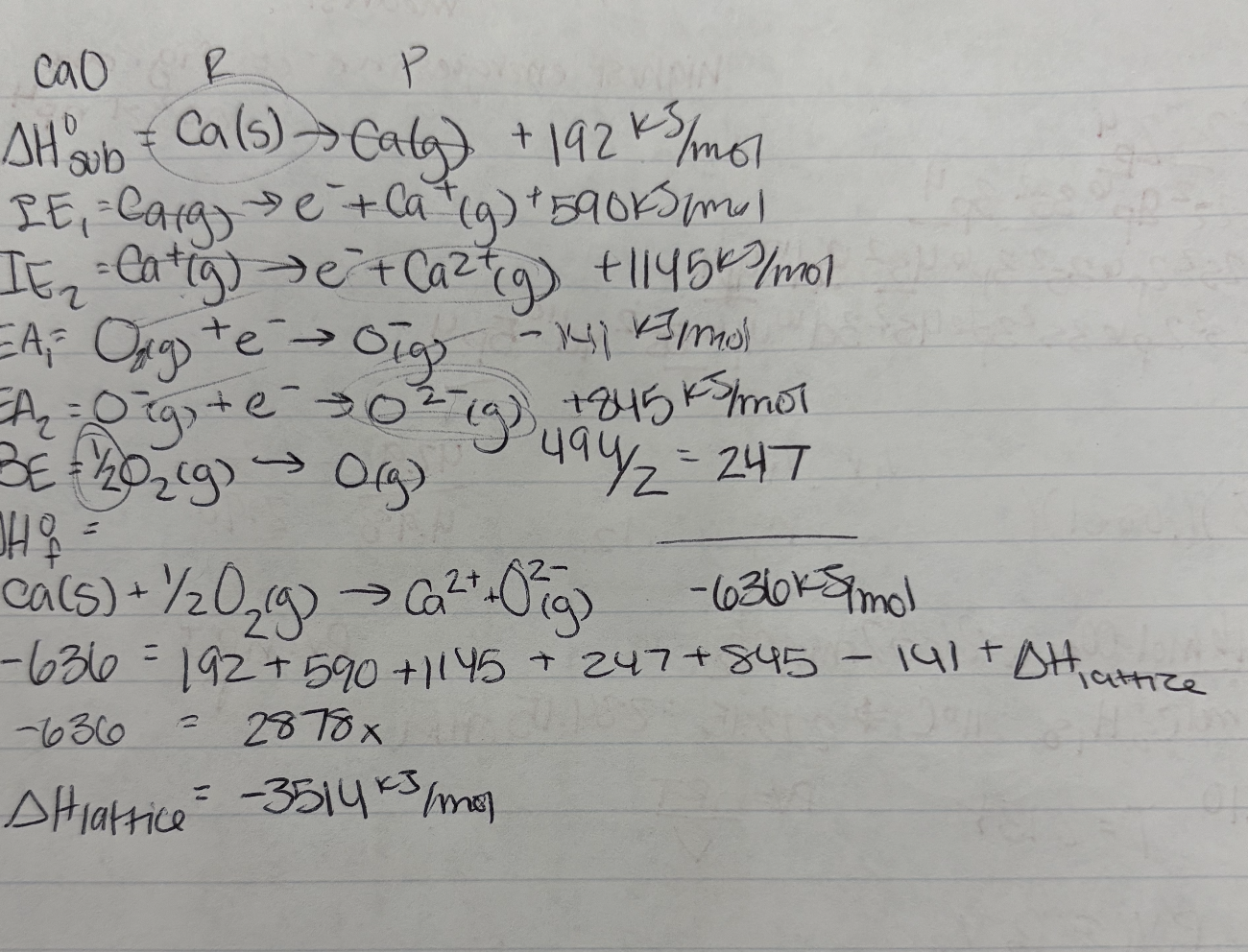Chapter 9: Periodicity and Ionic Bonding
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
Periodicity
the characteristic of something happening regularly after a certain interval (Monday every 7 days)
On the PT elements are arranged in increasing order of their atomic number so that elements repeat their properties aftera definite interval
Periodicity in Electron Configurations
(ns^1)

Valence and Core Electrons
Valence Electrons: highest occupied energy level (outermost shell)
Cl → 17e^- 7 valence e^- Core: 17-7
Core Electrons: all electrons that are not valence electrons
For Main group elements (1A - 8A) Valence Electrons = to the group number of theelement
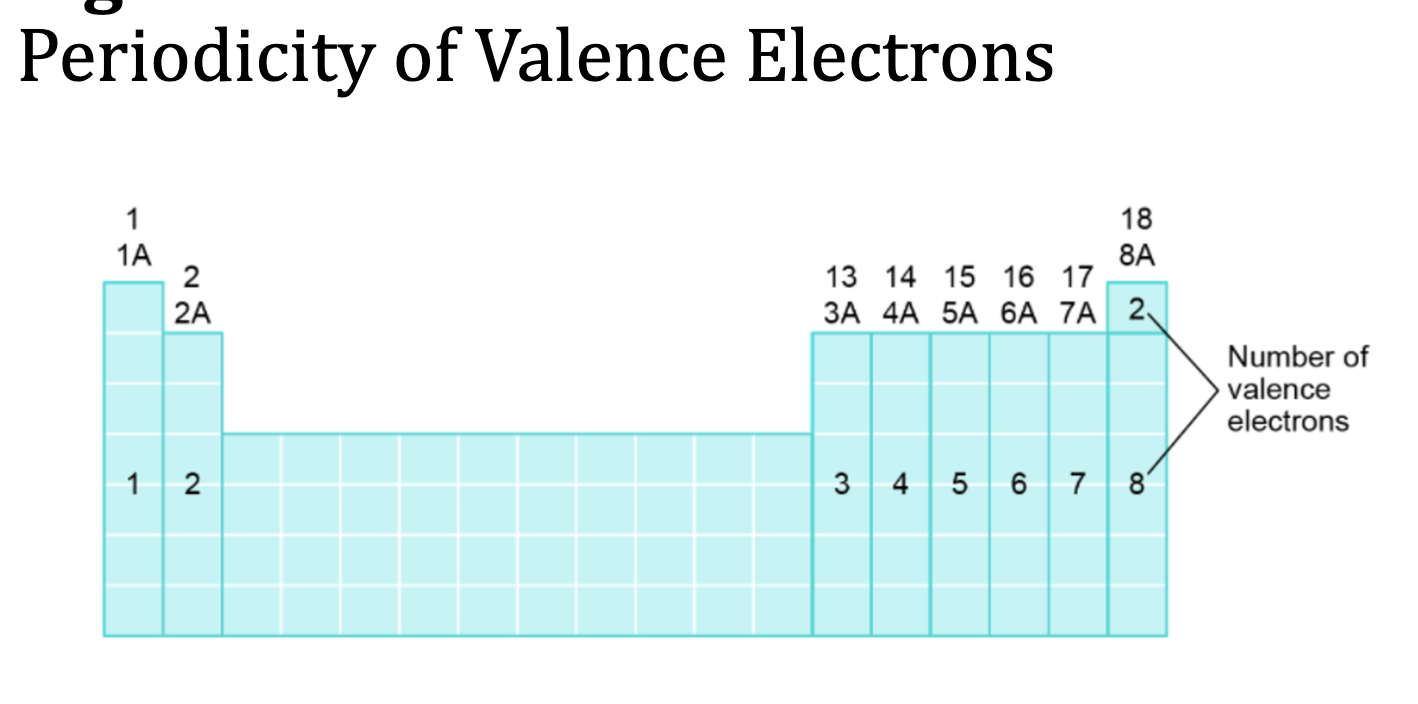
Valence Electrons
Valence electrons are involved in bonding, so elements
with the same valence electron configurations have
similar chemical properties.

