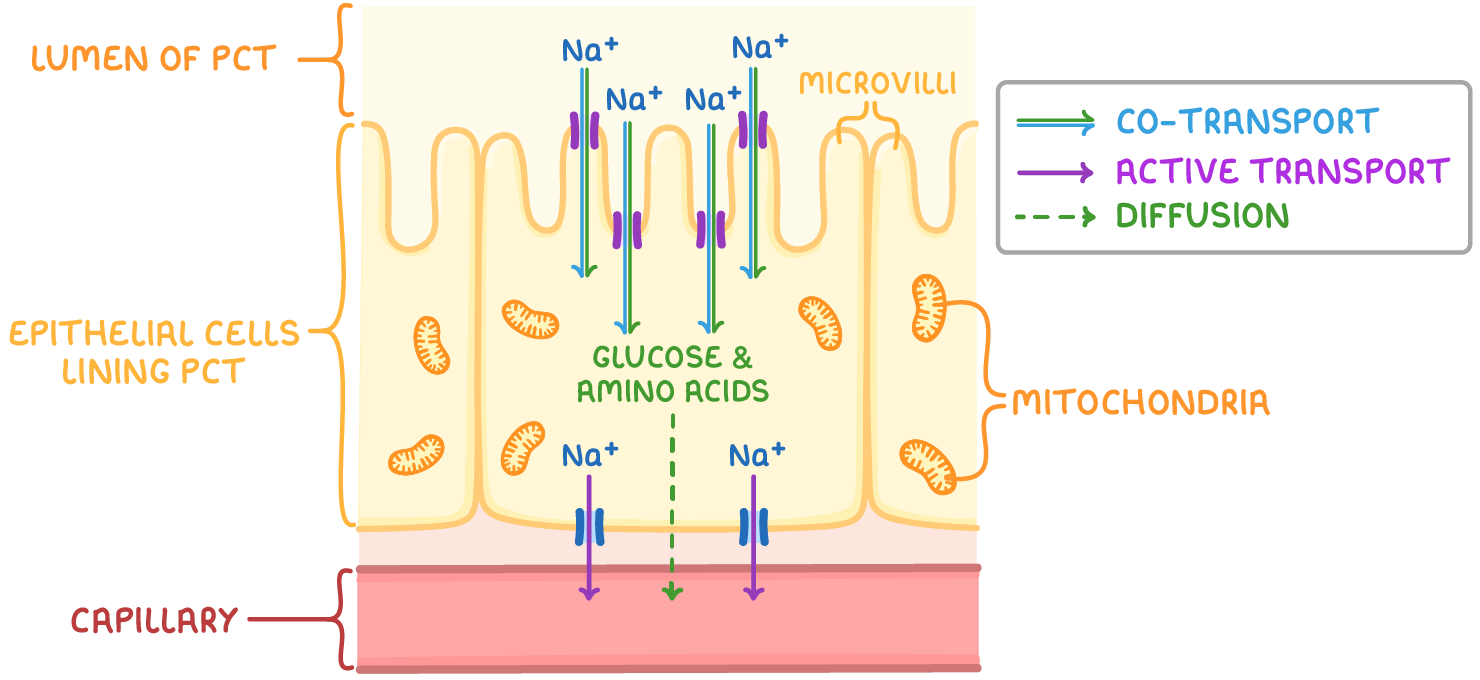
adaptations of the PCT
microvilli→ increase SA:V ratio
basal infoldings→ increase SA:V ratio
Lots of mitochondria→ provide ATP for Active transport
co-transporter proteins in plasma membrane→ allow for co-transport of substances
reabsorption in the PCT
Na+ actively transported into capillaries→ reduced Na+ conc. in epithelial cells lining PCT
Na+ moves from PCT lumen into epithelial cells , down conc. gradient
Na+ co-transported with substances e.g. glucose, amino acids into epithelial cell
reabsorbed molecules can diffuse into blood capillaries
role of the DCT
makes final adjustments to filtrates content, mainly by reabsorbing water and salts
involves:
alteration of DCT membrane permeability to regulate further absorption of water and solutes
regulation of blood pH by selectively reabsorbing certain ions
the loop of Henle
U-shaped tubule within kidney nephron
starts in the renal cortex and descends into the renal medulla
comprises of two main sections:
ascending limb
descending limb
descending limb
first section through which filtrate travels
narrow
highly permeable to water
impermeable to ions
ascending limb
second section, follows descending limb
wider than descending limb
impermeable to water
permeable to ions
water reabsorption in the loop of Henle
In descending limb, water leaves filtrate via osmosis into interstitial space
filtrate loses water as it moves down descending limb-reaching lowest WP at tip in medulla
Lost water is reabsorbed into blood in surrounding capillaries and is carried away
ascending limb→ impermeable to water but permeable to Na+ and Cl-
Na+ and Cl- diffuse out of filtrate into interstitial space at bottom of ascending limb due to low WP
WP in interstitial space of medulla is is v. low
Na+ and Cl- need to be actively transported out of top of ascending limb as WP increases as it ascends
water potential gradient created in interstitial→ highest WP in cortex and increasingly lower WP deeper into the medulla
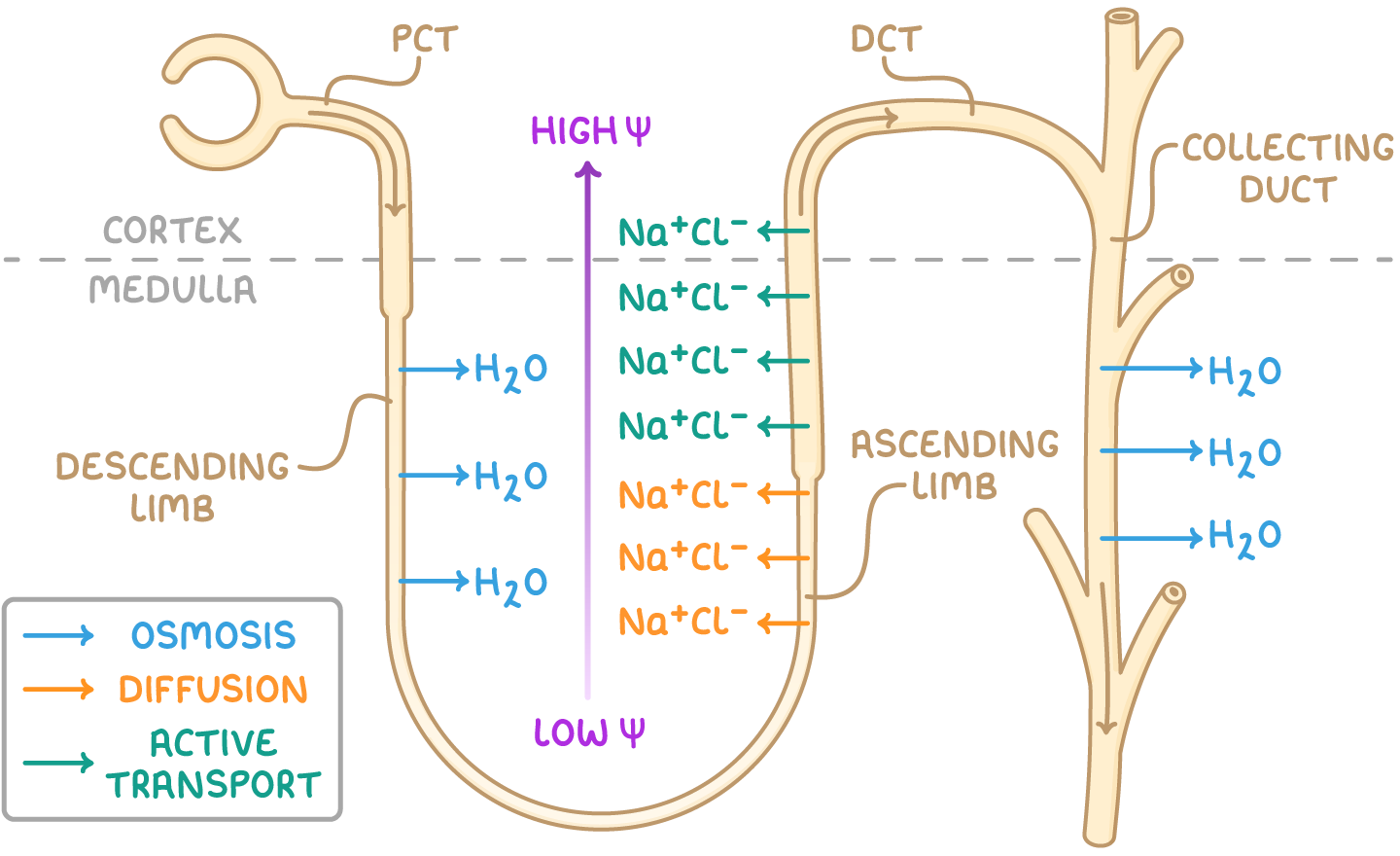
what happens when filtrate enters collecting duct
water moves from filtrate in collecting duct into interstitial space and then into surrounding capillaries
water continues to exit filtrate as it moves through the collecting duct
urine leaving collecting duct has very low WP as most water has been reabsorbed into blood
countercurrent multiplier
loop of Henle operates as a counter current multiplier system- intensifies salt gradient in medulla
concentrates urine
ensures there is always a WP gradient drawing water out of the collecting duct
If flows were parallel, less reabsorption would occur
how is the countercurrent multiplier set up
as filtrate moves down collecting duct, it loses water→ decreases WP
due to pumping of ions out of ascending limb of loop of Henle, WP of surrounding tissues in medulla is even lower than in collecting duct
allows water to continue to move out of filtrate down whole length of collecting duct
ADH
antidiuretic hormone
