kinetic theory equation
0.0(0)
0.0(0)
Card Sorting
1/11
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
12 Terms
1
New cards
Change in momentum of molecule colliding with walls of container is Δp =
Momentum before = mu
Momentum after = -mu
change in momentum = 2mu
2
New cards
Time taken between successive collisions of molecule with wall is t =
Distance/speed = 2L/u
3
New cards
Frequency of collisions with wall is f =
u/2L
4
New cards
Average force on (from) 1 molecule is F (1 molecule) =
2mu x u/2L = mu2/L
5
New cards
Average force on (from) N molecules is F =

6
New cards


7
New cards
Pressure on wall is p =
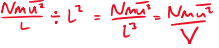
8
New cards
For one molecule, with velocity components u, v and w, square speed is c2 =
u2 + v2+ w2
9
New cards


10
New cards
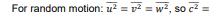

11
New cards


12
New cards
So finally
pV = 1/3 Nm(average c)2