Ex 9.1
valence = highest n / energy level and combine the e^-

Atomic Radius
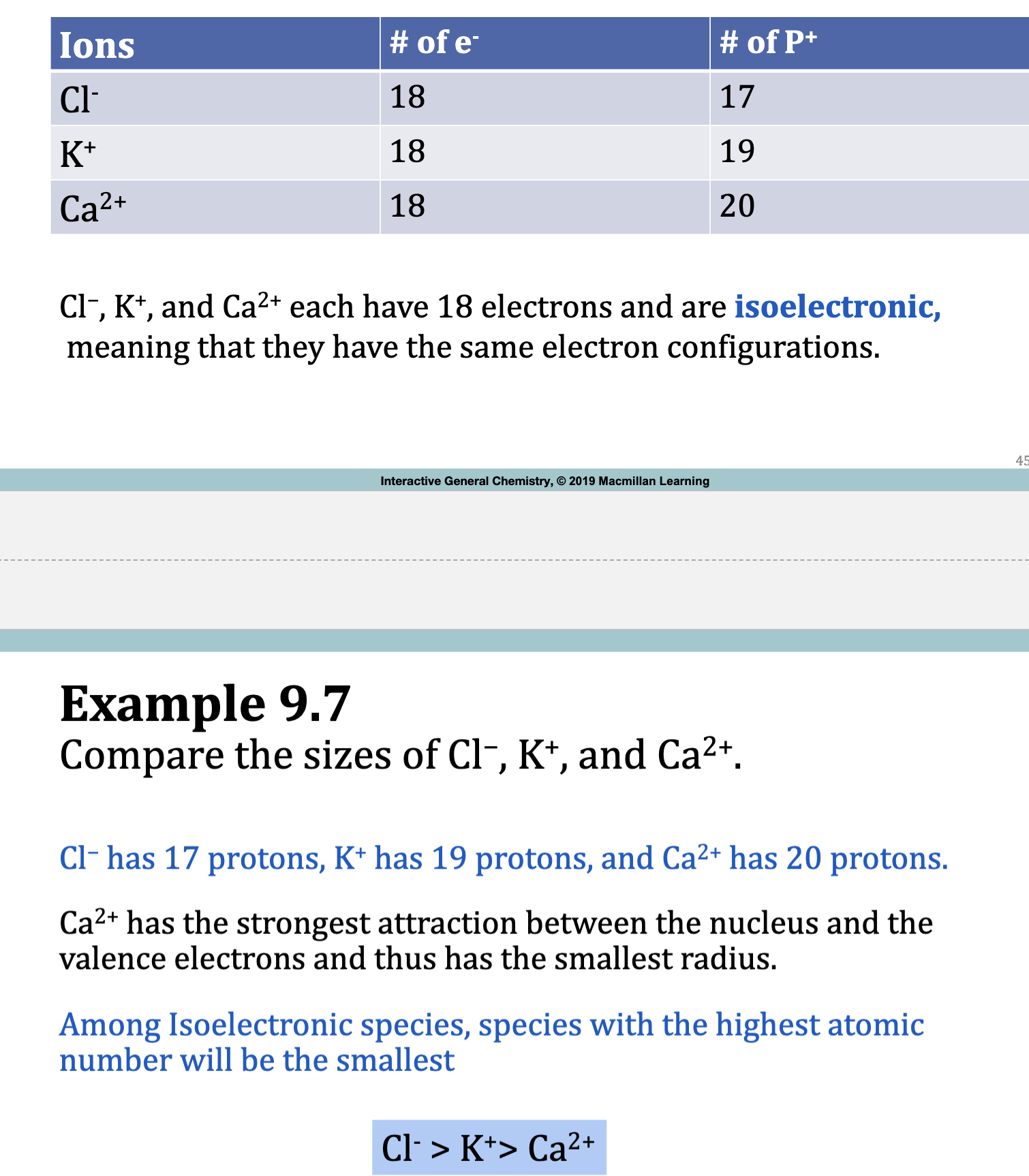
• Atomic radius generally decreases from left to right across a period.
• The nuclei of atoms become more and more strongly positive while the
number of core electrons remains the same, causing Zeff to increase.
• Valence electrons experiencing higher Zeff are pulled more tightly to the
nucleus, making the atomic radius smaller.(i.e. increasing atomic radius = smaller radius)
• This general trend is more complex for transition metals.
• Atoms and ions get larger as you move down a group on the periodic table,
due to increased size of the orbitals as n increases.

Ex 9.5

Ionic Radius: Cations
• Removing an electron decreases the total negative charge of an
atom while retaining the positive charge of the nucleus.
Na: 1s2 2s2 2p 6 3s1
Na+: 1s2 2s2 2p 6
• This increases the attraction between the valence electrons and
the nucleus.