key features of ADH
produced in hypothalamus
stored in posterior pituitary gland after production
target cells are those lining DCTs and collecting ducts in kidneys
mechanism of ADH action
ADH attaches to receptors on surface of cells in DCT and collecting duct
Triggers activation of cAMP→ second messenger that initiates cascade of reactions leading to phosphorylation of water channel proteins (aquaporins)
Aquaporin vesicles merge with cell surface membrane
water moves through aquaporins via osmosis from DCT and CD into surrounding interstitial space
water reabsorbed into surrounding blood vessels
response to lack of water
water moves from osmoreceptors into blood→ osmoreceptors shrink, detecting decrease in WP of blood. Respond by producing ADH
Nerve signals prompt release of ADH from posterior PG and ADH transported via blood to the kidneys
increase in aquaporins in DCT and CD cell membranes= greater permeability to water
More water reabsorbed into the blood
urine becomes more concentrated and is produced in smaller volumes
response to excess water
water moves into osmoreceptors from blood via osmosis and osmoreceptors detect an increase in WP of blood
nerve signals to posterior PG decrease, reducing release of ADH
DCT and collecting duct cell membranes become less permeable to water
less water reabsorbed into the blood
urine becomes more dilute and is produced in larger volumes
use of urine samples for medical diagnosis
key indicators of medical conditions found in urine:
presence of glucose can indicate diabetes
elevated creatinine in urine= muscle and/ or kidney damage
presence of blood/ proteins in urine may signal kidney disorders
use of urine samples for pregnancy testing
hCG is a hormone produced by placenta after embryo is implanted in uterus
presence of hCG in blood or urine indicates pregnancy
Modern pregnancy tests use monoclonal antibodies that specifically target hCG
how do pregnancy tests work
wick of test soaked in urine
mobile monoclonal antibodies with coloured beads bind to hCG to form antibody-hCG complex
urine carries complex to window with immobilised monoclonal antibodies that bind to antibody-hCG complex
creates a coloured line/ symbol, indicating pregnancy
other immobilised antibodies bind to mobile antibodies with or without hCG, forming control line to confirm test is working
the use of urine samples for drug detection
drugs filtered through the kidneys and are removed during urination→ urine typically contains evidence of drugs a person has used
E.g.:
anabolic steroids can be used illegally by athletes
illegal recreational drugs e.g. cocaine can be tested for in urine samples
how to urine drug tests work
carry out an immunoassay-. monoclonal antibodies bind to drug or its breakdown product to indicate whether urine sample contains them
vaporise urine sample with known solvent
separate components of sample using gas chromatography
use mass spec. to identify molecular structures in the sample.
causes of kidney failure