• All cations have smaller radii than their corresponding neutral
atoms.
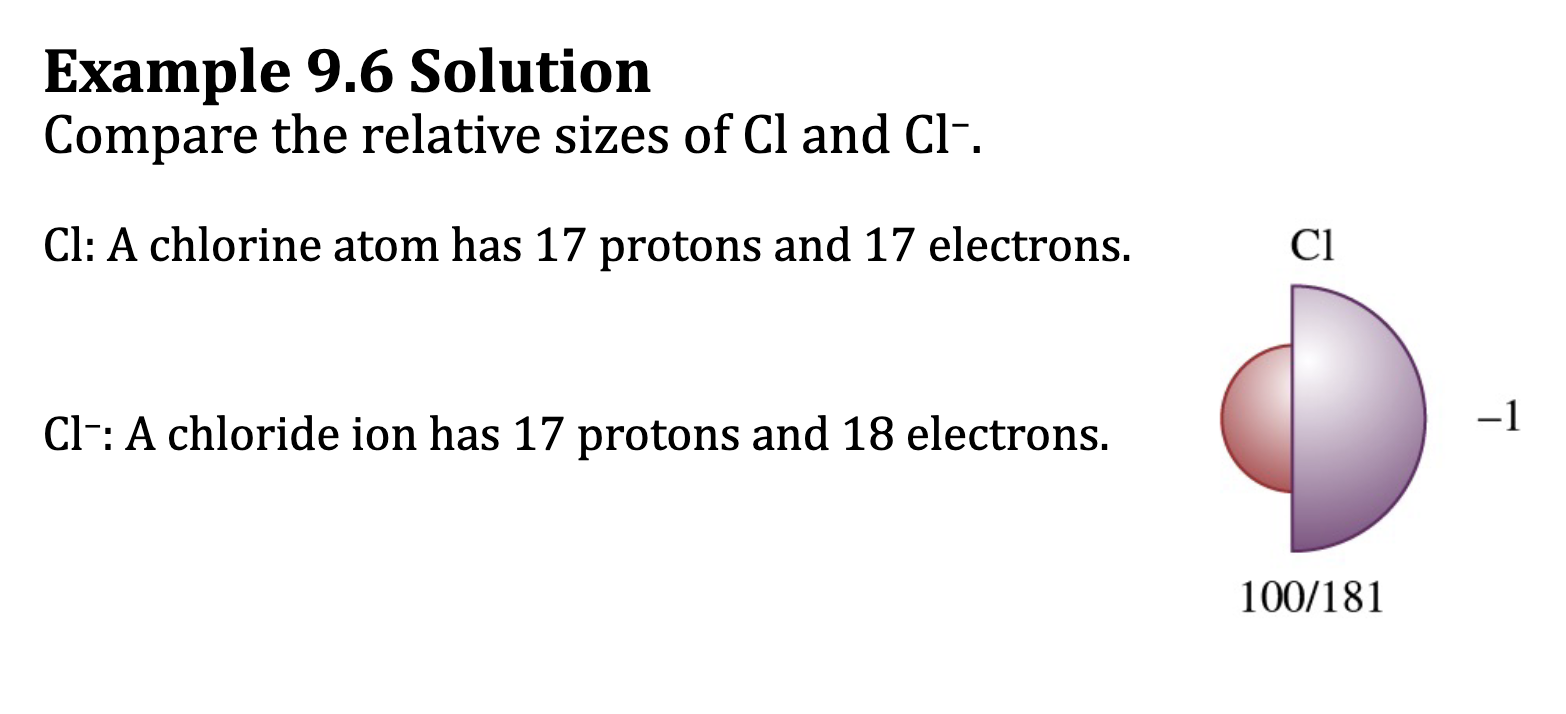
Ionic Radius: Anions
• Adding an electron to the valence shell increases the negative charge of
an atom and retains the positive charge of the nucleus.
O: 1s2 2s2 2p 4
O 2- : 1s2 2s2 2p 6
• This decreases the attraction between the valence electrons and the
nucleus and increases the repulsions between valence electrons.
• All anions have larger radii then their corresponding neutral atoms.

Ex 9.6
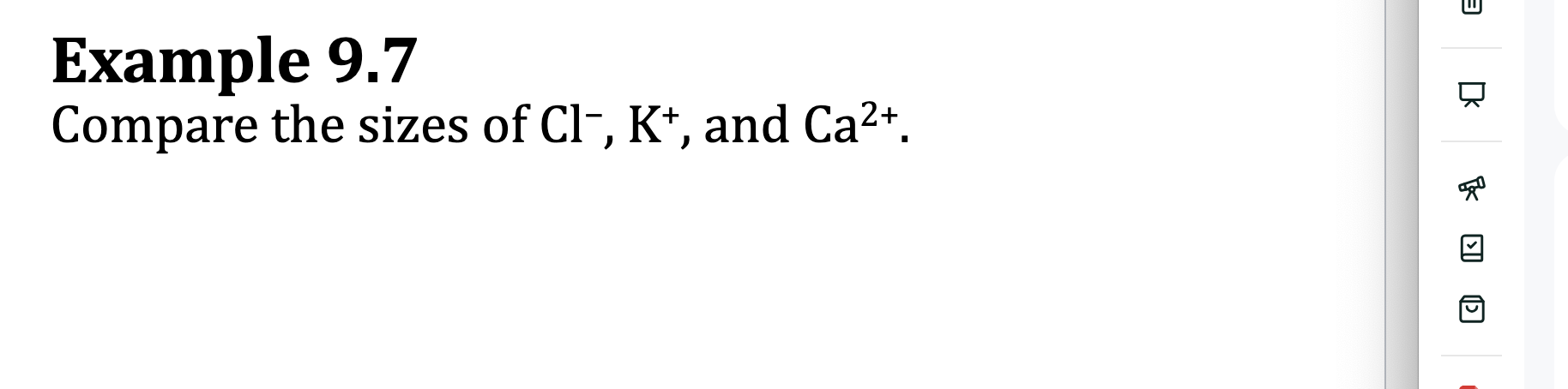
Ex 9.7
Species with highest atomic number would be the smallest
Ionization Energy
ionization energy, IE: the energy required to remove an electron from a gaseous atom to produce a gaseous cation.
Na(g) ⟶ Na+(g) + e– IE 1
IE is measure of nucleus–electron attraction. In general, smaller
atoms have high value for IE
Refer to Handout.
more stable = more energy to ionize
Fig. 9.7: Relative Periodic Trends in Ionization Energy and Atomic Radius
Exceptions to the General Trend in IE
When new subshells are occupied
E.x.: IE1 (BE) > IE1 (B)
Be: 1s²2s² and B: 1s²2s²2p^1
The 2s subshell is lower in energy than the 2p subshell, making it harder to remove an electron from the 2s subshell
Each time a new subshell begins, the IE of that element is lower (easier to ionize) than the previous one
Paired electrons in orbitals
E.x.: IE1 (N) > IE1 (O)
N: 1s²2s²2p³ and O: 1s²2s²2p^4
O has 4 electrons occupying 3 2p orbitals, so one orbital contains a pair of electrons, which repel each other. It takes less energy to remove one of a pair of electrons
Half-filled subshells are particularly stable
Second and Third Ionization Energies
First: energy required to remove an electron from a gaseous atom = +1 ion
Second: energy required to remove an electron from the +1 ion = +2 ion
Nuclear Charge, Z
equal to the number of the protons in the nucleus of the atom
Effective Nuclear Charge, Z subscript eff