occurs when kidneys are unable to function effectively→ cannot filter blood and eliminate waste products properly
Two main causes:
Kidney infections→ lead to inflammation and swelling in the kidneys, damaging cells responsible for filtering and reabsorption
High blood pressure→ can damage glomeruli capillaries so proteins and blood leak into urine
indicators of kidney failure
Glomerular filtration rate (GFR)→ measure of how much blood is filtered in Bowman’s capsules of the nephrons
used as an indicator of kidney failure:
low GFR= less effective blood filtration
blood test can measure level of creatinine in blood→ used to estimate GFR
high level of creatinine signals that kidneys are not working properly and could indicate kidney disease
effects of kidney failure
build-up of mineral ions in blood→ electrolyte and osmotic imbalances
build up of toxic urea in blood→ can poison cells
high blood pressure→ may cause heart problems and strokes
loss of calcium and phosphorus balance→ weakens bones
build up of abnormal proteins in blood→ causes pain and joint stiffness
anaemia→ causes tiredness and lethargy
how does dialysis filter the blood
patient’s blood passes along one side of a semi-permeable membrane
dialysis fluid contains normal plasma levels of mineral ions→ ions diffuse through dialysis tubing membrane into blood until normal ion levels
dialysis fluid has normal plasma levels of glucose→ glucose diffuses from fluid to blood until equal conc. reached
dialysis fluid has no urea→ urea diffuses into fluid from blood
larger molecules remain in blood→ cannot pas through membrane
types of renal dialysis
haemodialysis:
blood leaves patients body and flows into dialysis machine
blood filtered in dialysis machine and returned to body
peritoneal dialysis:
peritoneum→ membrane lining abdominal cavity
acts as surface across which substances exchanged between blood and dialysis fluid
dialysis fluid injected into and then drained from abdominal cavity so blood can be filtered within body itself
advantages and disadvantages of peritoneal dialysis
advantages:
no need for specialist equipment
can be done at home by patient
patient can be mobile during treatment
disadvantages:
risk of infection
required more frequently
advantages and disadvantages of haemodialysis
advantages:
lower risk of infection
required less frequently
disadvantages:
requires specialist equipment
must be done in hospital or medical centre
patient must be immobile during treatment
advantages of kidney transplants
no need for regular dialysis sessions
no need for dietary monitoring
prevents build up of waste products between dialysis sessions that cause long term damage
generally improves quality of life
one of cost rather than several long-term payments in dialysis
disadvantages of kidney transplants
risk of organ rejection if immune system recognises antigens as foreign and attacks
shortage of donor kidneys
necessitates use of medication to suppress immune system
involves associated risks of surgery