particles or atoms with more than 1 electron have repulsions and attractions
valence electron experience a nuclear charge, Zeff, that is lower than the actual nuclear charge, Z (# of protons.
Zeff < Z
Valence electrons do not shield each other as much
Elecrons most effective in shielding are the core electrons
Larger effective nuclear charge means stronger attraction to the nucleus
Larger effective nuclear charge, the smaller the atom
not asked: Zeff = Z - S (S = Core electrons)
E.x. 9.7: Compare the sizes of Cl-, K+, and Ca²+
which has the largest amount of protons? large amounts of protons means greatest attracttions to the nucleus (When isoelectronic: same electron configurations)

Table 9.1: Successive Ionization Energies for Some Main Group Elements (kJ/mol)
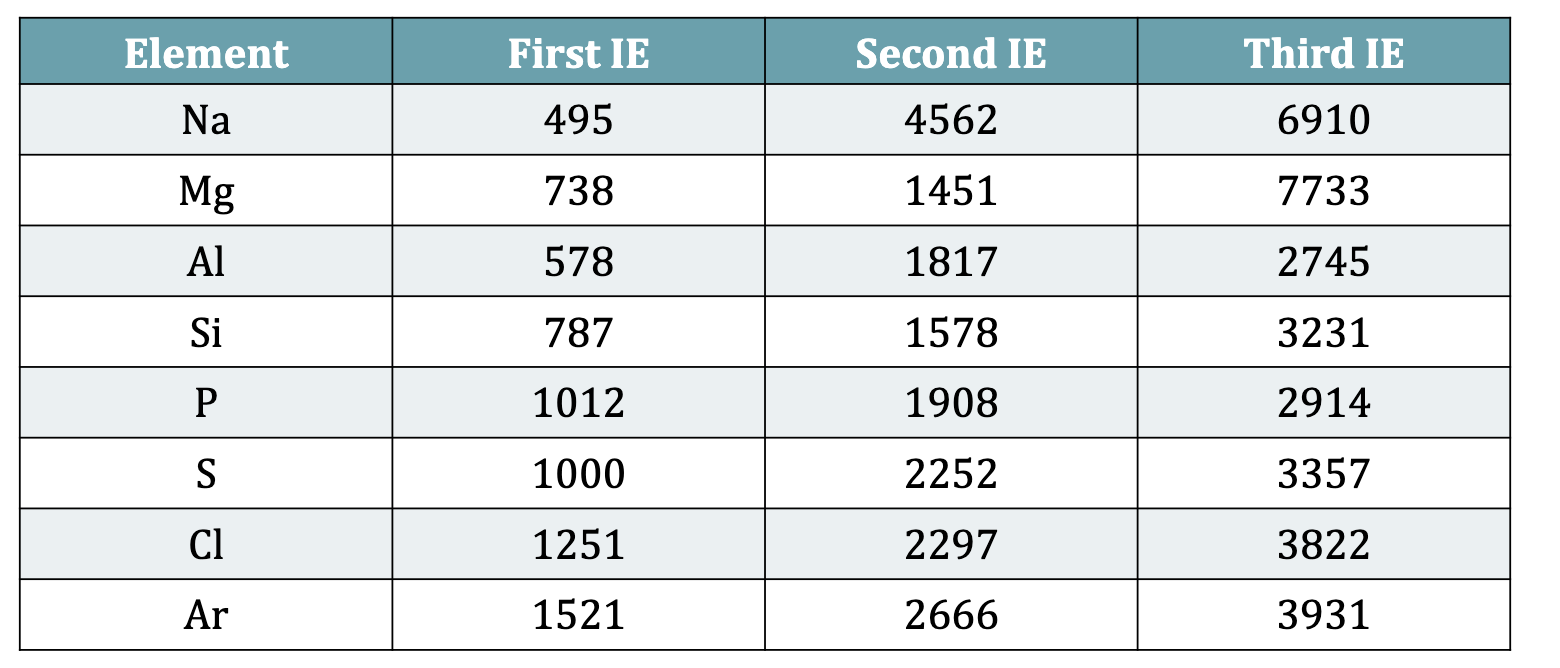
General Trends in subsequent Ionization Energies
IE3 > IE2 > IE1
removal of valence electrons is relatively easier, but removal of core electrons requires a lot more energy
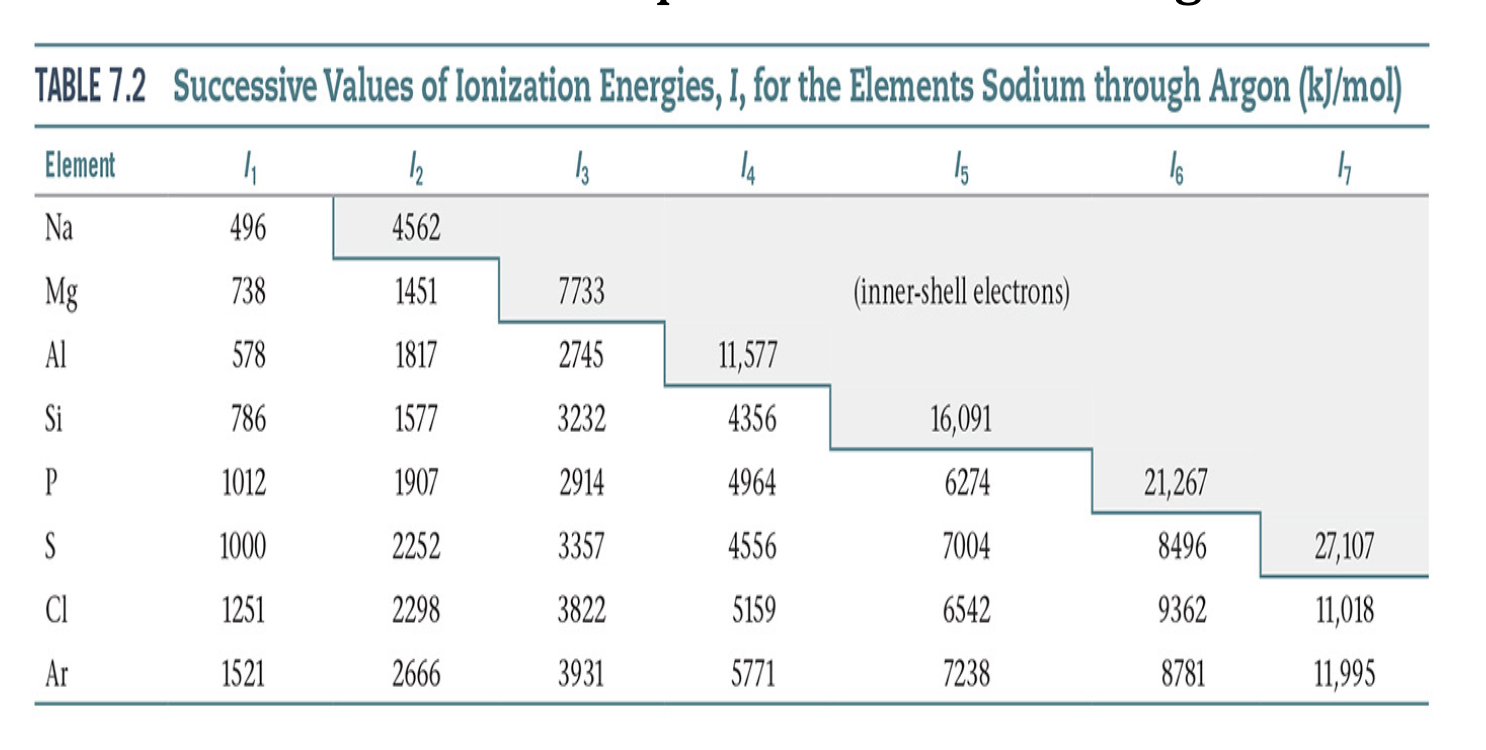

Ionization Energy: Example
difference in second and first is not as big as second to third

Electron Affinity, EA
the energy change associated w/ adding an electron to a gaseous atom
negative electron affinities = a decrease in energy when an electron is added. to a gas phase atom; exothermic
Positive electron affinities = endothermic process; energy is required to add an electron to the gaseous atoms
Figure 9.9: Electron Affinities of Some Elements (kJ/mol)
remember halogens are the most negative
noble gases have the most electronegative properties (idk i didnt hear her)
more electronegative (L to R)
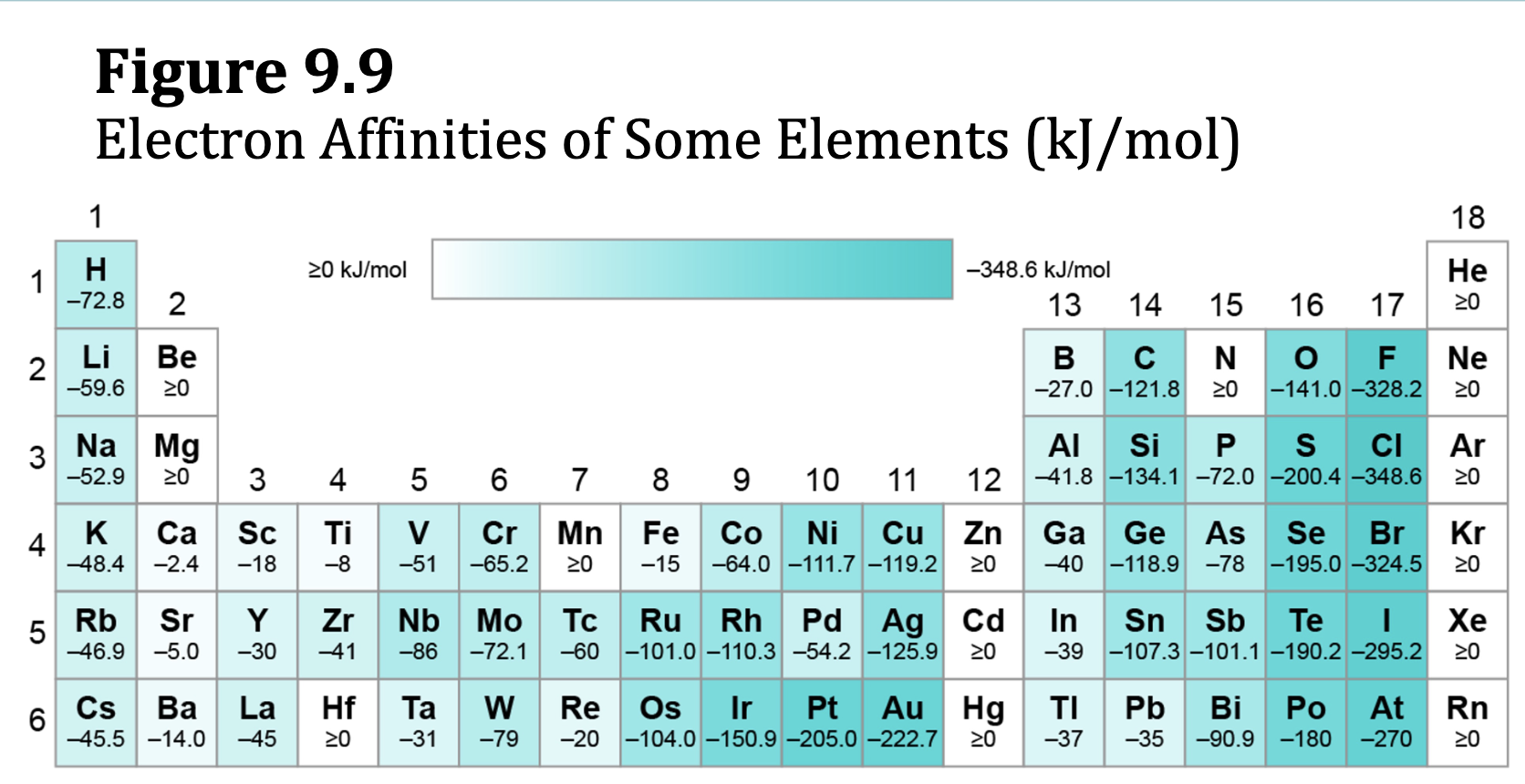

E.x. 9.10

Ions with Nobel Gas Configurations are Stable
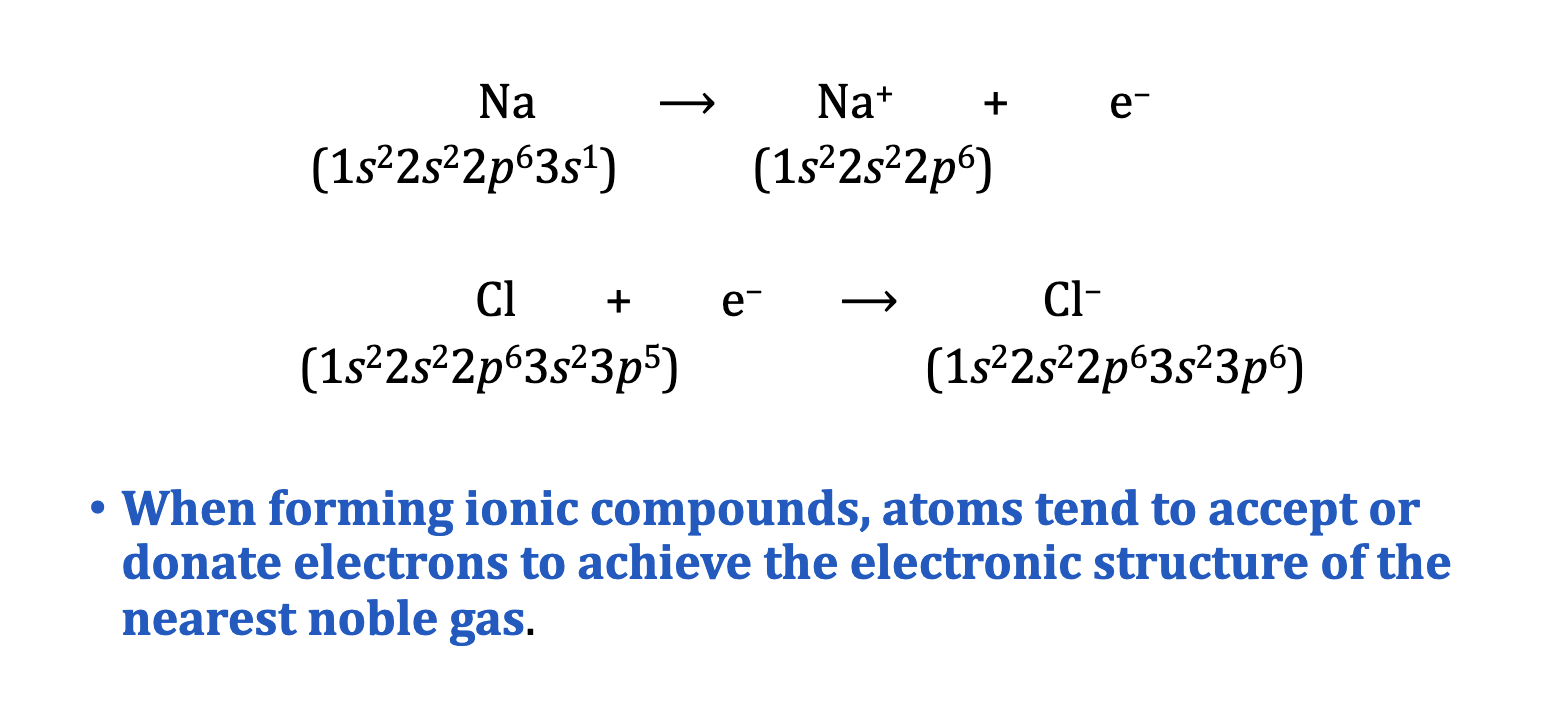
Ionic Lattice
ionic compounds do not have individual molecules
Lattice Energy
Energy released when gas-phase ions are converted into a solid compound
OR
Energy absorbed when a solid compound is converted into gas-phase ions
breaking = endothermic
K+(g) + Cl-(g) → KCl (s) deltaHL = -717kJ/mol
lattice neergy increasesas the charge of the ions increases
lattice energy decreases as the size of the ions increases
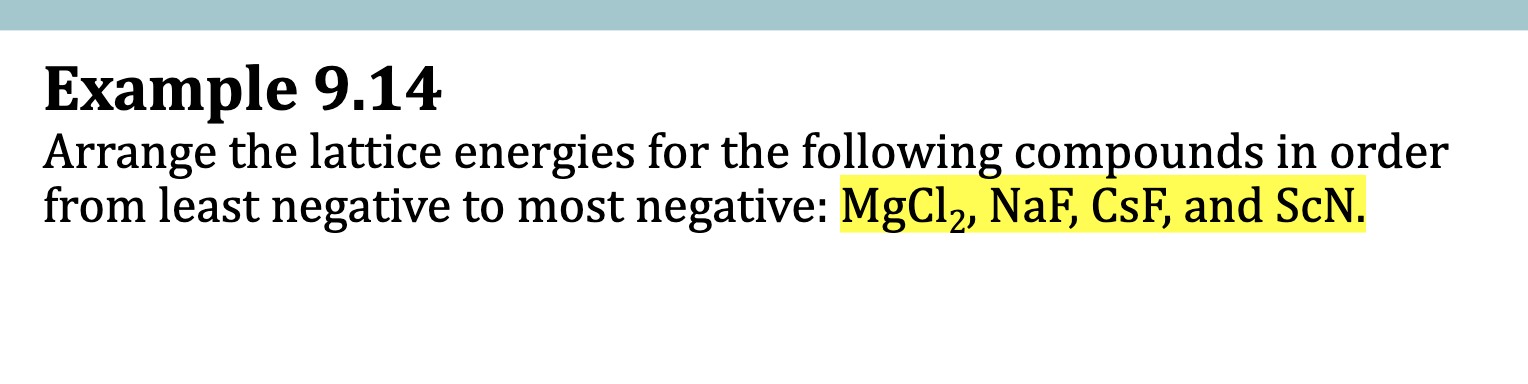
E.x. 9.14
Find the charges of each ion
Mg = 2+, Cl = -1
Na = + F = -
Cs = + F = -
Sc = 3+ N = 3-
Multiply the Charges
2 , 1 , 1, 9
Rank.
ScN > MgCl2 >idk
CsF < NaF < MgCl2 < ScN
Born-Haber Cycle
hypothetical reactions that use Hess’ Law to calculate lattice energies
Enthalpy of sublimination
Ionization energy (IE1, IE2, IE3 depending on the cation charge)
Bond energy, BE (energy required to break a chemical bond)
Electron affinity
Standard enthalpy of formation
Born-Haber Cycle Example: determine lattice energy of KCl from the given information
Start with solid state of metal that converts to gas (sublimination)
K (s) → K (g) 89 kJ
Formation of gas (ionization energy)
K (g) → K+(g) + e^- 418kJ
½ Cl2 (g) → Cl (g) 244kJ/2
Cl (g) + e- → Cl-(g) -349 kJ
Combine elements
K+ (g) + Cl-(g) → KCl (s) x kJ
Cancel like terms and add up all energies
K(s) + ½ Cl2(g) → KCl (s) = -437
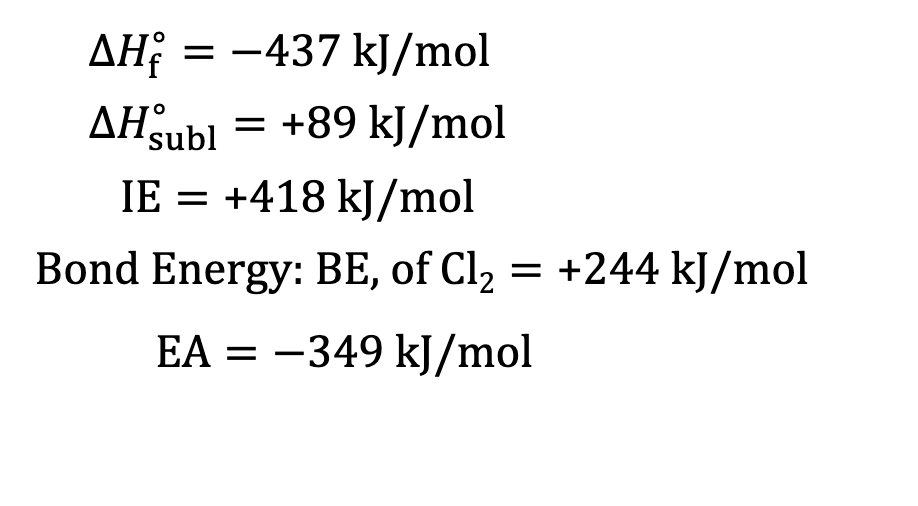
How to tell the Valence Electrons of an Element
look at the group number above the column
Isoelectronic
when a list of elements have the same amount of electrons,
to find effective nuclear charge: ion with the most protons
More protons = highest effective nuclear charge
As nuclear effective nuclear charge increases, electrons are pulled closer to nucleus, so radius decreases
How to determine a group element via successive ionization energies
Look at where the biggest jump occurs.
For instance, if this biggest jump is I2 to I3, that means the element is in group IIA because it is more stable at +2
How to Determine Elements of Which Group on PT RELEASE the most energy by gaining an electron?
Look at valence electron, if they have 7 valence electrons (i.e. Halogens), they only need 1 more to have a full outer shell.
Full outer shell = high electron affinity = larger amount of energy released when electron is gained
How to Determine Elements of Which Group on PT ABSORB the most energy by gaining an electron?
Look at valence electrons, if they have complete valence shell (8), they need to make a whole new shell = higher energy (they do not want to gain any more e because of this)
What does it mean to attain a noble gas electron configuration?
To have a full outer shell.
Metals have 1-3 electrons in their outer shell (valence) So, its easier for them to LOSE electrons to empty their outer shell (the next inner shell is full)
Nonmetals have 5-7 electrons in their outer shell (valence) So its easier for them to GAIN to fill up the shell
What does the Exception mean by ns2 or ns2np4
look at the electron config. of elements, if the highest energy subshell has an s and p subshell with the s full and p partially full (4 electrons), then they are a part of the exception
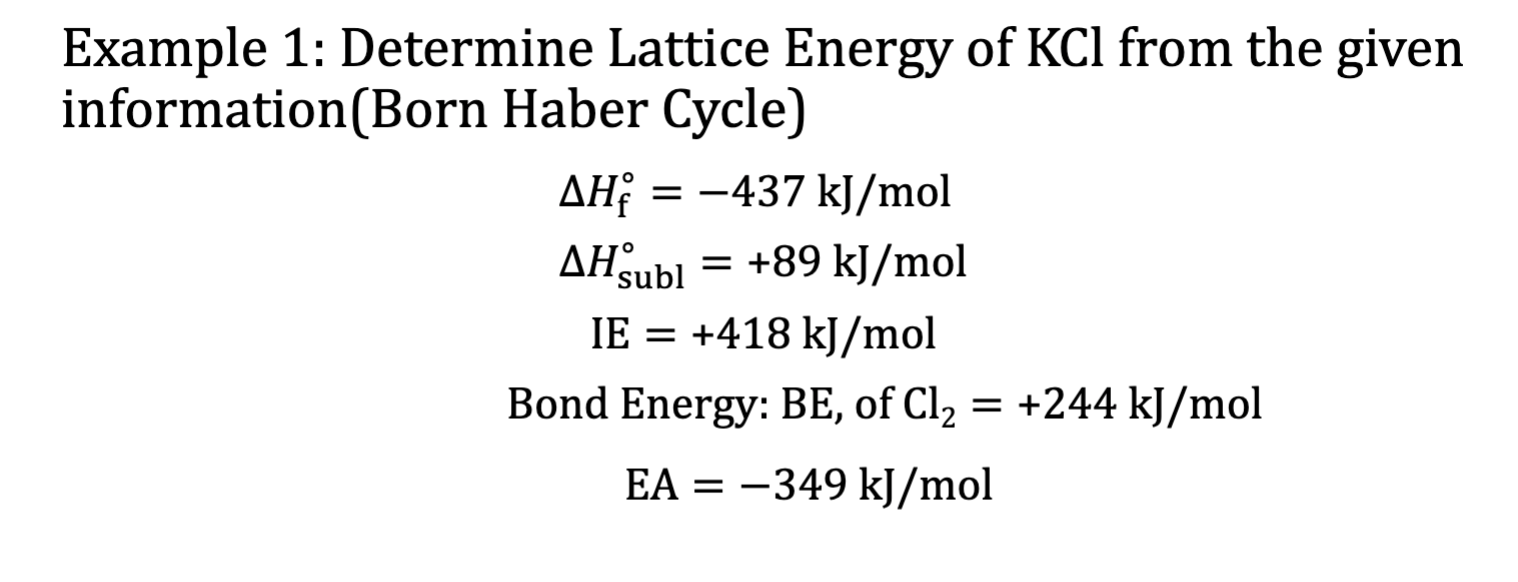
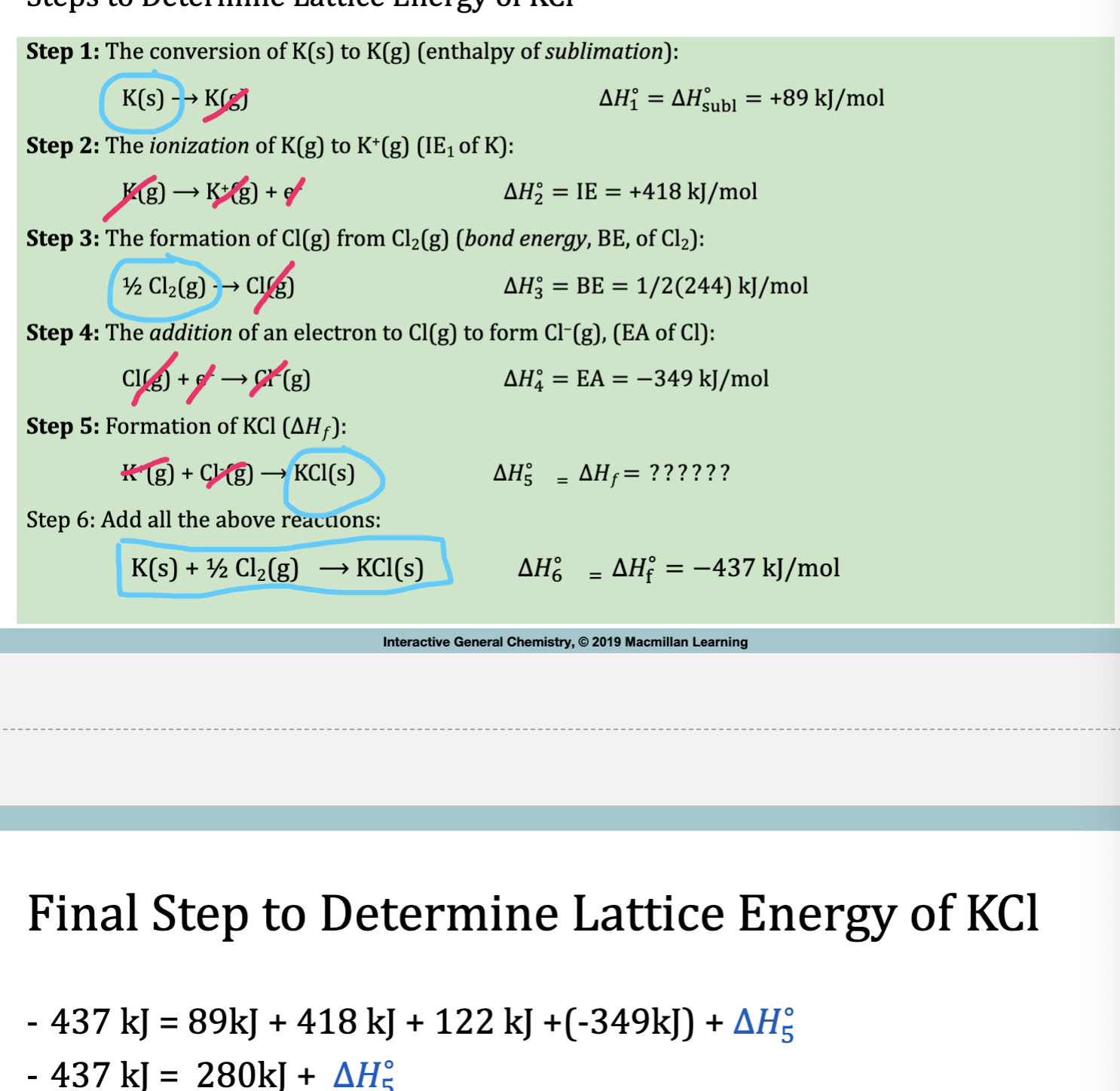
Example 1: Lattice Energy (Born Haber Cycle)
Recall what each variable means + their corresponding formula.
Hsubl = K(s) → K(g) 89kJ
IE = K(g) → e-+K+(g) 418 kJ
BE=½ Cl2(g) → Cl(g) (conversion of molecule to atom) 244/2 kJ
Cl needs to be an anion in order to combine
EA = Cl(g) + e- → Cl-(g) -349 kJ
K+(g)+Cl-(g) →KCl(s) xkJ
Add all equations and see what’s left over (cancel like terms on each side)
deltaHf= K(s) + ½ Cl- → KCl (s) = -437 kJ
-436 = 89+418+122+(-349)+ x
-437 = 280+x
-717 = deltaHlattice

Example 2
same as example 1